Abstract
We report a clinical case of a 39-year old male, without any known previous medical condition but with occupational exposure to paints and dust cement, who presented an autoimmune pulmonary alveolar proteinosis (PAP) triggered by exposure to toxic inhalation at his workplace. PAP is a rare lung disease characterized by intra-alveolar abnormal accumulation of surfactant. The presence of a crazy-paving pattern in high-resolution computed tomography scan brings the suspicion of PAP although histopathology results of bronchoalveolar lavage are always required for its final diagnosis. The autoimmune form of PAP due to toxic inhalation, such as the one here described, is rare and it is usually difficult to establish a causal relationship.
Keywords: Alveolar proteinosis, Hypoxemia, Toxic inhalation, Rare lung disease, Autoimmunity
1. Introduction
Pulmonary alveolar proteinosis (PAP) is a rare lung disease, first described as a distinct clinical entity in 1958 [1]. Its incidence is estimated to be 0.2–0.4 cases per million [[2], [3], [4]], with a male predisposition (2:1 to 3:1, male:female) [2,5]. Approximately 72% of patients are active smokers [2]. PAP is a surfactant-like disease characterized by intra-alveolar abnormal accumulation of surfactant. The complex surfactant homeostasis is disturbed in PAP [2,6,7], which can occur through different mechanisms, namely disruption of granulocyte-macrophage colony stimulating factor (GM-CSF) signaling (primary PAP), alveolar macrophages dysfunction (secondary PAP) or surfactant production disorders (congenital PAP) [3,5,8,9].
Autoimmune (aPAP) and secondary PAP (sPAP), occur typically in adulthood [3,5] and have similar clinical presentation [3]. Congenital PAP occurs in neonatal period causing acute respiratory distress (discussed somewhere else). The majority of cases are aPAP (approximately 90%) compared to sPAP which are only present in 4% of the cases [7,10]. The aPAP patients are slightly older, at the time of diagnosis, when compared to sPAP (between 39 and 51 years instead of 37–45 years, respectively) [11]. An aPAP is mediated by the formation of immunoglobulin G (IgG) auto-antibodies that block GM-CSF [12], whilst sPAP is associated with the presence of other conditions or co-morbidities, like infection, toxic inhalation, malignancy and immunodeficiency, without the formation of GM-CSF auto-antibodies [3,8,9].
Patients usually present a progressive exertional dyspnea of insidious onset associated with cough, tiredness, malaise or low grade fever. Hypoxemic respiratory failure is the major clinical finding [3], secondary to a right to left shunting effect of blood from an intact pulmonary capillary bed perfusing through poorly ventilated alveoli [13]. Unrecognizing this condition, especially if extensive disease is present, leads to progressive refractory hypoxemia and development of acute respiratory distress. Physical examination is usually normal with unspecific inspiratory crackles at pulmonary auscultation [2,5,13]. Differential diagnosis can be broad, like opportunistic diseases (e.g. pneumocystosis), pulmonary oedema, exogenous lipoid pneumonia, acute eosinophilic pneumonia, acute interstitial pneumonia, sarcoidosis, alveolar haemorrhage, invasive lepidic mucinous adenocarcinoma and drug-induced lung diseases. High-resolution computed tomography (HRCT) scan can be a helpful tool [14], showing ground-glass opacities combined with thickened polygonal septal lines and intralobular reticulations, predominantly in the lower lobes [15,16], resembling a cobblestone appearance, also known as a “crazy paving” pattern. This pattern is typical in PAP but unspecific and therefore not pathognomonic [14,16]. More rarely, PAP can also present an interstitial, focal, nodular or even fibrotic pattern [5,14]. BAL is useful to identify the presence of PAP [3,17,18].
2. Case presentation
We report a case of a 39-year-old Caucasian male with a 30 pack-year cigarette smoker, who denies other types of smoking. He has no relevant medical history and does not take any medication. He works as a maintenance worker, exposed to paints, sprays and cement dust. He has two cats at home. No medication allergies are known or other type of allergies.
