Abstract
Purpose of Review:
The goal of this review has been to elucidate the sex differences in cancer incidence and mortality in cutaneous melanoma. We have evaluated biological and behavioral research to determine where the critical questions exist.
Recent Findings:
The most recent findings, through 2015, are exploratory in nature but seem to indicate that the differences are more likely due to biological variations rather than behavioral. While behavioral studies do show that women are more likely than men to seek health care and practice healthy behaviors, these differences are not sufficiently strong to explain the variation in incidence and mortality in cutaneous melanoma. Evolved differences in the immune systems of females and the role of sex steroid hormones in immunomodulation are two promising avenues for research. Studies in mice demonstrate that the newer immunotherapies are more effective in females and sex steroid hormones, such as estrogen receptor beta are inversely associated with tumor aggressiveness while testosterone increases it.
Summary:
Our analysis indicates that biological factors need to be investigated more thoroughly to understand the variation in incidence and mortality in cutaneous melanoma. Such understanding could lead to reducing incidence and mortality for both males and females (male incidence is 27.4 per 100,000; female 16.8 per 100,000; male mortality is 3.9 per 100,000; female mortality 1.6 per 100,000). It is most likely that behavioral differences between the sexes cannot account for the preponderance of male mortality. In addition to the important role of genetic factors, it is critical to evaluate further additional biological factors and their interactions with genetics and behavior.
Keywords: Melanoma, sex, biology, genetics, behavior, sun exposure
Introduction
Sex differences in melanoma incidence and mortality are robust, consistent, and well-documented; overall, males have a higher risk of developing cancer and one-and-a-half times the risk of mortality than females1. This increased risk cuts across racial and ethnic groups and all cancer types 2–6. To the age of 50, females actually have approximately twice the incidence of melanoma as males, but thereafter this difference changes, and by the age of 60 males have twice the incidence of females and by age 70 this difference rises to three times as great for males.1 Although melanoma incidence over the last five years has increased for both sexes (1.2% for females and 2.3% for males), the good news is that mortality from melanoma has decreased by 2.8% for females and 3.0% for males4. Mortality from melanoma is relatively constant among males and somewhat greater than that for females until the age of 50 when male mortality consistently increases to almost three times that of females.1
In terms of sex differences in survival from cancer, cutaneous melanoma is one of the most striking 3. Using follow up data from four Phase III European Organisation for Research and treatment of Cancer (EORTC), Melanoma Group, females retain a 38% survival advantage compared to males after adjusting for stage at diagnosis 7–9. When compared to males, females have thinner tumors, less tumor ulceration, less progression to lymph node or organ metastases, a longer delay before relapse, and a higher cure rate 7,8,10.
Here we summarize the sex differences in melanoma, covering the spectrum from biologically-based pathways with immunological, hormonal, and genetic underpinnings, to behavioral-related pathways such as differences in UV exposure, primary care access, and skin awareness.
Immunity
Sex-specific differences in overall immune function are robust and well-documented. Women mount more effective cellular and humoral immune responses and are less likely to succumb to bacterial and viral infections than men 5,11–16.
Life history theory attributes these differences to sex-specific evolutionary trade-offs in energetic allocation to competing somatic demands such as reproduction 17–20. One notable example of sex-specific reproductive asymmetry is the nine-month period of pregnancy. The mother’s immune system must be sufficiently suppressed through a complex interplay between energetics, immune cells, and hormones to allow the antigenically distinct fetus to grow without triggering an immune response, but not so suppressed that the mother cannot fend off infections 21. Over evolutionary time, such asymmetries have led to sexually dimorphic systems such as immunity, and are seen across all females regardless of gravidity.
As an immunogenic disease, the immune system is especially critical to detecting and destroying melanoma tumors. For this reason, alterations to the immune system such as systemic immunosuppression after a major organ transplant, are major risk factors for the development of melanoma 22. In order to evade detection by the immune system, melanoma tumors down-regulate surface antigens, and secrete immunosuppressive cytokines in the tumor microenvironment, effectively suppressing the site-specific immune response 23,24. If successful, malignant melanomas evade detection and spread throughout the body and into the lymphatic system, further compromising host immunity25.
Melanoma, like many tumors, is chemoresistant, making previous treatments difficult and often ineffective. Fortunately, the recent development of successful alternatives to chemotherapy such as immune checkpoint inhibitors and targeted therapies are dramatically changing the treatment landscape for melanoma patients 26. Interestingly, in mice, immune checkpoint inhibitors are significantly more effective in female mice than male mice16. Whether this is true for humans will await the results of further trials.
Endocrine Factors
Other sex-specific immune-modulating factors such as steroid hormones like estrogen and testosterone have been heavily implicated in host immune activation and response 27,27–37, largely through sex-steroid receptors located on immune cells 38–40. Estrogen acts as a powerful immune-enhancer, potentiating antibody12 and likely inflammatory responses 41–43. Testosterone on the other hand, attenuates non-specific immune response 12.
