To understand the epidemiology and susceptibility patterns of yeast infections in Ontario, Canada, we examined 4,715 clinical yeast isolates submitted to our laboratory for antifungal susceptibility testing from 2014 to 2018. Candida albicans was the most frequently submitted species (43.0%), followed by C. glabrata (21.1%), C. parapsilosis (15.0%), and C. tropicalis (6.2%). Twenty-three other Candida spp.
KEYWORDS: antifungal resistance, antifungal susceptibility testing, azole, Candida, echinocandin, whole-genome sequencing, yeast
ABSTRACT
To understand the epidemiology and susceptibility patterns of yeast infections in Ontario, Canada, we examined 4,715 clinical yeast isolates submitted to our laboratory for antifungal susceptibility testing from 2014 to 2018. Candida albicans was the most frequently submitted species (43.0%), followed by C. glabrata (21.1%), C. parapsilosis (15.0%), and C. tropicalis (6.2%). Twenty-three other Candida spp. (11.6%) and 4 non-Candida species (3.1%) were also identified. Few changes in species distribution were observed from 2014 to 2018, but the total numbers of yeast isolates sent for testing increased, with an annual 7.4% change. According to CLSI clinical breakpoints, resistance rates remained low overall. Moderate fluconazole resistance was noted among C. glabrata (9%), C. parapsilosis (9%), and C. tropicalis (12%) isolates. Only 1% of C. glabrata isolates were resistant to caspofungin, micafungin, and anidulafungin. Whole-genome sequence analysis confirmed 11 cases of acquired resistance to azoles or echinocandins via in-host evolution. There were mutations in the gene for the catalytic subunit of 1,3-beta-glucan synthase-mediated echinocandin resistance in 3 of 3 C. albicans strains, 3 of 4 C. glabrata strains, and 1 strain of C. tropicalis. Azole resistance was likely caused by a homozygous ERG3 mutation in 1 C. albicans strain and a previously undescribed chromosomal-duplication event involving ERG11 and TAC1 orthologs in 1 C. tropicalis strain. While antifungal resistance rates remain low among yeast isolates in Ontario, ongoing surveillance is necessary to inform empirical therapy for optimal patient management and to guide antifungal stewardship.
INTRODUCTION
Invasive fungal infections are a significant cause of morbidity and mortality (1, 2). Among the fungi that can cause invasive fungal infections, Candida yeast species are the primary threat, particularly in health care settings, where they are among the top four most common nosocomial bloodstream pathogens (1, 3–5). Other yeasts, such as Saccharomyces spp., Trichosporon spp., Malassezia spp., Geotrichum candidum, and Rhodotorula spp., have also been implicated in invasive fungal infections but remain relatively rare (2, 6).
Several studies have described a changing epidemiology of candidiasis. Although species distributions of clinical yeast isolates differ based on geography and population, there has been a notable shift away from Candida albicans to non-albicans species; species such as C. glabrata, C. parapsilosis, and C. tropicalis are increasingly being encountered. Increased utilization of antifungal drugs for both treatment and prophylaxis in recent years has been identified as a cause of the changing epidemiology of candidiasis through selective pressure favoring resistant species (1, 7, 8). Overall, antifungal resistance remains relatively low; however, instances of resistance are increasingly being reported worldwide (9, 10). There has been a perceptible increase in resistance, particularly to azoles, among C. glabrata isolates in North America and C. tropicalis isolates in Asia (1, 11–14). C. glabrata isolates are generally susceptible to the echinocandins, but localized spikes in resistance are problematic and warrant attention (15, 16). Finally, infections due to rare Candida spp., many of which are intrinsically resistant, are increasingly being described (1, 9). Of these, C. auris is a major threat globally due to its pervasive nature and frequent resistance to one or more classes of antifungal agents (17).
Global and local surveillance studies are important to monitor antifungal resistance. Global surveillance is particularly good at detecting and defining emerging threats, while local studies provide useful data to inform empirical therapy and aid antifungal stewardship efforts (8).