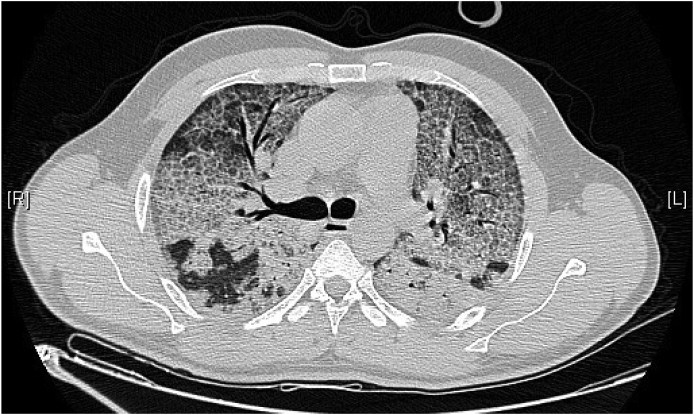
Since November 2019 he started to complain of dyspnea, on exertion only (mMRC 1 – modified Medical Research Council [Dyspnea Scale]). Three months later he started to refer progressive worsening of dyspnea (mMRC 3–4) associated with flu-like symptoms (dry cough and malaise). He denied having fever, night sweats or chills, hemoptysis, weight loss, anorexia, rashes, arthralgias or myalgias, recent travelling or injuries or sick contacts. He was first observed by his general practitioner and did a chest X-ray that revealed a diffuse bilateral heterogeneous alveolar infiltrate in the lower and middle lobes. He was started on antibiotic – amoxicillin 500mg – without any clinical improvements, which lead him to the emergency department. On physical examination, he presented dyspnea at rest and hypoxemic respiratory failure, with the following vital signs: blood pressure 153/83 mmHg, pulse rate 84 beats/min and oxygen saturation of 79% in room air. Arterial blood gas on room air revealed severe hypoxemia (paO2 44.4 mmHg; paCO2 36 mmHg; pH 7.410; HCO3− 22.3mmol/L – PaO2/FiO2 210). Cardiac, vascular, and abdominal examinations were all unremarkable. A HRCT scan was obtained and showed an irregular cobblestone pattern associated with ground-glass opacities with an anterior-posterior gradient, with superimposed intra and interlobular septal thickening (Fig. 1).
Fig. 1.
HRCT scan demonstrating combination of ground-glass opacities with thickened polygonal septal lines and intralobular reticulations compatible with a cobblestone appearance – “crazy paving” pattern.
He started high-flow nasal cannula (HFNC) remaining with peripheral oxygen saturation >94%. Amoxicillin plus clavulanic acid (1.2gr) and pulses of methylprednisolone (1gr/day) were started. Pulmonary hypertension was excluded with an echocardiogram. Flexible bronchoscopy with bronchoalveolar lavage (BAL) was performed. No endobronchial abnormalities were viewed and a milky lavage liquid was obtained. BAL cytology was normal and all microbiology testing was negative with an exception for Human Coronavirus NL63 (HCNL63). Serum findings were polycitemia (Hb 18g/dL), an elevated lactate dehydrogenase (LDH) (462 U/L) and a slightly elevated total IgE (215 U/ml). Additional blood and BAL analysis were performed which are resumed in Table 1. Histopathological testing from BAL was compatible with PAP. Antibiotic was suspended. He presented acne as a side effect from corticosteroids, which were also stopped. He remained without fever or any analytic markers of infection (protein C-reactive and procalcitonin were negative). After the diagnosis a whole lung lavage (WLL) was performed, with moderate tolerance but with some clinical improvement, after two lung lavages in two separate days. Re-evaluation through HRCT scan showed some radiologic improvement.
Table 1.
Laboratory results.
| Serum and BAL analyses | Relevant results |
|---|---|
| Kidney function, hepatic function, thyroid function, cardiac enzymes and NT-proBNP, D-dimer test | Normal |
| Autoimmunity, ACE, SV, Serum protein electrophoresis | Negative |
| HIV type 1 and 2, Parainfluenza virus type 1, 2 and 3, Influenza virus type A and B, IgM and IgG Mycoplasm, Legionella, Adenovirus, Metapneumovirus, Rhinovirus, Coronavirus type OC43, 229E and HKU1, Enterovirus, Human bocavírus, HBV, HCV, HAV | Negative |
| Mycobacterium tuberculosis and non-tuberculosis, Syphilis, Nocardia, Pneumocystis jirovecci, Rhodococcus | Negative |
| Immunophenotyping of BAL | Ratio CD4/CD8 2.66 |
| Galactomannan antigen | Negative |
HBV - Hepatitis B virus; HCV - Hepatitis C virus; HAV- Hepatitis A virus; HIV - Human immunodeficiency virus; ACE - Angiotensin-converting enzyme; SV – Sedimentation velocity; Hb – Hemoglobina.