The role of sex steroid hormones in melanoma is far from clear 44. The expression of the estrogen receptor beta (ER-ß) is negatively associated with melanoma invasiveness and tumor thickness 45. The exogenous addition of estrogen inhibits tumor growth in vitro 46 and in metastatic tumors 47, while testosterone increases tumor aggressiveness 44,48.
Reproductive status such as pregnancy and menopause have large within-sex differences in fluctuating sex-steroid hormones, conveniently presenting researchers with natural experimental conditions. Reproductive-age women have a more reactive inflammatory profile 5 and higher levels of T-lymphocytes when compared to post-menopausal women 12,49,50. There appears to be no association between pregnancy and melanoma 44. The relation between menopausal status and melanoma survival is conflicted, multiple studies have found no evidence of differences in post-menopausal groups 51–53, while others have documented significant differences in post-menopausal groups 39,54–58.
Genetic Factors
Phenotypic differences in both immunocompetence and steroid hormones are largely driven by differences in underlying genetic architecture. Until recently, the cost of genetic sequencing has been a major barrier to genomic research. However, decreasing sequencing costs has greatly increased the capacity to study the genotypic features of melanoma.
Several genetic factors are suggested as underlying a male survival disadvantage in melanoma outcome. The X chromosome alone has 1500 genes containing oncogenes and tumor suppressor genes whose regulation are critical to cancer progression and suppression compared to the 344 genes on the Y chromosome 44. Additionally, men carry the potential deleterious effects of X chromosome monosomy and oncogenes on the Y chromosome (TSPY) 44.
Differences in autosomal genetic variations are important in understanding the mechanisms of sex disparity in melanoma. Previous studies have reported significant differential genetic effects for melanoma by sex. A non-synonymous single nucleotide polymorphism (SNP), rs16891982, in the SLC45A2 gene is shown to be associated with much higher risk for melanoma in males (OR=5.5 in males vs OR=2.37 in females)59. A study of melanoma in a Spanish population discovered that SNPs relating to pigmentation constitutes one potential genetic cause underlying a higher rate for melanoma in males 60. SNPs in genes (TYR, GPR143, and F2RL1) were shown to increase melanoma predisposition in males as opposed to females, and these SNPs were also found to be associated with dark pigmentation and sun tolerance in females but not in males 60. A recent study reported that sex differences in outcomes of cutaneous melanoma patients were associated with inherited abnormalities on TP53, MDM2 and BCL2 genes in the apoptosis pathway 61. The MC1R red hair variants were demonstrated to contribute differentially to melanoma risks in males and females 62.
Melanoma has a high rate of missense mutations 63. These antigenic mutations increase the likelihood of detection by the immune system 63. Patients with these high tumor mutational burdens (TMB) have lower mortality rates 64,65 and greater prognosis with immunotherapy. Counterintuitively, Gupta et al. reported a higher burden of somatic missense mutations among men (median of 298) compared to women (median of 211.5) using data from 266 metastatic melanomas from The Cancer Genome Atlas (TCGA) project 63, despite a higher overall male mortality.
While driver mutations in BRAF 66–68, NRAS 68, and KIT 69 are essential in the development and progression of melanoma, very few studies have observed evidence of differential mutations between males and females for these important genes 68,70,71 warranting future larger studies performing comprehensive assessments of sex differences in driver mutations.
X-linked genes have been implicated in sex differences in melanoma outcome. The PPP2R3B gene, located on the X chromosome in females and the Y chromosome in males, was reported to have lower expression in males than in females and was independently associated with poor melanoma outcome 72. Autosomal gene expression levels differ between males and females and may also play an important role in exploring the mechanisms for the sex disparity in melanoma 44,73. The miR-221&222 and miR-506–514 clusters, which could be related to sex differences, were demonstrated to have oncogenic roles in melanoma progression and melanocyte transform 74,75. In addition, long non-coding RNAs are emerging as important modulators of melanoma proliferation, survival and metastatic behavior, which have the potential to be used as novel prognostic and diagnostic markers and contribute to understanding the sex differences in melanoma 13,76.
Behavioral Factors
The continued increase in melanoma-specific cancer incidence is often thought to be the result of a combination of increased detection (possibly over-diagnosis) and exposures such as tanning bed use and harmful recreational UV exposure 77. However, while women are more likely to use tanning beds 78–81, the relative risk for melanoma from using tanning beds is similar for each sex 82.
Health Behavior
One of the hypothesized causes of later tumor stages at the time of diagnosis among males is the fact that they are less likely to go to the doctor 83–85 or find a tumor until it has grown deeper, while females tend to utilize more primary care than men 83,86.
Skin awareness, skin self-examination and physician examination are all associated with the discovery of thinner lesions by both males and females 87. However, females are more likely to be aware of their skin and to conduct skin self-screening as well as visit physicians for health reasons.
