Abstract
The rapid spread of coronavirus disease 2019 (COVID-19) has exceeded the standard capacity of many hospital systems and led to an unprecedented scarcity of resources, including the already limited resource of extracorporeal membrane oxygenation (ECMO). With the large amount of critically ill patients and the highly contagious nature of the virus, significant consideration of ECMO candidacy is crucial for both appropriate allocation of resources as well as ensuring protection of health care personnel. As a leading pediatric ECMO program in the epicenter of the pandemic, we established new protocols and guidelines in order to continue caring for our pediatric patients while accepting adult patients to lessen the burden of our hospital system which was above capacity. This article describes our changes in consultation, cannulation, and daily care of COVID-19 positive patients requiring ECMO as well as discusses strategies for ensuring safety of our ECMO healthcare personnel and optimal allocation of resources.
Level of Evidence
Level V.
Key words: COVID-19, Extracorporeal membrane oxygenation (ECMO), Acute respiratory distress syndrome (ARDS), Pandemic, SARS-CoV-2
The first cases of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were diagnosed in December 2019 in Wuhan, China, the initial epicenter of the pandemic [1]. As the virus spread worldwide, the World Health Organization (WHO) declared a pandemic in February 2020, with New York City (NYC) becoming the epicenter in March. As of May 26, 2020, 196,623 cases have been diagnosed in NYC, 51,287 cases requiring hospitalization with 16,565 confirmed deaths [2]. As was experienced internationally, the rapid spread of COVID-19 resulted in a scarcity of medical equipment, intermittent medication shortages, lack of medical personnel, and notable in NYC the inadequate space to treat patients resulting in significant expansion of intensive care unit (ICU) and inpatient facilities both within hospital systems and with the addition of new health care facilities.
Although less commonly required than ventilators and dialysis machines during this pandemic, extracorporeal membrane oxygenation (ECMO) is another scarce resource causing concern regarding resource allocation for the most critically ill population. The use of ECMO for patients with severe acute respiratory distress syndrome (ARDS) despite maximal medical treatment gained popularity during the influenza A (H1N1) pandemic of 2009 with several studies demonstrating the benefit of ECMO in this patient population [[3], [4], [5]]. Notably, Noah et al. demonstrated that hospital mortality rate for patients with H1N1-related ARDS receiving ECMO was significantly less than matched patients who were not cannulated onto ECMO (23.7% vs 52.5%, P = 0.006) [4]. A retrospective review by Beurtheret et al. noted that out of 12 patients cannulated onto veno-venous ECMO secondary to H1N1 associated respiratory failure, 10 patients (83.3%) were alive at 12 months follow up [5]. Subsequently, multiple studies have further supported the use of ECMO in patients with respiratory failure outside of the H1N1 pandemic [[6], [7], [8], [9]]. In 2018, a retrospective study on Middle East respiratory syndrome (MERS) patients with refractory hypoxemic respiratory failure indicated that ECMO should be used as a rescue therapy as ECMO use was associated with significantly lower mortality than those treated with conventional medical therapies (65% vs 100%, P = 0.02) [9]. ECMO use for patients with refractory respiratory failure secondary to COVID-19 was initially reported in Wuhan, China [[10], [11], [12], [13]]. Although outcomes for ECMO in COVID-19 patients in the United States have not yet been reported, this advanced form of life support has continued to be used to treat the most critically ill patients during the current pandemic.
Although the number of centers providing ECMO in the United States has increased since the H1N1 pandemic in 2009, few are considered high volume centers. Subsequently, ECMO remains a limited resource. As data supports that ECMO outcomes are better in higher-volume centers, referral to such centers during times of increased need has been the experience during the COVID-19 pandemic [14]. New York-Presbyterian Columbia and Morgan Stanley Children's Hospital of New York-Presbyterian (MSCHONY) are both high volume ECMO centers accepting transfers from hospitals both within and separate from the New York-Presbyterian (NYP) hospital system. As the pandemic peaked in April, MSCHONY started to accommodate adult patients requiring intensive care as our partner adult hospital was over capacity. Among these patients were several critically ill patients requiring ECMO assistance. Aside from the adjustment of pediatric healthcare personnel caring for adult patients, allocation of an already scarce resource in high demand had to be meticulously decided upon.