3. Discussion
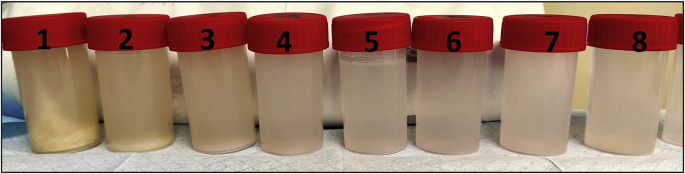
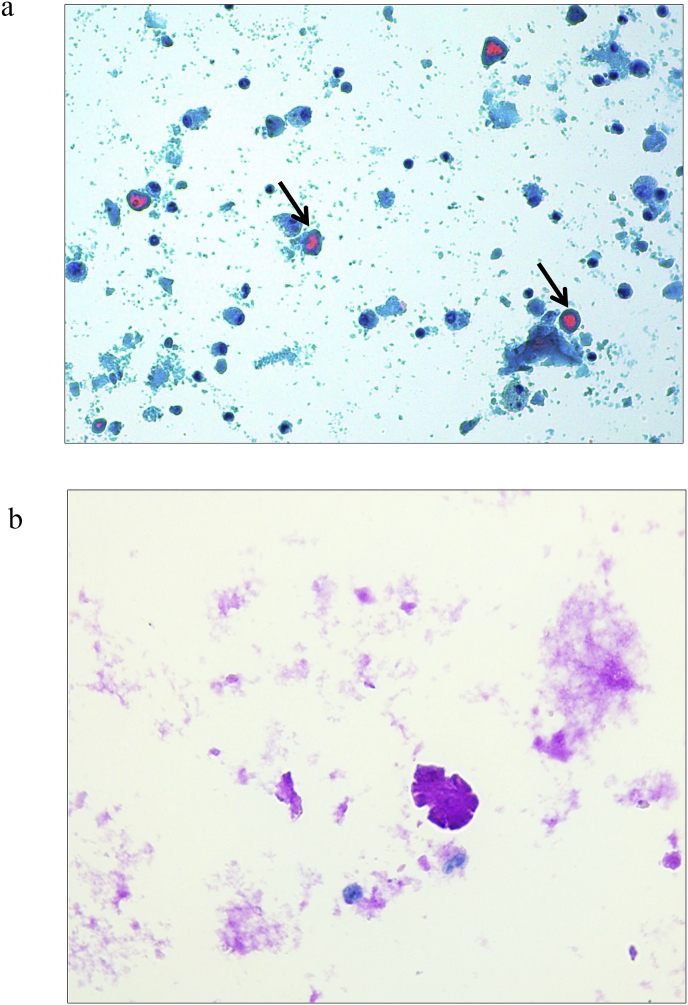
Diagnosing PAP is challenging. Clinical history and demographic features of our patient combined with lifestyle behaviors (active smoker), occupational exposure and the presence of a “crazy paving” pattern in HRCT scan – Fig. 1 – enhanced the suspicion for a PAP diagnosis [6,19]. However, exclusion of other diseases was needed in order to consider PAP's diagnosis. For that, serological and microbiology testing, in the serum and in the BAL, were performed. Some serological markers such as an elevated LDH, which is observed in 80% of PAP patients, and polycythemia, were present although these are unspecific [20]. Macroscopically, BAL had a milky appearance – Fig. 2 – which is typical in PAP patients. Histopathological examination showed noncellular, homogeneous, globular structures (basophilic stain) and lipoproteinaceous material staining positive for both May-Grünwald-Giemsa stain and Periodic acid-Schiff (PAS) stain [3,6,9,21] – Fig. 3a and 3b. These results are compatible with the filling of alveoli with noncellular, finely granular and eosinophilic material which represents lipoproteinaceous alveolar accumulation [9]. Pneumocystis jirovecci pneumonia and pulmonary oedema were both excluded as they stained negative for PAS. Pulmonary oedema lacks the coarse granules seen in PAP thru PAS staining; Grocott-Gomori methenamine silver staining was negative for Pneumocystis jirovecci [22]. Other infectious were excluded (Table 1), with the only exception for HCNL63. Histopathological findings from BAL made the final diagnosis of PAP [3,17,18].
Fig. 2.
Milky appearance of the bronchoalveolar lavage fluid acquired during first WLL – tubes ordered from the beginning of procedure (no. 1) to the end (no. 8), demonstrating that the milky fluid is becoming lighter as the lipoproteinaceos material is being washed out from the lungs during the procedure.