Paddock et al found that in females the practice of routine skin self-examination increased the likelihood that the lesion would be self-discovered; females were significantly more likely to conduct skin self-examination than males (58.1 percent vs 41.9 percent, p = 0.03) 88.
“Skin awareness” (that is, being aware of one’s skin for medical or cosmetic reasons) was associated with a 50% decrease in melanoma mortality 89. Females reported being approximately twice as likely to be aware of their skin and any problems. However, once a lesion was noticed, there was no difference by sex in the delay to seeing a clinician 90.
Brady et al, showed that females were more likely to self-detect melanoma lesions than men91; others have shown that females are also more aware of their skin 88 and more likely to utilize sunscreen92,93.
Sun Exposure
Ultraviolet radiation, including both sun exposure and tanning bed use has been clearly and definitively associated with the development of melanoma by the International Agency for Cancer Research 94. There are different types of sun exposure with “intermittent” sun exposure, the type of exposure one gets on weekends after being inside all week, often leading to sunburns, considered the major type of sun exposure associated with the development of melanoma 95. This type of sun exposure is in contrast to chronic exposure, the type of sun exposure received on an almost daily basis, such as in occupation or gardening. Chronic exposure, in meta-analyses, does not increase the risk of melanoma 96.
The incidence pattern for melanoma differs dramatically by age and sex (Figure 1), such that females have a slightly higher rate of melanoma until around the age of 50 and then the incidence increases slowly while male incidence increases dramatically at that point97. Further, the anatomic site of melanoma differs by sex. These differences are consistent across latitudes. Males tend to have the highest incidence of melanoma on the trunk and a higher incidence of melanoma on the head and neck than females. Females have a high incidence of melanoma on the leg. It is not clear whether these differences are a function of sun exposure patterns, a genetically controlled sex-linked characteristic, or a combination of both.
Figure 1.
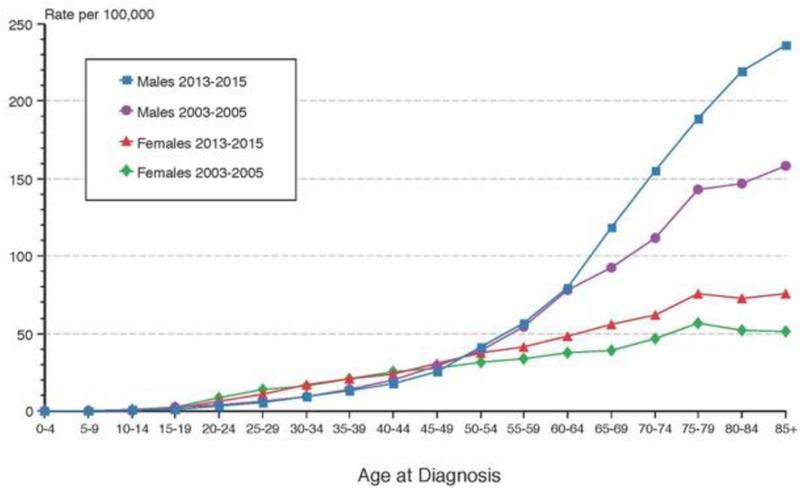
Melanoma incidence comparing 2003–2005 to 2013–2015 among whites (including Hispanics) by age and sex. From SEER.
Gordon et al, analysed sun exposure data from Stockholm-Gotland healthcare region (average population 1.7 million), representing 20% of the Swedish population during the time period 1977 to 200198. Their data demonstrates clearly that males received more UV radiation (intermittent overall and intermittent on the core) than females. This is in contrast to the lack of a difference between the sexes for ultraviolet radiation on the extremities (peripheral) and chronic. Such information underlines the differences between intermittent sun exposure and chronic exposure and their respective risks for developing melanoma.
Sun Protection
It is important to evaluate the association between sunscreen and other sun protection methods in reducing melanoma risk. The most persuasive evidence for the use of sunscreens comes from a randomized clinical trial conducted in Queensland, Australia, where there appeared to be a large reduction in the rate of invasive melanoma incidence in the group who used sunscreen (HR 0.29, 95% CI 0.08,0.97), three melanomas in the intervention group and 11 in the control group. 99
A National Health Interview Survey interviewed a representative sample of 31,162 US adults in 2015. It appears that females generally use more sun protection than males (shade, sunscreen and sun avoidance). However, males appeared to use more clothing in the sun.
A large systematic review and meta-analysis involving 313,731 participants, 10,813 cases or non-melanoma skin cancer 857 cases found no association between the use of sunscreen and the development of melanoma100. A contradictory study by Rueegg et al, found protective associations for use of sunscreen in hospital-based studies, one ecological study and a randomized controlled trial101 although there was large heterogeneity among study designs and among the case-control studies. When adjusted for confounding by sun exposure, sunburns and patient phenotype, all estimates moved to lower melanoma risk among sunscreen users.. However, population-based cases control and cohort studies found significant positive associations between the use of sunscreen and the development of melanoma. A case-control study of more than 1,000 participants in Minnesota also evaluated the use of sunscreens and other methods of sun protection and found that sunscreens were associated with a reduced risk of developing melanoma but that other sun protection methods, such as seeking shade and using clothing were far stronger in their association with a reduced risk of melanoma 93.