As noted by Ramanathan et al., ECMO preparedness in times of high demand such as during the COVID-19 pandemic is essential [15]. As a pediatric hospital in the epicenter of the current international pandemic, systems were created to continue providing care for our pediatric patients while supporting our colleagues throughout the NYP healthcare system. This article will describe the sustainable protocols and guidelines created by our pediatric ECMO team that ensure quality care for both pediatric and adult patients, appropriate resources for our non-COVID-19 positive patients, and safety of all healthcare personnel. This article was approved by our institutional review board (IRB).
1. General ECMO
1.1. Pediatric ECMO program
As a pediatric hospital, our primary mission is to provide medical treatment for pediatric patients, which cannot be overlooked even in a time of crisis. During this pandemic, although we have canceled all elective surgeries in guidance with the American College of Surgeons (ACS) for pediatric surgery and the American Pediatric Surgical Association (APSA) guidelines, we have continued with emergent surgical cases, the majority of which are congenital cardiac cases which contribute significantly to our population of patients who require ECMO [16]. We regionalized our enterprise's pediatric care to our children's hospital so that our satellite hospitals could surge their capacity for adult care. With the potential increase of our pediatric needs, our ECMO guidelines had to account for appropriate triaging of both pediatric and adult cases.
The pediatric ECMO team consists of two ECMO fellows, multiple pediatric surgeons, pediatric intensivists, and a team of pediatric perfusionists. The average ECMO census at MSCHONY at any given time is two to three patients with capacity at five to six cannulated patients. In April, we accepted our first adult, COVID-19 positive patient cannulated onto ECMO. At that time, we had four pediatric ECMO patients including one COVID-19 positive patient and two neonates being treated as a patient under investigation (PUI) as they were born to COVID-19 positive mothers. As we remained close to maximum capacity for total ECMO patients, consideration of allocation of resources was crucial.
1.2. Adult ECMO patients
Although the need for ECMO in pediatric patients with COVID-19 is rare, this already scarce resource was utilized more frequently in adult patients with hypoxemia despite maximal medical management. As the cases in NYC began to exponentially increase, requests for transfer of patients requiring ECMO to our adult partner hospital increased, surpassing their capacity to provide the necessary intensive care that is required for so many critically ill patients. Although our pediatric hospital was caring for multiple COVID-19 positive children including one patient requiring ECMO, the burden of disease was much less, resulting in our ability to take adult patients requiring ICU care. Our pediatric intensive care units (PICU) managed numerous adult patients with the ECMO service accepting patients on ECMO ranging in age from 24 to 52 years of age. All of the adult ECMO patients were either cannulated at our adult partner hospital or at the referring hospital by our adult ECMO team before transfer to our facility (Table 1 ). The pediatric ECMO team actively consulted on all critically ill adults cared for in the PICU. Upon admission to our ICU, these patients were cared for primarily by the pediatric ICU and ECMO teams with peripheral assistance from the corresponding adult teams. All patients were cannulated onto veno-venous ECMO with a femoral and an internal jugular cannula. All patients have arrived on pressors and have required chest tubes secondary to spontaneous pneumothoraces, with two patients also requiring continuous veno-venous hemofiltration (CVVH) secondary to renal failure.
Table 1.
Patient and cannulation characteristics for COVID-19 positive and PUI patients requiring ECMO support and treated at MSCHONY.