Fig. 3.
a Bronchoalveolar lavage filled with globular, homogeneous, eosinophilic proteinaceous material (arrow) (Thin prep, May-Grünwald-Giemsa stain). b The proteinaceous material, from bronchoalveolar lavage, stained positive with PAS, consistent with Pulmonary Alveolar Proteinosis (PAS histochemical stain).
Further investigations were performed to determine the nature of PAP. Immunodeficiencies, hematologic disorders or major toxic disorders were not detected. High titers of auto-antibodies (IgG) against GM-CSF were detected in the serum of our patient, when compared to positive controls (in serial dilutions - 1/50; 1/250; 1/1000), reported to us by an outside laboratory (INSERM UMR 1163, see acknowledgments).
Upon these results we consider to be in the presence of aPAP [7,12] that was triggered by cement dust and paints exposure. As previously reported, occupational exposures are important risk factors for development of autoimmune diseases [23]. Some cohort PAP studies have reported positive GM-CSF auto-antibodies from toxic inhalation [4,24,25] suggesting that an unknown autoimmune disease could be triggered by it, like an aPAP [25,26]. The true understanding of the role of occupational exposure in aPAP remains to be elucidated, but occupational agents can act as adjuvants, or induce apoptosis of cells resulting in increased exposure to sequestered auto-antigens, leading to immune system reactivity in the form of auto-antibodies, in this case thru formation of GM-CSF auto-antibodies [23,25], thus promoting lung injury. Also gene-environment interactions including the presence of gene polymorphisms may play a critical role [25].
Viruses are usually frequent complications in PAP [2]. HCNL63 is mainly responsible for mild respiratory symptoms in healthy individuals [27], but in immunocompromised patients it can lead to serious respiratory distress. The presence of HCNL63 has likely contributed to further promote a significant clinical deterioration in our patient. As previously recognized, GM-CSF acts as part of signaling pathways in innate and adaptive immune response, being particularly important for differential maturation and function of alveolar macrophages in the lungs and basal functional capacity of neutrophils [12]. Therefore, very high levels of GM-CSF auto-antibodies, like in aPAP, dramatically reduce GM-CSF bioactivity, thereby diminishing GM-CSF activation of myeloid cell functions [12], which are important for antiviral responses and viral clearance [28]. Opportunistic infections may then arise due to host multifactorial susceptibility (macrophage and neutrophils dysfunction, impaired host defense owing to abnormalities in surfactant and intra-alveolar accumulations promoting microorganism growth) or secondary to the use of corticosteroids. Because of this, corticosteroids must be avoided as empirical treatment in PAP [13].
Standard treatment for symptomatic PAP is still WLL [29], which was first developed in the 1960s. This technique allows removal of lipoproteinaceous material accumulated in the lungs resulting in significant clinical improvement and respiratory insufficiency resolution [30,31]. Even though associated with some adverse effects [32], usually 60% of the patients have a good response within two washes per lung with almost complete recovery [3,13]. Long term results have been reported in aPAP after WLL [29]. WLL associated or not with GM-CSF replacement therapy has shown good results in aPAP [31,[33], [34], [35]]. Nevertheless, prevention of contact with the suspected occupational agent is required to avoid future flares [8].
4. Conclusion
Our clinical case demonstrates the importance of clinical suspicion in rare lung diseases, such as PAP. Autoimmune PAP triggered by toxic inhalation in professional settings is rare but it has been increasingly noticed [2,24,25]. How occupational exposure triggers the development of aPAP is still unknown and controversial. Although different possible mechanisms have been proposed further investigations are still needed. The case presented here further supports the link between toxic inhalation of dust cement and paints with development of an aPAP.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None
Acknowledgements
We want to thank the Pneumonology Department from Hospital Garcia de Orta, Almada, Portugal ;Pneumonology Department and Respiratory Critical Care Unit from Centro Hospitalar Lisboa Norte, Lisbon, Portugal, for supporting this work.
We also thank Doctor Anne Puel and her research team, Génétique Humaine des Maladies Infectieuses/Human Genetics of Infectious Diseases, at INSERM UMR 1163, Université de Paris, France, for the analysis of the GM-CSF antibodies and interpretation of laboratory results.