Increased access to whole-genome sequencing (WGS) provides an additional opportunity to characterize the genetic mechanisms of antifungal resistance acquired by resistant isolates identified in antifungal surveillance studies. Echinocandin resistance in Candida spp. is largely caused by mutations in the 2 hot-spot regions of the genes encoding the catalytic subunit of 1,3-beta-glucan synthase, required for cell wall growth. These mutations render 1,3-beta-glucan synthase impervious to echinocandin activity (7, 18, 19). The genetic alterations causing azole resistance are more difficult to define, since it can involve multiple possible mechanisms affecting ergosterol synthesis and/or augmenting drug efflux pump activity (7). Understanding the mechanisms of resistance encountered in resistant clinical isolates can aid drug discovery and therapeutic guidelines.
In this study, we describe the epidemiology and antifungal susceptibilities of 4,715 clinical yeast isolates, including Candida spp., as well as Cryptococcus neoformans, Trichosporon asahii, Saccharomyces cerevisiae, and Rhodotorula mucilaginosa, recovered from Ontario patients from 2014 to 2018. Additionally, we used WGS analysis to investigate susceptible-turned-resistant (within a 6-month time frame) pairs of isolates obtained from individual patients to confirm instances of acquired resistance and to elucidate the genetic mechanisms underlying the resistant phenotypes. This study complements other recent Canadian (20) and North American (8, 21) data sets to describe antifungal resistance in Canada’s most populous province.
RESULTS
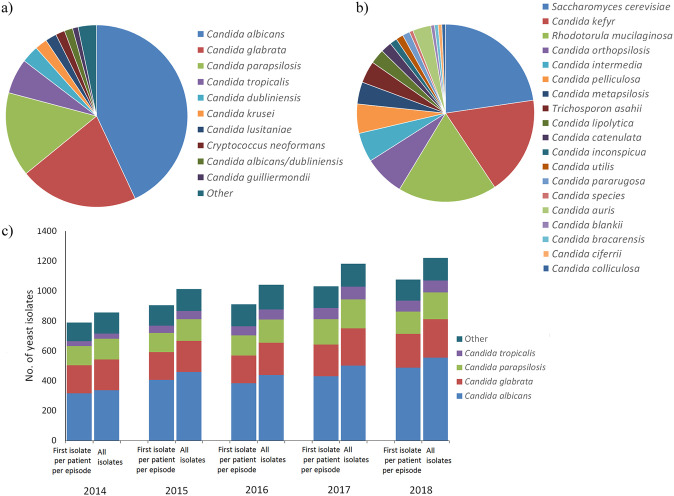
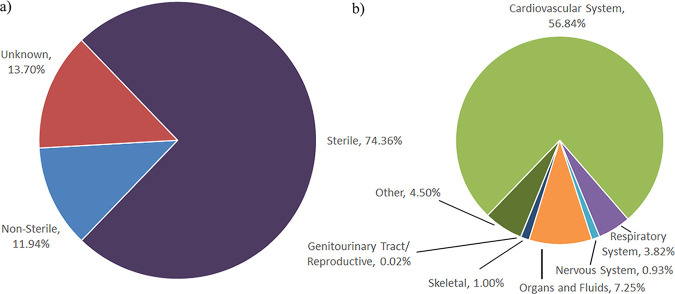
From 2014 to 2018, a total of 5,171 clinical yeast isolates were evaluated for antifungal susceptibility. A data set of 4,715 isolates representing the first isolate of a species received from a patient and submitted to Public Health Ontario (PHO) for antifungal susceptibility testing (AFST) within a 6-month period (selected, for the purposes of this paper, to define an infection episode) was used for the statistics described below. Figure 1a and b show the range of yeast species isolated from the specimens, with C. albicans isolated most frequently (43.0%), followed by C. glabrata (21.1%), C. parapsilosis (15.0%), and C. tropicalis (6.2%). The relative species distributions of C. albicans, C. glabrata, and C. parapsilosis remained constant over the study time frame (P = 0.14, P = 0.21, and P = 0.51, respectively), but the proportion of C. tropicalis increased from 2014 (4.3%) to 2018 (7.0%) (P = 0.004). An additional 23 Candida spp. and 4 non-Candida yeasts, encompassing 11.6% and 3.1% of the isolates, respectively, were identified (Fig. 1a and b). The total numbers of yeast isolates submitted for AFST increased from 2014 to 2018, with an annual change in the number of yeast isolates per 100,000 population (22) of 7.4% (Fig. 1c). Most isolates were recovered from sterile sites (81%), with blood the most frequent specimen source (57%) (Fig. 2; see Table S1 in the supplemental material).