| COVID-19 positive ECMO patients cared for at MSCHONY | |||||||
|---|---|---|---|---|---|---|---|
| Age (years) | COVID-19 status | Comorbidities | Indication | Cannulation Protocol | Configuration | Location of Cannulation | Cannulating Team |
| 14 | Positive | Dilated cardiomyopathy | Worsening heart failure | Modified COVID protocol | V-A (femoral vein/artery) | MSCHONY | Pediatric ECMO |
| 24 | Positive | None | Respiratory failure | Modified COVID protocol | V-V (femoral vein, IJ) | Outside hospital | Adult ECMO |
| 45 | Positive | Obesity, hypertension | Respiratory failure | Modified COVID protocol | V-V (femoral vein, IJ) | Outside hospital | Adult ECMO |
| 52 | Positive | Diabetes mellitus type 2, obesity, asthma | Respiratory failure | Modified COVID protocol | V-V (femoral vein, IJ) | Adult ICU | Adult ECMO |
| Age (days) | COVID-19 status | Comorbidities | Indication | Cannulation protocol | Configuration | Location of cannulation | Cannulating team |
| 1 | PUI – treated as positive | None | Respiratory failure due to meconium aspiration | Modified COVID protocol | V-A (common carotid artery, IJ) | MSCHONY | Pediatric ECMO |
| 1 | PUI – treated as positive | Congenital diaphragmatic hernia | Respiratory failure | Modified COVID protocol | V-A (common carotid artery, IJ) | MSCHONY | Pediatric ECMO |
V-A = venoarterial.
V-V = veno-venous.
IJ = internal jugular vein.
PUI = patient under investigation.
2. SARS-CoV-2 testing guidelines
All adult ICU and ECMO patients transferred to our hospital have a confirmed diagnosis of SARS-CoV-2 prior to transfer. In regard to pediatric patients, all patients admitted from the emergency department were tested for SARS-CoV-2 and results received before admission to a unit. Patients transferred from outside hospitals were retested upon arrival regardless of prior results. This allowed for immediate knowledge of SARS-CoV-2 status on all new patients admitted to the hospital. A more challenging population in regard to testing is our neonates. The risk of vertical transmission from SARS-CoV-2 positive mothers remains unknown as well as accuracy and ideal timing of testing in the neonate population. In the obstetrics and gynecology department at NYP Allen Hospital and Columbia University Irving Medical Center between March 22 and April 4, 15.4% of women who delivered infants tested positive for SARS-CoV-2, 87.9% of which were asymptomatic at presentation [17]. All women admitted for delivery were tested with nasopharyngeal swabs and a quantitative polymerase-chain-reaction test to detect SARS-CoV-2 infection. All newborns of positive mothers were tested at 24 h of life to avoid potential false positives from maternal secretions and retested at 14 days of life. Regardless of the result of the initial test at 24 h, all neonates of positive mothers were treated as PUI until tested again at 14 days old. These patients are placed in negative pressure rooms and cannulation guidelines for SARS-CoV-2 positive patients as well as standard contact and droplet precautions including N95 respirator, surgical mask, eye protection, gown and gloves were used. Only after a negative test result at 14 days of life is a neonate considered not to be infected and removed from the negative pressure room as well as contact and droplet precautions.
3. Determining candidacy
Extracorporeal Life Support Organization (ELSO) presented recommendations for absolute and relative contraindications to ECMO for supporting adult patients with COVID-19 [18]. Notable absolute contraindications included significant underlying comorbid medical conditions and ongoing CPR including extracorporeal cardiopulmonary resuscitation (ECPR) cannulations. Additionally, this consensus document recommended that COVID-19 is not a contraindication for ECMO in the neonatal and pediatric population, and that candidacy should be determined according to previously published guidelines for pediatric respiratory and cardiac cannulation. However, even for pediatric patients, ELSO recommends referencing the list of relative and absolute contraindications recommended for adult population, taking these factors into consideration when determining candidacy. Further, although ELSO recommends against the employment of ECPR for neonatal and pediatric patients with severe ARDS caused by COVID-19, candidacy determination is left to each ELSO center. Institution specific adaptations to these recommended guidelines were determined by the pediatric and adult ECMO teams as well as intensive care physicians. Additional involvement of an institution's ethics committee when determining how to best allocate this scare resource may provide benefit.