Conclusion
Despite major progress in melanoma treatment in the development of immunotherapy, understanding the relation between mutational loads and the immune system, and the rich literature documenting marked sex differences in immunocompetence, hormones, and genetics in melanoma, sex disparities in survival persist.
Although there are multiple differences between men and women in terms of behavior in the sun and in utilization of primary care; differences in these behaviors would suggest higher incidence in women not lower. Thus, it seems much more likely that the biological differences are critical in terms of developing advanced melanoma and that more investigation of these is needed.
Biological sex is a fundamental factor in melanoma. It is critical that future studies be powered sufficiently to account for sex differences. These sex disparities in melanoma outcomes provide an excellent opportunity to test clear hypotheses and to develop new insights into the underlying mechanisms of melanoma, cancer, and the immune system.
Acknowledgements:
This work was supported by P01 CA 206980-01A1 to MB and 3P01CA206980-02SZ to MS.
Matthew Robert Schwartz reports grants from NCI 3P01CA206980-01 - Diversity Supplement to Schwartz - during the conduct of the study.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Compliance with Ethical Standards
Conflict of Interest
Li Luo and Marianne Berwick each declare no potential conflicts of interest.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutions/national research committee standards, and international/national/institutional guidelines).
Contributor Information
Matthew Robert Schwartz, Anthropology Department and University of New Mexico Comprehensive Cancer Center.
Li Luo, University of New Mexico Comprehensive Cancer Center, Department of Internal Medicine.
Marianne Berwick, University of New Mexico Comprehensive Cancer Center, Department of Internal Medicine and Department of Dermatology.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Noone A, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2015 Natl Cancer Inst; Bethesda MD: [Internet]. Available from: https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- 2.Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2009. April;18(4):1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2011. August;20(8):1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ••.Cronin KA, Lake AJ, Scott S, Sherman RL, Noone A, Howlader N, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018. July 1;124(13):2785–800.The most recent statistics on cancer in the United States, demonstrating a decline in melanoma mortality among both sexes.
- 5. ••.Gubbels Bupp MR, Potluri T, Fink AL, Klein SL. The Confluence of Sex Hormones and Aging on Immunity. Front Immunol [Internet] 2018. [cited 2018 Oct 23];9 Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2018.01269/fullRecent, comprehensive, and well-written review detailing the role of sex hormones on immunity.
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018. January;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 7.Joosse A, de Vries E, Eckel R, Nijsten T, Eggermont AMM, Hölzel D, et al. Gender Differences in Melanoma Survival: Female Patients Have a Decreased Risk of Metastasis. J Invest Dermatol 2011. March 1;131(3):719–26. [DOI] [PubMed] [Google Scholar]
- 8.Joosse A, Collette S, Suciu S, Nijsten T, Lejeune F, Kleeberg UR, et al. Superior Outcome of Women With Stage I/II Cutaneous Melanoma: Pooled Analysis of Four European Organisation for Research and Treatment of Cancer Phase III Trials. J Clin Oncol 2012. June 20;30(18):2240–7. [DOI] [PubMed] [Google Scholar]
- 9.Ramchandran K, Patel JD. Sex differences in susceptibility to carcinogens. Semin Oncol 2009. December;36(6):516–23. [DOI] [PubMed] [Google Scholar]
- 10.Scoggins CR, Ross MI, Reintgen DS, Noyes RD, Goydos JS, Beitsch PD, et al. Gender-Related Differences in Outcome for Melanoma Patients. Ann Surg 2006. May;243(5):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansar Ahmed S, Penhale WJ, Talal N. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol 1985. December;121(3):531–51. [PMC free article] [PubMed] [Google Scholar]
- 12.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update 2005. August;11(4):411–23. [DOI] [PubMed] [Google Scholar]
- 13. ••.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016. August 22;16:626.Comprehensive and recent review article detailing sexually dimorphic immunity.