FIG 1.
(a and b) Species distribution of common (a) and less common (b) yeast isolates from patient specimens representing the first isolate per patient per infection episode submitted for AFST in Ontario. (c) Species distribution per year from 2014 to 2018, including all isolates and the first isolate per patient per infection episode.
FIG 2.
Specimen sources of yeast isolates representing the first isolate per patient per infection episode submitted for AFST in Ontario. (a) Distribution of sterile, nonsterile, and unknown specimens. (b) Distribution of specimens categorized into major organ systems.
MICs based on broth microdilution (BMD) results for susceptibility to echinocandins, azoles, and amphotericin B are summarized in Table 1 for the more commonly encountered yeast species, i.e., C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. dubliniensis, C. krusei, C. lusitaniae, C. neoformans, and C. guilliermondii. Where CLSI breakpoints or Sensititre YeastOne (ThermoFisher, Waltham, MA) epidemiological cutoff values (ECVs) exist, the percent susceptible and resistant or wild-type and non-wild type were calculated (23–25).
TABLE 1.
Activities of antifungal drugs against common yeast species according to CLSI clinical breakpoints (23) and/or recently determined Sensititre YeastOne ECVs (24, 25)
| Organism, no. of isolates tested, and antifungal agent | Breakpoints (S, I, R)a (μg/ml) | ECV (μg/ml) | Range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | % of isolatesb |
|||
|---|---|---|---|---|---|---|---|---|---|
| CLSI method |
ECV |
||||||||
| S | R | WT | NWT | ||||||
| C. albicans, n = 2,029 | |||||||||
| Fluconazole | ≤2, SDD = 4, ≥8 | 0.5 | 0.12 to >256 | 0.5 | 0.5 | 98 | 1 | 97 | 3 |
| Voriconazole | ≤0.12, 0.25–0.5, ≥1 | 0.03 | 0.008 to >8 | 0.008 | 0.015 | 98 | 1 | 93 | 7 |
| Posaconazole | 0.06 | 0.008 to >8 | 0.03 | 0.03 | 98 | 2 | |||
| Itraconazole | 0.008 to 16 | 0.06 | 0.06 | 98 | 2 | ||||
| Amphotericin B | 2 | 0.12 to 2 | 0.5 | 1 | |||||
| Caspofungin | ≤0.25, 0.5, ≥1 | 0.008 to 8 | 0.06 | 0.06 | 100 | 0 | 100 | 0 | |
| Micafungin | ≤0.25, 0.5, ≥1 | 0.03 | 0.008 to 2 | 0.008 | 0.015 | 100 | 0 | 100 | 0 |
| Anidulafungin | ≤0.25, 0.5, ≥1 | 0.12 | 0.015 to 1 | 0.015 | 0.06 | 100 | 0 | 100 | 0 |
| C. glabrata, n = 994 | |||||||||
| Fluconazole | SDD ≤ 32, ≥64 | 8 | 0.12 to >256 | 16 | 32 | 91c | 9 | 93 | 7 |
| Voriconazole | 0.25 | 0.015 to 8 | 0.5 | 1 | 96 | 4 | |||
| Posaconazole | 1 | 0.008 to >8 | 1 | 2 | 92 | 8 | |||
| Itraconazole | 4 | 0.015 to >16 | 0.5 | 1 | 93 | 7 | |||
| Amphotericin B | 2 | 0.12 to 2 | 1 | 1 | |||||
| Caspofungin | ≤0.12, 0.25, ≥0.5 | 0.008 to 8 | 0.06 | 0.12 | 93 | 1 | 99 | 1 | |
| Micafungin | ≤0.06, 0.12, ≥0.25 | 0.03 | 0.008 to 4 | 0.015 | 0.015 | 99 | 1 | 99 | 1 |
| Anidulafungin | ≤0.12, 0.25, ≥0.5 | 0.25 | 0.015 to 2 | 0.03 | 0.06 | 99 | 1 | 99 | 1 |
| C. parapsilosis, n = 708 | |||||||||