For all COVID-19 positive adult and pediatric patients as well as PUI, ECPR and ECMO candidacy is determined upon admission. When evaluating patients with COVID-19, we are not altering our guidelines for ECMO candidacy in neonatal and pediatric patients. Ultimately, the decision was made to offer ECPR to pediatric COVID-19 positive patients on a case by case basis. All clinical teams are cognizant to initiate ECMO cannulation in these patients with early signs of clinical decline to allow for controlled cannulations and to avoid ECPR. For adult patients, ECPR is strongly discouraged although each patient is considered individually with significant focus on the presence of comorbidities, septic shock, and multisystem organ dysfunction as disqualifying conditions. All consideration for ECPR candidacy is done in consultation with the ECMO service, with adult patient candidacy also discussed with the adult ECMO service. At this time, we have not cannulated any COVID-19 positive patients via ECPR. Similar to ECPR, candidacy for ECMO cannulation in pediatric COVID-19 positive patients and PUI is determined by our standard candidacy guidelines while adult patients require multidisciplinary discussion with age, comorbidities, duration of intubation, and status of other organ systems considered. In all cases, availability of resources, most notably ECMO circuits, was considered in order to maintain available resources for our non-COVID pediatric patients.
4. Consultation protocol
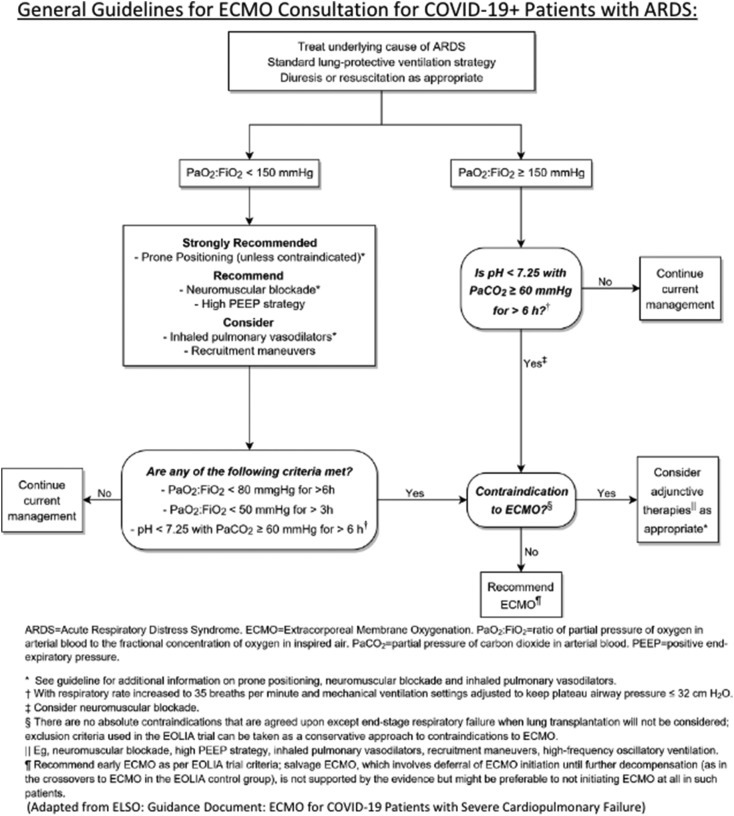
In anticipation of the surge of patients, our institution drafted guidelines for prompting an ECMO consult in pediatric and adult patients with COVID-19 related ARDS, based on the algorithm put forth by ELSO and adapted for use by our adult ICU for ARDS management, as seen in Fig. 1 [[18], [19]]. An ECMO consult on COVID-19 positive patients is obtained if:
-
·
PaO2:FiO2 ≥ 150 mmHg and pH < 7.25 with PaCO2 ≥ 60 mmHg for > 6 h; OR
-
·
PaO2:FiO2 < 80 mmHg for > 6 h; OR
-
·
PaO2:FiO2 < 50 mmHg for > 3 h; OR
-
·
PaO2:FiO2 < 150 and pH < 7.25 with PaCO2 ≥ 60 mmHg for > 6 h
Fig. 1.
Guidelines for ECMO Consultation for COVID-19 Positive Patients with ARDS used at New York-Presbyterian Columbia and Morgan Stanley Children's Hospital of New York.