- 14.Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 2015. January 1;109(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 2015. January;109(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin P-Y, Sun L, Thibodeaux SR, Ludwig SM, Vadlamudi RK, Hurez VJ, et al. B7-H1-Dependent Sex-Related Differences in Tumor Immunity and Immunotherapy Responses. J Immunol Baltim Md 1950 2010. September 1;185(5):2747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stearns SC. The Evolution of Life Histories Oxford University Press; 1992. 262 p. [Google Scholar]
- 18.Roff D, editor. Evolution Of Life Histories: Theory and Analysis [Internet] Springer; US; 1993. [cited 2018 Nov 28]. Available from: //www.springer.com/us/book/9780412023910 [Google Scholar]
- 19.Zuk M The Sicker Sex. PLOS Pathog 2009. January 30;5(1):e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuk M, Stoehr AM. Immune Defense and Host Life History. Am Nat 2002. October 1;160(S4):S9–22. [DOI] [PubMed] [Google Scholar]
- 21.Medawar PB, Medawar PB, Medawar P, Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates 1953. January 1 [cited 2018 Nov 20]; Available from: https://www.scienceopen.com/document?vid=d66cb799-1041-4ed0-81c6-c3d639a78be5
- 22.Robbins HA, Clarke CA, Arron ST, Tatalovich Z, Kahn AR, Hernandez BY, et al. Melanoma risk and survival among organ transplant recipients. J Invest Dermatol 2015. November;135(11):2657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumgartner J, Wilson C, Palmer B, Richter D, Banerjee A, McCarter M. Melanoma Induces Immunosuppression by Upregulating FOXP3+ Regulatory T Cells. J Surg Res 2007. July;141(1):72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarter MD, Baumgartner J, Escobar GA, Richter D, Lewis K, Robinson W, et al. Immunosuppressive dendritic and regulatory T cells are upregulated in melanoma patients. Ann Surg Oncol 2007. October;14(10):2854–60. [DOI] [PubMed] [Google Scholar]
- 25.Wolchok JD, Saenger Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. The Oncologist 2008;13 Suppl 4:2–9. [DOI] [PubMed] [Google Scholar]
- 26.Franklin C, Livingstone E, Roesch A, Schilling B, Schadendorf D. Immunotherapy in melanoma: Recent advances and future directions. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2017. March;43(3):604–11. [DOI] [PubMed] [Google Scholar]
- 27.La Rocca C, Carbone F, Longobardi S, Matarese G. The immunology of pregnancy: Regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett 2014. November;162(1, Part A):41–8. [DOI] [PubMed] [Google Scholar]
- 28.Warning JC, McCracken SA, Morris JM. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 2011. June 1;141(6):715–24. [DOI] [PubMed] [Google Scholar]
- 29.Whitacre CC, Reingold SC, O’Looney PA, Blankenhorn E, Brinley F, Collier E, et al. A Gender Gap in Autoimmunity: Task Force on Gender, Multiple Sclerosis and Autoimmunity*. Science 1999. February 26;283(5406):1277–8. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Loo KK, Palaszynski K, Ashouri J, Lubahn DB, Voskuhl RR. Estrogen Receptor α Mediates Estrogen’s Immune Protection in Autoimmune Disease. J Immunol 2003. December 15;171(12):6936–40. [DOI] [PubMed] [Google Scholar]
- 31. •.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 2015. April 1;294(2):63–9.Recent work identifying the role of estrogen receptors in the immune system.
- 32.Grossman CJ. Interactions between the gonadal steroids and the immune system. Science 1985. January 18;227(4684):257–61. [DOI] [PubMed] [Google Scholar]
- 33.Guleria I, Sayegh MH. Maternal Acceptance of the Fetus: True Human Tolerance. J Immunol 2007. March 15;178(6):3345–51. [DOI] [PubMed] [Google Scholar]
- 34.Gobert M, Lafaille JJ. Maternal-Fetal Immune Tolerance, Block by Block. Cell 2012. July 6;150(1):7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu B, Li X, Sun R, Tong X, Ling B, Tian Z, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal–fetal interface. Proc Natl Acad Sci 2013. January 15;110(3):E231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thellin O, Heinen E. Pregnancy and the immune system: between tolerance and rejection. Toxicology 2003. April 1;185(3):179–84. [DOI] [PubMed] [Google Scholar]
- 37.Billington WD. The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar. J Reprod Immunol 2003. October;60(1):1–11. [DOI] [PubMed] [Google Scholar]
- 38.Jung K-W, Park S, Shin A, Oh C-M, Kong H-J, Jun JK, et al. Do Female Cancer Patients Display Better Survival Rates Compared with Males? Analysis of the Korean National Registry Data, 2005–2009. Gorlova OY, editor. PLoS ONE 2012. December 26;7(12):e52457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Micheli A, Ciampichini R, Oberaigner W, Ciccolallo L, de Vries E, Izarzugaza I, et al. The advantage of women in cancer survival: An analysis of EUROCARE-4 data. Eur J Cancer 2009. April 1;45(6):1017–27. [DOI] [PubMed] [Google Scholar]
- 40.Shang Y. Hormones and cancer. Cell Res 2007. April 16;17:277–9. [DOI] [PubMed] [Google Scholar]