References
- 1.Rosen Samuel H., Castleman Benjamin, Liebow Averill A. Pulmonary alveolar proteinosis. N. Engl. J. Med. 1958;258(23) doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- 2.Seymour J.F., Presneill J.J. State of the art: pulmonary alveolar proteinosis, progress in the first 44 years. Am. J. Respir. Crit. Care Med. 2002;166:215–235. doi: 10.1164/rccm.2109105. [DOI] [PubMed] [Google Scholar]
- 3.Borie R., Danel C., Debray M., Taille C., Dombret M., Aubier M. Pulmonary alveolar proteinosis. Eur. Respir. J. 2011;20(120):98–107. doi: 10.1183/09059180.00001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue Y.1, Trapnell B.C., Tazawa R., Arai T., Takada T., Hizawa N., Kasahara Y., Tatsumi K., Hojo M., Ichiwata T., Tanaka N., Yamaguchi E., Eda R., Oishi K., Tsuchihashi Y., Kaneko C., Nukiwa T., Sakatani M., Krischer J.P., NKJC of the RLDC Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am. J. Respir. Crit. Care Med. 2008;177(7):752. doi: 10.1164/rccm.200708-1271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juvet S.C., Hwang D., Waddell T.K., Downey G.P. Rare lung diseases II : pulmonary alveolar proteinosis (Review) Canc. Res. J. 2008;15(4):203–210. doi: 10.1155/2008/528948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trapnell B.C., Nakata K., Bonella F. Pulmonary alveolar proteinosis. Nat Rev Dis Prim. 2019;5(16) doi: 10.1038/s41572-019-0066-3. [DOI] [PubMed] [Google Scholar]
- 7.Trapnell B.C., Whitsett J.A., Nakata K. Pulmonary alveolar proteinosis. N. Engl. J. Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 8.Blirando K. Pulmonary alveolar proteinosis management: current and future therapeutic strategies. EC Pulmonol Respir Med. 2019;8(2):140–158. [Google Scholar]
- 9.WDCT T., MN K., ML R.-C., NL M., TE K.J. vol. 3. American Registry of Pathology; Washington, DC: 2002. Pulmonary alveolar proteinosis; pp. 169–176. (Non-Neoplastic Disorders of the Lower Respiratory Tract. Atlas of Non-Tumor Pathology). [Google Scholar]
- 10.Campo I., Mariani F., Rodi G. Assessment and management of pulmonary alveolar proteinosis in a reference center. Orphanet J. Rare Dis. 2013;8(1):1–7. doi: 10.1186/1750-1172-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D., Tian X., Feng R. Secondary pulmonary alveolar proteinosis: a single-center retrospective study (a case series and literature review) BMC Pulm. Med. 2018;18(15):1–7. doi: 10.1186/s12890-018-0590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchida K., Nakata K., Suzuki T. Granulocyte/macrophage – colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects. Blood. 2009;113(11):2547–2556. doi: 10.1182/blood-2008-05-155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah P.L., Hansell D., Lawson P.R., Reid K.B.M., Morgan C.V. Pulmonary alveolar proteinosis : clinical aspects and current concepts on pathogenesis. Thorax. 2000;55:67–77. doi: 10.1136/thorax.55.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holbert J.M., Costello P., Hoffman R.M., Rogers R.M. CT features of pulmonary alveolar proteinosis. Am. Journal. Rev. 2001;176:1287–1294. doi: 10.2214/ajr.176.5.1761287. [DOI] [PubMed] [Google Scholar]
- 15.Rossi S.E., Erasmus J.J., Volpacchio M., Franquet T., Castiglioni T., Page H. “Crazy-Paving” pattern at thin-section CT of the lungs: radiologic- pathologic overview. Radiographics. 2003;23:1509–1519. doi: 10.1148/rg.236035101. [DOI] [PubMed] [Google Scholar]
- 16.Wever W De, Meersschaert J., Coolen J. The crazy-paving pattern : a radiological-pathological correlation. Insights Imaging. 2011;2:117–132. doi: 10.1007/s13244-010-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maygarden S.J., Iacocca M.V., Funkhouser W.K., Novotny D.B. Pulmonary alveolar proteinosis: a spectrum of cytologic, histochemical, and ultrastructural findings in bronchoalveolar lavage fluid. Diagn. Cytopathol. 1999;24(6):389–395. doi: 10.1002/dc.1086. [DOI] [PubMed] [Google Scholar]
- 18.Wells A.U. The clinical utility of bronchoalveolar lavage in diffuse parenchymal lung disease. Eur. Respir. Rev. 2010;19(117):237–241. doi: 10.1183/09059180.00005510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allwood B.W., Stellenbosch University Stellenbosch S.A. Crazy paving in pulmonary alveolar proteinosis. N. Engl. J. Med. 2020:2020. doi: 10.1056/NEJMicm1908563. [DOI] [PubMed] [Google Scholar]
- 20.Aguiar M., Monteiro P., Marques M.M., Feijó S., Rosal J.M., Almeida AB De. Whole lung lavage – report of four cases of alveolar proteinosis. Rev. Port. Pneumol. 2009;15(1):77–88. doi: 10.1016/S0873-2159(15)30111-2. [DOI] [PubMed] [Google Scholar]
- 21.Thrall M. Pulmonary alveolar proteinosis and mimics. 2020. https://app.expertpath.com/document/pulmonary-alveolar-proteinosis-and-%0A/066f5663-baf7-4702-84fd-3d42e096989a%0A ExpertPath. Published.