| Fluconazole | ≤2, SDD = 4, ≥8 | 1 | 0.12 to 128 | 0.5 | 4 | 83 | 9 | 83d | 17d |
| Voriconazole | ≤0.12, 0.25–0.5, ≥1 | 0.03 | 0.008 to 2 | 0.015 | 0.06 | 99 | 0 | 85d | 15d |
| Posaconazole | 0.25 | 0.008 to 0.25 | 0.03 | 0.06 | 100d | 0d | |||
| Itraconazole | 0.5 | 0.015 to 0.5 | 0.06 | 0.12 | 100d | 0d | |||
| Amphotericin B | 1 | 0.12 to 2 | 0.5 | 0.5 | |||||
| Caspofungin | ≤2, 4, ≥8 | 0.06 to 1 | 0.5 | 1 | 100 | 0 | 100e | 0e | |
| Micafungin | ≤2, 4, ≥8 | 4 | 0.03 to 4 | 1 | 2 | 100 | 0 | 100e | 0e |
| Anidulafungin | ≤2, 4, ≥8 | 8 | 0.03 to 4 | 1 | 2 | 100 | 0 | 100e | 0e |
| C. tropicalis, n = 294 | |||||||||
| Fluconazole | ≤2, SDD = 4, ≥8 | 1 | 0.25 to >256 | 2 | 8 | 79 | 12 | 88 | 12 |
| Voriconazole | ≤0.12, 0.25–0.5, ≥1 | 0.12 | 0.008 to >8 | 0.12 | 0.5 | 61 | 8 | 92 | 8 |
| Posaconazole | 0.12 | 0.03 to >8 | 0.25 | 0.5 | 97 | 3 | |||
| Itraconazole | 0.5 | 0.03 to 16 | 0.25 | 0.5 | 94 | 6 | |||
| Amphotericin B | 2 | 0.25 to 2 | 1 | 1 | |||||
| Caspofungin | ≤0.25, 0.5, ≥1 | 0.008 to 2 | 0.06 | 0.12 | 100 | 0 | 100 | 0 | |
| Micafungin | ≤0.25, 0.5, ≥1 | 0.06 | 0.008 to 0.06 | 0.03 | 0.03 | 100 | 0 | 100 | 0 |
| Anidulafungin | ≤0.25, 0.5, ≥1 | 0.12 | 0.015 to 0.25 | 0.06 | 0.12 | 100 | 0 | 100 | 0 |
| C. dubliniensis, n = 145 | |||||||||
| Fluconazole | 0.5 | 0.12 to 1 | 0.25 | 0.5 | 100 | 0 | |||
| Voriconazole | 0.008 to 0.03 | 0.008 | 0.008 | 99 | 1 | ||||
| Posaconazole | 0.125 | 0.008 to 0.12 | 0.03 | 0.06 | 100 | 0 | |||
| Itraconazole | 0.25 | 0.015 to 0.12 | 0.03 | 0.06 | 100 | 0 | |||
| Amphotericin B | 0.5 | 0.12 to 1 | 0.5 | 0.5 | |||||
| Caspofungin | 0.015 to 2 | 0.06 | 0.12 | 99 | 1 | ||||
| Micafungin | 0.12 | 0.008 to 1 | 0.03 | 0.03 | 99 | 1 | |||
| Anidulafungin | 0.12 | 0.015 to 0.12 | 0.12 | 0.12 | 100 | 0 | |||
| C. krusei, n = 103 | |||||||||
| Fluconazole | 0.25 to 128 | 32 | 64 | 100 | 0 | ||||
| Voriconazole | ≤0.5, 1, ≥2 | 0.5 | 0.008 to 8 | 0.25 | 0.5 | 92 | 4 | 96 | 4 |
| Posaconazole | 0.5 | 0.03 to 1 | 0.25 | 0.5 | 100 | 0 | |||
| Itraconazole | 1 | 0.06 to 1 | 0.25 | 0.5 | 100 | 0 | |||
| Amphotericin B | 2 | 0.25 to 2 | 1 | 1 | |||||
| Caspofungin | ≤0.25, 0.5, ≥1 | 0.03 to 2 | 0.25 | 0.5 | 85 | 1 | 99 | 1 | |
| Micafungin | ≤0.25, 0.5, ≥1 | 0.25 | 0.008 to 1 | 0.12 | 0.12 | 99 | 1 | 99 | 1 |
| Anidulafungin | ≤0.25, 0.5, ≥1 | 0.25 | 0.015 to 0.5 | 0.06 | 0.06 | 99 | 0 | 99 | 1 |
| C. lusitaniae, n = 94 | |||||||||
| Fluconazole | 1 | 0.12 to 32 | 0.5 | 2 | 98 | 2 | |||
| Voriconazole | 0.008 to 0.25 | 0.008 | 0.03 | 95 | 5 | ||||
| Posaconazole | 0.06 | 0.008 to 0.25 | 0.03 | 0.06 | 98 | 2 | |||
| Itraconazole | 1 | 0.015 to 0.5 | 0.06 | 0.12 | 100 | 0 | |||
| Amphotericin B | 2 | 0.12 to 8 | 0.5 | 0.5 | |||||
| Caspofungin | 1 | 0.015 to 8 | 0.25 | 0.5 | 99 | 1 | |||
| Micafungin | 0.5 | 0.008 to 8 | 0.06 | 0.12 | 98 | 2 | |||
| Anidulafungin | 1 | 0.015 to 2 | 0.12 | 0.25 | 98 | 2 | |||