- 41.Barzi A, Lenz AM, Labonte MJ, Lenz H-J. Molecular pathways: Estrogen pathway in colorectal cancer. Clin Cancer Res Off J Am Assoc Cancer Res 2013. November 1;19(21):5842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caiazza F, Ryan EJ, Doherty G, Winter DC, Sheahan K. Estrogen receptors and their implications in colorectal carcinogenesis. Front Oncol 2015;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Kozloski M. Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. J Gerontol A Biol Sci Med Sci 2011. May;66(5):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nosrati A, Wei ML. Sex disparities in melanoma outcomes: The role of biology. Arch Biochem Biophys 2014. December 1;563:42–50. [DOI] [PubMed] [Google Scholar]
- 45.de Giorgi V, Gori A, Gandini S, Papi F, Grazzini M, Rossari S, et al. Oestrogen receptor beta and melanoma: a comparative study. Br J Dermatol 2013. March 1;168(3):513–9. [DOI] [PubMed] [Google Scholar]
- 46.Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol 1999. February;103(2):282–8. [DOI] [PubMed] [Google Scholar]
- 47.de Giorgi V, Mavilia C, Massi D, Gozzini A, Aragona P, Tanini A, et al. Estrogen receptor expression in cutaneous melanoma: a real-time reverse transcriptase-polymerase chain reaction and immunohistochemical study. Arch Dermatol 2009. January;145(1):30–6. [DOI] [PubMed] [Google Scholar]
- 48.Shahabi S, He S, Kopf M, Mariani M, Petrini J, Scambia G, et al. Free Testosterone Drives Cancer Aggressiveness: Evidence from US Population Studies. PLOS ONE 2013. April 24;8(4):e61955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giglio T, Imro MA, Filaci G, Scudeletti M, Puppo F, De Cecco L, et al. Immune cell circulating subsets are affected by gonadal function. Life Sci 1994;54(18):1305–12. [DOI] [PubMed] [Google Scholar]
- 50.Yang JH, Chen CD, Wu MY, Chao KH, Yang YS, Ho HN. Hormone replacement therapy reverses the decrease in natural killer cytotoxicity but does not reverse the decreases in the T-cell subpopulation or interferon-gamma production in postmenopausal women. Fertil Steril 2000. August;74(2):261–7. [DOI] [PubMed] [Google Scholar]
- 51.Joosse A, Collette S, Suciu S, Nijsten T, Lejeune F, Kleeberg UR, et al. Superior Outcome of Women With Stage I/II Cutaneous Melanoma: Pooled Analysis of Four European Organisation for Research and Treatment of Cancer Phase III Trials. J Clin Oncol 2012. June 20;30(18):2240–7. [DOI] [PubMed] [Google Scholar]
- 52.Kemeny MM, Busch E, Stewart AK, Menck HR. Superior survival of young women with malignant melanoma. Am J Surg 1998. June;175(6):437–44; discussion 444–445. [DOI] [PubMed] [Google Scholar]
- 53.Lasithiotakis K, Leiter U, Meier F, Eigentler T, Metzler G, Moehrle M, et al. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer 2008. April 15;112(8):1795–804. [DOI] [PubMed] [Google Scholar]
- 54.Chirlaque MD, Salmerón D, Ardanaz E, Galceran J, Martínez R, Marcos-Gragera R, et al. Cancer survival in Spain: estimate for nine major cancers. Ann Oncol Off J Eur Soc Med Oncol 2010. May;21 Suppl 3:iii21–29. [DOI] [PubMed] [Google Scholar]
- 55.Joosse A, de Vries E, Eckel R, Nijsten T, Eggermont AMM, Hölzel D, et al. Gender Differences in Melanoma Survival: Female Patients Have a Decreased Risk of Metastasis. J Invest Dermatol 2011. March 1;131(3):719–26. [DOI] [PubMed] [Google Scholar]
- 56.Måsbäck A, Olsson H, Westerdahl J, Ingvar C, Jonsson N. Prognostic factors in invasive cutaneous malignant melanoma: a population-based study and review. Melanoma Res 2001. October;11(5):435–45. [DOI] [PubMed] [Google Scholar]
- 57.Stidham KR, Johnson JL, Seigler HF. Survival Superiority of Females With Melanoma: A Multivariate Analysis of 6383 Patients Exploring the Significance of Gender in Prognostic Outcome. Arch Surg 1994. March 1;129(3):316–24. [DOI] [PubMed] [Google Scholar]
- 58.de Vries E, Nijsten TEC, Visser O, Bastiaannet E, van Hattem S, Janssen-Heijnen ML, et al. Superior survival of females among 10 538 Dutch melanoma patients is independent of Breslow thickness, histologic type and tumor site. Ann Oncol 2008. March 1;19(3):583–9. [DOI] [PubMed] [Google Scholar]
- 59.Kocarnik JM, Park SL, Han J, Dumitrescu L, Cheng I, Wilkens LR, et al. Replication of associations between GWAS SNPs and melanoma risk in the Population Architecture Using Genomics and Epidemiology (PAGE) Study. J Invest Dermatol 2014. July;134(7):2049–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hernando B, Ibarrola-Villava M, Fernandez LP, Peña-Chilet M, Llorca-Cardeñosa M, Oltra SS, et al. Sex-specific genetic effects associated with pigmentation, sensitivity to sunlight, and melanoma in a population of Spanish origin. Biol Sex Differ 2016;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oliveira C, Lourenço GJ, Rinck-Junior JA, de Moraes AM, Lima CSP. Polymorphisms in apoptosis-related genes in cutaneous melanoma prognosis: sex disparity. Med Oncol Northwood Lond Engl 2017. February;34(2):19. [DOI] [PubMed] [Google Scholar]
- 62.Wendt J, Mueller C, Rauscher S, Fae I, Fischer G, Okamoto I. Contributions by MC1R Variants to Melanoma Risk in Males and Females. JAMA Dermatol 2018. July 1;154(7):789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupta S, Artomov M, Goggins W, Daly M, Tsao H. Gender Disparity and Mutation Burden in Metastatic Melanoma. JNCI J Natl Cancer Inst [Internet] 2015. August 20 [cited 2018 Mar 23];107(11). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4643631/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther 2017. November 1;16(11):2598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simpson D, Ferguson R, Martinez CN, Kazlow E, Moran U, Heguy A, et al. Mutation burden as a potential prognostic marker of melanoma progression and survival. J Clin Oncol 2017. May 20;35(15_suppl):9567–9567. [Google Scholar]