ECMO consultation for circulatory support followed our previously established institutional algorithms. Due to the highly contagious nature of the virus, coupled with the severe shortages of personal protective equipment (PPE), exposure to COVID-19 positive patients is minimized by implementing restrictions to our typical consult evaluation and workup for ECMO cannulation strategy. Physical exam of these patients require consideration of health care worker safety and PPE scarcity. Due to the wide variety of patient size across the spectrum of neonatal and pediatric patients, ultrasound upon consultation is typically obtained to guide the selection of cannula size. During the pandemic, ultrasounds of target vessels are not routinely performed; instead cannula sizes are determined strictly on maximal flow. Our cannulation strategy for adult patients is standardized with all patients being cannulated onto veno-venous ECMO using a 25 French Bio-Medicus NextGen multiport drainage cannula (Medtronic, Minneapolis, Minnesota) in the common femoral vein and a 20 French Elongated One-Piece Arterial (EOPA) outflow cannula (Medtronic, Minneapolis, Minnesota) in the internal jugular vein, with no utility of bedside vascular ultrasound exams.
Although initially we received very few consultations for pediatric patients with a strictly COVID-19 related disease process, more recently we have been consulted on multiple pediatric patients with a COVID-19 multi-system inflammatory syndrome. These patients have a history of symptoms consistent with COVID-19 or prior known exposure and are presenting with symptoms including but not limited to fever, rash, gastrointestinal symptoms, and shock with and without myocardial depression. Most patients presenting with this syndrome require intensive care admission and treatment with immunomodulators such as steroids and intravenous immunoglobulin. At this time, no patient presenting with post-COVID-19 multi-system inflammatory syndrome to MSCHONY has required ECMO support.
5. Cannulation protocol for COVID-19 positive patients and PUI
During an ECMO cannulation, the physical space in the patient's room becomes quickly occupied by the personnel, equipment, and machinery necessary for cannulation. Our practice is to cannulate exclusively in intensive care units (ICUs), or in operating theaters (including the operating rooms, cardiac catheterization labs, or interventional radiology suites). In the pediatric ICUs at our institution during the COVID-19 pandemic, there was a shortage of negative pressure rooms required for COVID-19 positive patients, resulting in many rooms being retrofitted with this feature, greatly expanding our capacity to house, treat, and cannulate COVID-19 patients.
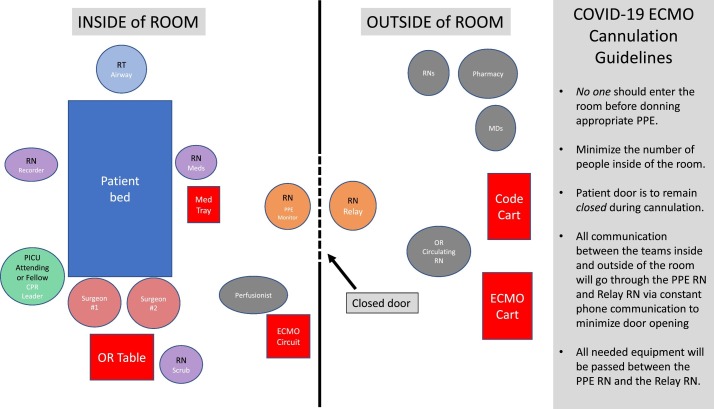
Patients with COVID-19 and PUI required specific modifications to the cannulation protocols in place at our institution. Guidelines were implemented in order to limit the number of personnel exposed to the patient and to limit the use of PPE given the nationwide equipment shortages. As with any emergency procedure, assigning clear and distinct roles is paramount, and this was the focus when preparing for ECMO cannulation of COVID-19 patients (Fig. 2 ). As many healthcare personnel are redeployed to the ICU during the pandemic, it is essential to ensure that only those with prior ECMO experience assist in the cannulation of a COVID-19 positive patient. Personnel were assigned roles either within or outside of the patient room, with no traffic into or out of the room once the procedure had begun. Roles inside the room were as follows:
-
·
ECMO Surgical team: comprised of primary surgeon, one surgical assistant, one scrub nurse
-
·
ICU Leader: ICU attending or fellow
-
·
Two ICU nurses, including one PPE monitor nurse
-
·
Respiratory therapist (one)
-
·
Perfusionist (one) with the circuit
-
·
Cardiology Attending: for intraoperative echocardiogram to determine cannula placement for pediatric patients cannulated via neck vessels.