| C. neoformans, n = 77 | |||||||||
| Fluconazole | 8 | 0.5 to 8 | 2 | 4 | |||||
| Voriconazole | 0.25 | 0.008 to 0.06 | 0.03 | 0.06 | |||||
| Posaconazole | 0.25 | 0.008 to 0.25 | 0.06 | 0.12 | |||||
| Itraconazole | 0.25 | 0.015 to 0.12 | 0.03 | 0.06 | |||||
| Amphotericin B | 0.5 | 0.12 to 1 | 0.5 | 1 | |||||
| Caspofungin | 8 | 8 | 8 | ||||||
| Micafungin | 4 to >8 | 8 | 8 | ||||||
| Anidulafungin | 2 to >8 | 8 | 8 | ||||||
| C. guilliermondii, n = 47 | |||||||||
| Fluconazole | 8 | 0.12 to 32 | 2 | 8 | 98 | 2 | |||
| Voriconazole | 0.008 to 1 | 0.06 | 0.25 | 98 | 2 | ||||
| Posaconazole | 0.5 | 0.03 to 1 | 0.12 | 0.5 | 100 | 0 | |||
| Itraconazole | 2 | 0.06 to 2 | 0.25 | 0.5 | 98 | 2 | |||
| Amphotericin B | 2 | 0.12 to 2 | 0.25 | 0.5 | |||||
| Caspofungin | ≤2, 4, ≥8 | 0.06 to 1 | 0.25 | 0.5 | 100 | 0 | 100 | 0 | |
| Micafungin | ≤2, 4, ≥8 | 2 | 0.06 to 1 | 0.5 | 1 | 100 | 0 | 100 | 0 |
| Anidulafungin | ≤2, 4, ≥8 | 8 | 0.12 to 2 | 1 | 2 | 100 | 0 | 100 | 0 |
S, susceptible; I, intermediate; R, resistant.
Isolates described are the first isolate tested per patient per infection episode, 2014 to 2018. WT, wild type; NWT, non-wild type.
SDD, susceptible dose dependent.
ECVs for fluconazole, voriconazole, posaconazole, and itraconazole are for C. parapsilosis sensu stricto.
ECVs for caspofungin, micafungin, and anidulafungin are for C. parapsilosis species complex.
When the first isolates submitted for AFST per patient per infection episode were examined, 98% of C. albicans isolates were susceptible to fluconazole and voriconazole; however, resistance to azoles was noted among non-albicans species. Nine percent of C. glabrata isolates were resistant to fluconazole. Similarly, 9% of C. parapsilosis isolates were resistant to fluconazole, and 83% were susceptible to the drug (8% were classified as susceptible dose dependent [SDD]) (Table 1). Azole resistance was more pronounced for C. tropicalis isolates, with 12% resistant (79% susceptible; 9% SDD) to fluconazole and 8% resistant (61% susceptible; 31% SDD) to voriconazole (Table 1). Caspofungin, micafungin, and anidulafungin exhibited good activity against Candida spp. Resistance rates for the echinocandins ranged from 0% to 1% for C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. krusei, and C. guilliermondii; of note, 7% of C. glabrata isolates and 15% of C. krusei isolates were nonsusceptible to caspofungin (Table 1). From 2014 to 2018 no statistically significant trends were detected pertaining to an increase or decrease in the percentage of the commonly isolated Candida spp. susceptible to various antifungal agents (Table 2). Isolates that were nonsusceptible to azoles or echinocandins were derived from a broad range of specimens (see Table S2 in the supplemental material) but were predominately bloodstream isolates of C. parapsilosis, C. tropicalis (azoles), and C. glabrata (echinocandins).