- 66.Bauer J, Büttner P, Murali R, Okamoto I, Kolaitis NA, Landi MT, et al. BRAF mutations in cutaneous melanoma are independently associated with age, anatomic site of the primary tumor, and the degree of solar elastosis at the primary tumor site. Pigment Cell Melanoma Res 2011. April;24(2):345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qi R-Q, He L, Zheng S, Hong Y, Ma L, Zhang S, et al. BRAF exon 15 T1799A mutation is common in melanocytic nevi, but less prevalent in cutaneous malignant melanoma, in Chinese Han. J Invest Dermatol 2011. May;131(5):1129–38. [DOI] [PubMed] [Google Scholar]
- 68.Thomas NE, Edmiston SN, Alexander A, Millikan RC, Groben PA, Hao H, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2007. May;16(5):991–7. [DOI] [PubMed] [Google Scholar]
- 69.Kong Y, Si L, Zhu Y, Xu X, Corless CL, Flaherty KT, et al. Large-scale analysis of KIT aberrations in Chinese patients with melanoma. Clin Cancer Res Off J Am Assoc Cancer Res 2011. April 1;17(7):1684–91. [DOI] [PubMed] [Google Scholar]
- 70.Gabriele L, Buoncervello M, Ascione B, Bellenghi M, Matarrese P, Carè A. The gender perspective in cancer research and therapy: novel insights and on-going hypotheses. Ann Ist Super Sanita 2016. June;52(2):213–22. [DOI] [PubMed] [Google Scholar]
- 71.Lee J-H, Choi J-W, Kim Y-S. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol 2011. April;164(4):776–84. [DOI] [PubMed] [Google Scholar]
- 72.van Kempen LCL, Redpath M, Elchebly M, Klein KO, Papadakis AI, Wilmott JS, et al. The protein phosphatase 2A regulatory subunit PR70 is a gonosomal melanoma tumor suppressor gene. Sci Transl Med 2016. 14;8(369):369ra177. [DOI] [PubMed] [Google Scholar]
- 73.Dorak MT, Karpuzoglu E. Gender Differences in Cancer Susceptibility: An Inadequately Addressed Issue. Front Genet [Internet] 2012. November 28 [cited 2018 Oct 23];3 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3508426/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Felicetti F, De Feo A, Coscia C, Puglisi R, Pedini F, Pasquini L, et al. Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma. J Transl Med 2016. February 24;14:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Streicher KL, Zhu W, Lehmann KP, Georgantas RW, Morehouse CA, Brohawn P, et al. A novel oncogenic role for the miRNA-506–514 cluster in initiating melanocyte transformation and promoting melanoma growth. Oncogene 2012. March 22;31(12):1558–70. [DOI] [PubMed] [Google Scholar]
- 76.Leucci E, Vendramin R, Spinazzi M, Laurette P, Fiers M, Wouters J, et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature 2016. March 24;531(7595):518–22. [DOI] [PubMed] [Google Scholar]
- 77.Jemal A, Saraiya M, Patel P, Cherala SS, Barnholtz-Sloan J, Kim J, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol 2011. November;65(5 Suppl 1):S17–25.e1–3. [DOI] [PubMed] [Google Scholar]
- 78.Boldeman C, Jansson B, Nilsson B, Ullén H. Sunbed use in Swedish urban adolescents related to behavioral characteristics. Prev Med 1997. February;26(1):114–9. [DOI] [PubMed] [Google Scholar]
- 79.Fears TR, Sagebiel RW, Halpern A, Elder DE, Holly EA, Guerry D, et al. Sunbeds and sunlamps: who used them and their risk for melanoma. Pigment Cell Melanoma Res 2011. June;24(3):574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Group BMJP. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ 2012. December 13;345:e8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schneider S, Diehl K, Bock C, Schlüter M, Breitbart EW, Volkmer B, et al. Sunbed use, user characteristics, and motivations for tanning: results from the German population-based SUN-Study 2012. JAMA Dermatol 2013. January;149(1):43–9. [DOI] [PubMed] [Google Scholar]
- 82.Lazovich D, Vogel RI, Berwick M, Weinstock MA, Anderson KE, Warshaw EM. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2010. June;19(6):1557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cleary PD, Mechanic D, Greenley JR. Sex Differences in Medical Care Utilization: An Empirical Investigation. J Health Soc Behav 1982;23(2):106–19. [PubMed] [Google Scholar]
- 84.Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs 2005. March 1;49(6):616–23. [DOI] [PubMed] [Google Scholar]
- 85.Robinson JK, Mallett KA, Turrisi R, Stapleton J. Engaging Patients and Their Partners in Preventive Health Behaviors. Arch Dermatol 2009. April;145(4):469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. •.Burnside C, Hudson T, Williams C, Lawson W, Laiyemo AO. Sex differences in the use of healthcare services among US adults with and without a cancer diagnosis. Turk J Urol 2018. July;44(4):298–302.This paper illustrates important differences by sex in the use of healthcare services. As it is in a minor journal, it may well be overlooked. However, it is very well written and an excellent reference.