Fig. 2.
Schematic for ECMO Cannulation of COVID-19 Positive Patients and Patients Under Investigation. (Credit: Anita Sen, MD).
When compared to a cannulation prior to the pandemic, the ECMO surgical team previously consisted of a primary surgeon, two surgical assistants one of which is the ECMO fellow, a scrub nurse, and a circulating operating room nurse. Additionally, two perfusionist are typically in the room while setting up the circuit and initiating ECMO however to decrease exposure, only one perfusionist enters the room at this time. The roles of individuals who remain outside of the room are also specified. In an attempt to limit to only essential personnel, the roles outside of the room were as follows:
-
·
Pediatric ECMO Fellow
-
·
Perfusionist
-
·
Operating Room Circulating Nurse
-
·
Relay Nurse
-
·
Front Line Provider: MD or nurse practitioner
All ECMO cannulations of COVID-19 positive patients and PUIs are to take place in negative pressure rooms in the ICU, with any exceptions to be approved by the ECMO and ICU leadership. In order to minimize the number of times the door was opened, transfer of equipment and information was handled by the relay nurse outside of the room and the PPE monitor nurse inside the room who were in constant communication via cell phone using wireless headphones or handheld whiteboards visible through the glass doors. Any equipment needed by the surgical team was obtained by the circulating operating room nurse or ECMO fellow outside of the room, handed to the relay nurse to be passed through the door to the PPE monitor nurse, who would then give to the scrub nurse. This system, while requiring multiple individuals, was designed to limit the number of personnel accessing an open door. This technique decreased the number of individuals in a patient's room by on average five people at any given time.
6. Equipment and changes in daily care
At both MSCHONY and New York-Presbyterian Columbia, our ECMO circuits are comprised of Cardiohelp (Maquet Cardiopulmonary AG, Germany), Cardiohelp disposables with 5.0 oxygenators used for the majority of pediatric patients while 7.0 oxygenators are used for larger children and for all adult patients (Maquet Cardiopulmonary AG, Germany), CardioQuip MCH-1000(m) heater-cooler (CardioQuip LLC, College Station, TX) and thermo-electric cooling module MCH-11TEC (CardioQuip LLC, College Station, TX). Our Cardiohelp circuits consist of either 3/8-in. tubing used for pediatric patients above 30 kg and all adult patients or custom 1/4-in. tubing (LivaNova, London, United Kingdom) that includes a shunt from post-oxygenator output to the pre-oxygenator venous line for patients < 19 kg. A hybrid circuit is used for patients between 19 and 30 kg. The shunt is designed to ensure the minimum required flow through the oxygenator (500 ml/min), with flow through the shunt controlled by a partial occluding clamp. Additionally, more unique to our circuit setup is the use of the Quantum Workstation and Quantum sensor module (Spectrum Medical, Fort Mill, South Carolina). The Quantum Workstation provides constant live monitoring of circuit flows, cardiac index, inflow and outflow pressures, fraction of delivered oxygen (FdO2), and sweep as well as draws data from patient monitors including saturation, mean arterial pressure, mixed venous oxygen saturation, partial pressure of oxygen, and partial pressure of carbon dioxide. All data for the entire ECMO run is stored in the workstation, can be viewed as trends, and is automatically uploaded into our electronic medical record system. Quantum Workstation also allows for remote monitoring of each patient via LiveVue, allowing perfusionist and ECMO fellows to quickly monitor multiple patients while preventing the necessity of continuous bedside care.
We currently have 16 Cardiohelp circuits available to support patients in both the adult and pediatric population at New York-Presbyterian Columbia and MSCHONY. Circuits are shared between the two hospitals with typically four to five circuits housed in the pediatric hospital and the remaining circuits at our adult partner hospital. Circuits are moved based on demand with attempts to have at least one backup circuit available at the adult hospital and two available at the pediatric hospital (one with 1/4-in. tubing and one with 3/8-in. tubing) for emergent circuit exchanges. In preparation for the pandemic, our previously used RotaFlow circuits were prepared for use. In all we have 11 RotaFlow circuits, five of which were sent to New York University (NYU) Langone Health to help support their high demand for ECMO during the pandemic while six were reserved to expand our ECMO support if needed.