TABLE 2.
Percentages of Candida spp. susceptible to various antifungals for which CLSI breakpoints exist, 2014 to 2018
| Organism and antifungal agent | % susceptiblea |
Cochran-Armitage P valueb |
||||
|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | ||
| C. albicans | ||||||
| Fluconazole | 98 | 98 | 98 | 98 | 98 | 0.792 |
| Voriconazole | 98 | 99 | 98 | 99 | 98 | 0.966 |
| Caspofungin | 100 | 100 | 100 | 100 | 99 | 0.122 |
| Micafungin | 100 | 100 | 100 | 100 | 99 | 0.085 |
| Anidulafungin | 100 | 100 | 100 | 100 | 100 | 0.192 |
| C. glabrata | ||||||
| Caspofungin | 99 | 87 | 95 | 93 | 92 | 0.206 |
| Micafungin | 99 | 99 | 98 | 99 | 100 | 0.659 |
| Anidulafungin | 99 | 99 | 98 | 99 | 100 | 0.825 |
| C. parapsilosis | ||||||
| Fluconazole | 90 | 81 | 79 | 82 | 83 | 0.254 |
| Voriconazole | 98 | 98 | 99 | 99 | 99 | 0.345 |
| Caspofungin | 100 | 100 | 100 | 100 | 100 | NA |
| Micafungin | 100 | 99 | 99 | 100 | 100 | 0.533 |
| Anidulafungin | 100 | 99 | 100 | 100 | 100 | 0.425 |
| C. tropicalis | ||||||
| Fluconazole | 76 | 77 | 85 | 75 | 79 | 0.963 |
| Voriconazole | 71 | 50 | 66 | 57 | 64 | 0.998 |
| Caspofungin | 100 | 100 | 100 | 100 | 99 | 0.220 |
| Micafungin | 100 | 100 | 100 | 100 | 100 | NA |
| Anidulafungin | 100 | 100 | 100 | 100 | 100 | NA |
| C. krusei | ||||||
| Fluconazole | 95 | 95 | 86 | 95 | 89 | 0.584 |
| Caspofungin | 100 | 89 | 82 | 68 | 89 | 0.083 |
| Micafungin | 100 | 95 | 100 | 100 | 100 | 0.476 |
| Anidulafungin | 100 | 95 | 100 | 100 | 100 | 0.476 |
| C. guilliermondii | ||||||
| Caspofungin | 100 | 100 | 100 | 100 | 100 | NA |
| Micafungin | 100 | 100 | 100 | 100 | 100 | NA |
| Anidulafungin | 100 | 100 | 100 | 100 | 100 | NA |
The numbers represent the first isolate of each species per patient per infection episode.
NA, not applicable.
ECVs for use with Sensititre YeastOne panels for the detection of resistance among Candida spp. to triazoles (25) and echinocandins (24) have recently been determined. Using these values, a higher percentage (>10%) of isolates with non-wild-type voriconazole MICs were noted for C. parapsilosis. Likewise, >10% of C. parapsilosis and C. tropicalis isolates had non-wild-type fluconazole MICs (Table 1).
From 2014 to 2018, antimicrobial susceptibility testing was performed on 145 isolates of 23 less common Candida spp. and non-Candida yeasts representing the first isolates submitted for AFST per patient per infection episode. For species with ≥3 isolates, MIC distributions showing the number of isolates at each MIC for each species-drug combination are displayed in Table 3. As previously suggested, combining these data with similarly derived data from other studies would provide a more robust description of the MIC profiles of these rarely encountered species (21).
TABLE 3.