- 22.Suster S., Moran C. Alveolar proteinosis. ExpertPath. 2020. https://app.expertpath.com/document/alveolar-proteinosis/e7064404-6d7e-4e58-be3c-b2abc35ee6aa Published.
- 23.Cooper G.S., Miller F.W., Germolec D.R. Occupational exposures and autoimmune diseases. Int. Immunopharm. 2002;2(2–3):303–313. doi: 10.1016/s1567-5769(01)00181-3. [DOI] [PubMed] [Google Scholar]
- 24.Bonella F., Bauer P.C., Griese M., Ohshimo S., Guzman J., Costabel U. Pulmonary alveolar proteinosis: new insights from a single-center cohort of 70 patients. Respir. Med. 2011;105(12):1908–1916. doi: 10.1016/j.rmed.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Costabel U., Nakata K. Pulmonary alveolar proteinosis associated with dust inhalation not secondary but. Autoimmune ? 2010;11:427–428. doi: 10.1164/rccm.200912-1821ED. [DOI] [PubMed] [Google Scholar]
- 26.Villar A., Rojo R. Alveolar proteinosis: the role of anti-GM-CSF antibodies. Arch. Bronconeumol. 2018;54(12):601–602. doi: 10.1016/j.arbres.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Abdul-rasool S., Fielding B.C. Understanding human Coronavirus HCoV-NL63. Open Virol. J. 2010;4:76–84. doi: 10.2174/1874357901004010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sever-chroneos Z., Murthy A., Davis J. GM-CSF modulates pulmonary resistance to influenza A infection. Antivir. Res. 2011;92(2):319–328. doi: 10.1016/j.antiviral.2011.08.022.GM-CSF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beccaria M., Luisetti M., Rodi G. 2004. Long-term Durable Benefit after Whole Lung Lavage in Pulmonary Alveolar Proteinosis; pp. 526–531. [DOI] [PubMed] [Google Scholar]
- 30.Gay P., Nowak S., Anevlavis S., Lovis A. Efficacy of whole-lung lavage in pulmonary alveolar Proteinosis : a multicenter international study of GELF. Respiration. 2017;2:198–206. doi: 10.1159/000455179. [DOI] [PubMed] [Google Scholar]
- 31.Awab A., Khan M.S., Youness H.A. Whole lung lavage — technical details , challenges and management of complications. J. Thorac. Dis. 2017;9(6):1697–1706. doi: 10.21037/jtd.2017.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi S., Chen L., Qiu X., Zhao Q., Xiao Y. Valuable serum markers in pulmonary alveolar proteinosis. Hindawi. 2019;2019 doi: 10.1155/2019/9709531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheng G., Chen P., Wei Y., Chu J., Cao X., Zhang H. Better approach for autoimmune pulmonary alveolar proteinosis treatment: inhaled or subcutaneous granulocyte-macrophage colony-stimulating factor: a meta-analyses. Respir. Res. 2018;19(163):1–11. doi: 10.1186/s12931-018-0862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohkouchi S., Akasaka K., Ichiwata T. Sequential granulocyte-macrophage colony-stimulating factor inhalation after whole-lung lavage for pulmonary alveolar proteinosis. A report of five intractable cases. Ann Am Thorac Soc. 2017;8:1298–1304. doi: 10.1513/AnnalsATS.201611-892BC. [DOI] [PubMed] [Google Scholar]
- 35.Tazawa R., Trapnell B.C., Inoue Y. Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am. J. Respir. Crit. Care Med. 2010;181:1345–1354. doi: 10.1164/rccm.200906-0978OC. [DOI] [PMC free article] [PubMed] [Google Scholar]