- 87.Swetter SM, Johnson TM, Miller DR, Layton CJ, Brooks KR, Geller AC. Melanoma in middle-aged and older men: a multi-institutional survey study of factors related to tumor thickness. Arch Dermatol 2009. April;145(4):397–404. [DOI] [PubMed] [Google Scholar]
- 88. •.Paddock LE, Lu SE, Bandera EV, Rhoads GG, Fine J, Paine S, et al. Skin self-examination and long-term melanoma survival. Melanoma Res 2016;26(4):401–8.Long term follow-up of melanoma patients based on their health behaviors.
- 89.Berwick M, Armstrong BK, Ben-Porat L, Fine J, Kricker A, Eberle C, et al. Sun exposure and mortality from melanoma. J Natl Cancer Inst 2005. February 2;97(3):195–9. [DOI] [PubMed] [Google Scholar]
- 90.Oliveria SA, Christos PJ, Halpern AC, Fine JA, Barnhill RL, Berwick M. Patient knowledge, awareness, and delay in seeking medical attention for malignant melanoma. J Clin Epidemiol 1999. November;52(11):1111–6. [DOI] [PubMed] [Google Scholar]
- 91.rady MS, Oliveria SA, Christos PJ, Berwick M, Coit DG, Katz J, et al. Patterns of detection in patients with cutaneous melanoma. Cancer 2000. July 15;89(2):342–7. [DOI] [PubMed] [Google Scholar]
- 92.Holman DM, Ding H, Guy GP, Watson M, Hartman AM, Perna FM. Prevalence of Sun Protection Use and Sunburn and Association of Demographic and Behaviorial Characteristics With Sunburn Among US Adults. JAMA Dermatol 2018. May 1;154(5):561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lazovich D, Vogel RI, Berwick M, Weinstock MA, Warshaw EM, Anderson KE. Melanoma risk in relation to use of sunscreen or other sun protection methods. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2011. December;20(12):2583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, et al. A review of human carcinogens--part D: radiation. Lancet Oncol 2009. August;10(8):751–2. [DOI] [PubMed] [Google Scholar]
- 95.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B 2001. October;63(1–3):8–18. [DOI] [PubMed] [Google Scholar]
- 96.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer Oxf Engl 1990 2005. January;41(1):45–60. [DOI] [PubMed] [Google Scholar]
- 97.SEER*Explorer: An interactive website for SEER cancer statistics [Internet] Surveillance Research Program, National Cancer Institute; [Cited 2018 Dec 12]. Available from https://seer.cancer.gov/explorer/. [Google Scholar]
- 98.Gordon D, Gillgren P, Eloranta S, Olsson H, Gordon M, Hansson J, et al. Time trends in incidence of cutaneous melanoma by detailed anatomical location and patterns of ultraviolet radiation exposure: a retrospective population-based study. Melanoma Res 2015. August;25(4):348–56. [DOI] [PubMed] [Google Scholar]
- 99.Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol Off J Am Soc Clin Oncol 2011. January 20;29(3):257–63. [DOI] [PubMed] [Google Scholar]
- 100.Silva ES da, Tavares R, Paulitsch F da S, Zhang L. Use of sunscreen and risk of melanoma and non-melanoma skin cancer: a systematic review and meta-analysis. Eur J Dermatol EJD 2018. April 1;28(2):186–201. [DOI] [PubMed] [Google Scholar]
- 101.Rueegg CS, Stenehjem JS, Egger M, Ghiasvand R, Cho E, Lund E, et al. Challenges in Assessing the Sunscreen-Melanoma Association. Int J Cancer 2018. November 16; [DOI] [PMC free article] [PubMed]