With the advent of COVID-19 infection spreading in the New York metropolitan area, a number of changes were made to our ECMO workflow from an equipment and perfusion care standpoint to ensure the same level of excellent care to our patients while also protecting all staff caring for the patients and preserve the limited resource of PPE. When taking care of multiple ECMO patients including patients who are not COVID-19 positive, the ECMO fellow and perfusionist round on all non COVID-19 patients prior to rounding on those patients who are positive. All care requiring assistance from these individuals including assistance turning the patients as well as lab draws are coordinated and all completed on morning rounds so as to decrease exposure to the ECMO fellow and perfusionist as well as preserve PPE. Additionally, labs that are typically drawn from the circuit daily are spaced to two to three times a week pending clinician determination including oxygenator gasses and circuit cultures. Additionally, since the start of the pandemic, all of our oxygenator exhaust containers used to collect condensation from the exhaust port on the oxygenator have been replaced with larger containers, thereby limiting the need to empty the container daily. Most beneficial to decreasing exposure of the ECMO team members and preserving PPE is the addition of a wireless keyboard connected to the Quantum workstation. This allows for all basic charting to be done and review of data to occur without entering the patient room. As previously mentioned, we have continued to use LiveVue as a means to monitor the circuit settings on multiple patients from the perfusion office, again decreasing healthcare exposure.
7. Allocation of resources
As is commonly seen in a pandemic or mass casualty setting, scarcity of resources has been a challenge while working in the epicenter of the current pandemic. Specifically, in regard to ECMO, these resources include personnel trained to care for such critically ill patients, PPE, and ECMO equipment. Numerous steps have been taken to ensure the safety of our medical professionals as well as preserve the extremely scarce resource of PPE as discussed above. In addition, several aspects are considered when determining our ability to accept additional adult ECMO patients. Although adult patients are more commonly hospitalized secondary to COVID-19 illness, as a pediatric hospital our priority is to continue treating our pediatric patients in need. With all requests for transfer of adult ECMO patients as well as consultations for cannulation of adult patients in our care, the total number of available circuits is considered in order to maintain circuit availability for our non-COVID pediatric patients. At times when circuits are unavailable, transfers for adult ECMO cannulation are not accepted. Additionally, the urgency of cannulation of in-house consultations is thoroughly analyzed in attempts to preserve circuits.
8. Conclusion
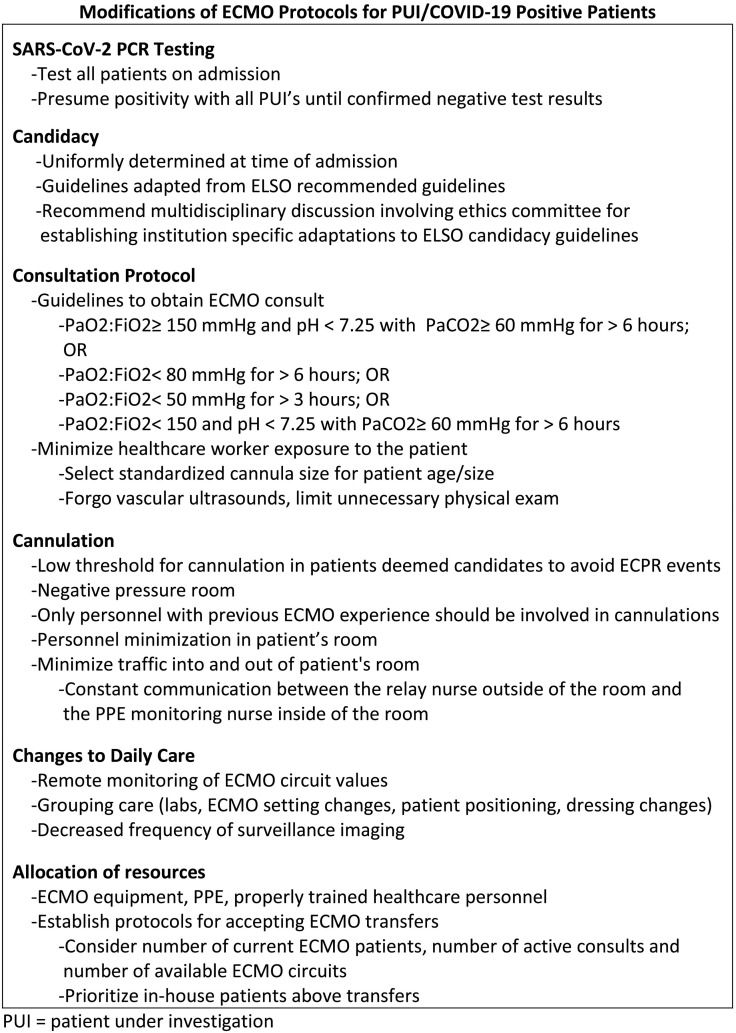
Although during the initial surge of COVID-19 diagnoses, pediatric patients were not requiring hospitalization to the degree of adult patients, our hospital continued to treat pediatric emergencies while learning quickly to adapt to the treatment of adult patients, helping to offload our overwhelmed hospital system. Sustainable changes in protocols and guidelines were created that ensure safety of healthcare personnel, maximize hospital resource utilization, and guarantee optimal care for all patients, as seen in Fig. 3 . Although further research on SARS-CoV-19 is crucial for future treatment and prevention, systematic changes and lessons learned from an ECMO department during this trying time can be utilized to further guide clinicians in the setting of a pandemic.