Activities of antifungal drugs against uncommon yeast species with ≥3 isolates
| Organism, no. of isolates tested, and antifungal agent | No. of isolatesa at MIC (μg/ml) of: |
MIC50 (μg/ml) | MIC90 (μg/ml) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | >128 | |||
| S. cerevisiae, n = 34 | ||||||||||||||||||
| Fluconazole | 6 | 3 | 9 | 6 | 4 | 4 | 1 | 1 | 2 | 16 | ||||||||
| Voriconazole | 1 | 10 | 12 | 4 | 3 | 3 | 1 | 0.06 | 0.5 | |||||||||
| Posaconazole | 2 | 4 | 11 | 10 | 2 | 4 | 1 | 0.5 | 2 | |||||||||
| Itraconazole | 3 | 8 | 13 | 5 | 2 | 3 | 0.25 | 2 | ||||||||||
| Amphotericin B | 2 | 8 | 18 | 6 | 0.5 | 1 | ||||||||||||
| Caspofungin | 1 | 2 | 10 | 15 | 6 | 0.25 | 0.5 | |||||||||||
| Micafungin | 1 | 4 | 18 | 10 | 1 | 0.12 | 0.25 | |||||||||||
| Anidulafungin | 1 | 2 | 4 | 18 | 6 | 3 | 0.12 | 0.25 | ||||||||||
| C. kefyr, n = 27 | ||||||||||||||||||
| Fluconazole | 8 | 13 | 6 | 0.25 | 0.5 | |||||||||||||
| Voriconazole | 21 | 6 | 0.008 | 0.015 | ||||||||||||||
| Posaconazole | 2 | 1 | 6 | 9 | 7 | 1 | 1 | 0.06 | 0.12 | |||||||||
| Itraconazole | 2 | 5 | 16 | 2 | 2 | 0.06 | 0.12 | |||||||||||
| Amphotericin B | 1 | 2 | 17 | 7 | 1 | 2 | ||||||||||||
| Caspofungin | 8 | 11 | 4 | 2 | 1 | 1 | 0.03 | 0.12 | ||||||||||
| Micafungin | 1 | 8 | 12 | 3 | 1 | 1 | 1 | 0.06 | 0.25 | |||||||||
| Anidulafungin | 2 | 3 | 8 | 8 | 3 | 1 | 1 | 1 | 0.12 | 0.5 | ||||||||
| R. mucilaginosa, n = 27 | ||||||||||||||||||
| Fluconazole | 1 | 1 | 1 | 1 | 5 | 18 | 256 | >256 | ||||||||||
| Voriconazole | 1 | 1 | 1 | 2 | 1 | 4 | 7 | 9 | 1 | 2 | 4 | |||||||
| Posaconazole | 1 | 2 | 2 | 2 | 10 | 7 | 3 | 1 | 8 | |||||||||
| Itraconazole | 1 | 1 | 2 | 2 | 6 | 12 | 3 | 1 | 16 | |||||||||
| Amphotericin B | 1 | 4 | 16 | 6 | 0.5 | 1 | ||||||||||||
| Caspofungin | 1 | 1 | 1 | 21 | 3 | 8 | >8 | |||||||||||
| Micafungin | 1 | 1 | 2 | 20 | 3 | 8 | >8 | |||||||||||
| Anidulafungin | 1 | 1 | 1 | 21 | 3 | 8 | >8 | |||||||||||
| C. orthopsilosis, n = 10 | ||||||||||||||||||
| Fluconazole | 1 | 5 | 2 | 2 | 1 | 1 | 8 | |||||||||||
| Voriconazole | 1 | 2 | 5 | 2 | 1 | 0.03 | 0.5 | |||||||||||
| Posaconazole | 1 | 2 | 2 | 6 | 0.12 | 0.12 | ||||||||||||
| Itraconazole | 1 | 1 | 2 | 6 | 1 | 0.12 | 0.25 | |||||||||||
| Amphotericin B | 2 | 8 | 1 | 0.5 | 1 | |||||||||||||
| Caspofungin | 1 | 3 | 6 | 1 | 0.5 | 1 | ||||||||||||
| Micafungin | 2 | 7 | 2 | 0.5 | 1 | |||||||||||||
| Anidulafungin | 1 | 5 | 4 | 1 | 0.5 | 2 | ||||||||||||
| C. intermedia, n = 8 | ||||||||||||||||||