Fig. 3.
Summary of the modifications of ECMO protocols for PUI and COVID-19 positive patients.
Acknowledgments
Acknowledgements
Protocols described above were authored by Eva Cheung, MD, medical director of Pediatric ECMO at MSCHONY. We would like to acknowledge Anita Sen, MD for her assistance in developing the ECMO cannulation strategy for COVID-19 patients and PUI as well as for the schematic drawing in Fig. 2.
Funding
None.
Declaration of interest
The authors declare that there are no conflicts of interest.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in china. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NYC Department of Health COVID-19: Data 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page [accessed 26 May 2020]
- 3.The Australian and New Zeland Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 4.Noah M., Peek G., Finney S., et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2001;306(15):1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 5.Beurtheret S., Mastroianni C., Pozzi M., et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome: single-center experience with 1-year follow-up. Eur J Cardiothorac Surg. 2012;41(3):691–695. doi: 10.1093/ejcts/ezr082. [DOI] [PubMed] [Google Scholar]
- 6.Peek G., Mugford M., Tiruvoipati R., et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicenter randomized controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 7.Combes A., Hajage D., Capellier G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 8.Goligher E., Tomlinson G., Hajage D., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA. 2018;320(21):2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 9.Alshahrani M., Sindi A., Alshamsi F., et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8(1) doi: 10.1186/s13613-017-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X., Yuan Y., Xi J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang G., Hu C., Luo L., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbaro R., Odetola F., Kidwell K., et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality: Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191(8):894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramanathan K., Antognini D., Combes A., et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious disease. Lancet. 2020;8(5):518–526. doi: 10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American College of Surgeons (ACS) COVID-19 Guidelines for Triage of Pediatric Patients https://www.facs.org/covid-19/clinical-guidance/elective-case/pediatric-surgery
- 17.Sutton D., Fuchs K., D'Alton M., et al. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020 doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Extracorporeal Life Support Organization Extracorporeal Life Support Organization COVID-19 Interim Guidelines; A consensus document from an international group of interdisciplinary ECMO providers. https://www.elso.org/Portals/0/Files/pdf/ELSO%20covid%20guidelines%20final.pdf [DOI] [PMC free article] [PubMed]
- 19.New York-Presbyterian Hospital System. COVID-19 and ARDS Management: Evidence Based Guidelines. [accessed 5 May 2020].