| Fluconazole | 2 | 3 | 2 | 1 | 0.25 | 1 | ||||||||||||
| Voriconazole | 8 | 0.008 | 0.008 | |||||||||||||||
| Posaconazole | 5 | 3 | 0.008 | 0.015 | ||||||||||||||
| Itraconazole | 2 | 4 | 2 | 0.03 | 0.06 | |||||||||||||
| Amphotericin B | 3 | 2 | 3 | 0.25 | 0.5 | |||||||||||||
| Caspofungin | 6 | 1 | 1 | 0.06 | 0.25 | |||||||||||||
| Micafungin | 2 | 6 | 0.03 | 0.03 | ||||||||||||||
| Anidulafungin | 3 | 3 | 1 | 1 | 0.03 | 0.12 | ||||||||||||
| C. pelliculosa, n = 8 | ||||||||||||||||||
| Fluconazole | 3 | 4 | 1 | 4 | 8 | |||||||||||||
| Voriconazole | 1 | 6 | 1 | 0.12 | 0.25 | |||||||||||||
| Posaconazole | 1 | 3 | 3 | 1 | 0.5 | 1 | ||||||||||||
| Itraconazole | 3 | 4 | 1 | 0.25 | 0.5 | |||||||||||||
| Amphotericin B | 1 | 2 | 4 | 1 | 0.5 | 1 | ||||||||||||
| Caspofungin | 1 | 3 | 3 | 1 | 0.06 | 0.12 | ||||||||||||
| Micafungin | 1 | 3 | 4 | 0.03 | 0.03 | |||||||||||||
| Anidulafungin | 8 | 0.015 | 0.015 | |||||||||||||||
| C. metapsilosis, n = 6 | ||||||||||||||||||
| Fluconazole | 1 | 4 | 1 | 1 | 4 | |||||||||||||
| Voriconazole | 1 | 4 | 1 | 0.015 | 0.06 | |||||||||||||
| Posaconazole | 1 | 4 | 1 | 0.03 | 0.06 | |||||||||||||
| Itraconazole | 3 | 2 | 1 | 0.06 | 0.12 | |||||||||||||
| Amphotericin B | 1 | 5 | 0.5 | 0.5 | ||||||||||||||
| Caspofungin | 3 | 2 | 1 | 0.25 | 0.5 | |||||||||||||
| Micafungin | 2 | 4 | 0.5 | 0.5 | ||||||||||||||
| Anidulafungin | 1 | 4 | 1 | 0.12 | 0.5 | |||||||||||||
| T. asahii, n = 6 | ||||||||||||||||||
| Fluconazole | 3 | 2 | 1 | 4 | 8 | |||||||||||||
| Voriconazole | 1 | 3 | 2 | 0.06 | 0.12 | |||||||||||||
| Posaconazole | 4 | 2 | 0.25 | 0.5 | ||||||||||||||
| Itraconazole | 1 | 5 | 0.25 | 0.25 | ||||||||||||||
| Amphotericin B | 1 | 5 | 0.5 | 0.5 | ||||||||||||||
| Caspofungin | 6 | 8 | 8 | |||||||||||||||
| Micafungin | 6 | 8 | 8 | |||||||||||||||
| Anidulafungin | 6 | 8 | 8 | |||||||||||||||
| C. auris, n = 5 | ||||||||||||||||||
| Fluconazole | 1 | 3 | 1 | 1 | 2 | |||||||||||||
| Voriconazole | 2 | 3 | 0.015 | 0.015 | ||||||||||||||
| Posaconazole | 1 | 4 | 0.015 | 0.015 | ||||||||||||||
| Itraconazole | 1 | 1 | 3 | 0.06 | 0.06 | |||||||||||||
| Amphotericin B | 1 | 3 | 1 | 1 | 2 | |||||||||||||
| Caspofungin | 1 | 1 | 3 | 0.25 | 0.25 | |||||||||||||
| Micafungin | 2 | 3 | 0.03 | 0.06 | ||||||||||||||
| Anidulafungin | 5 | 0.12 | 0.12 | |||||||||||||||
| C. lipolytica, n = 4 | ||||||||||||||||||
| Fluconazole | 2 | 1 | 1 | 4 | >256 | |||||||||||||
| Voriconazole | 2 | 1 | 1 | 0.06 | 8 | |||||||||||||
| Posaconazole | 1 | 1 | 1 | 1 | 0.5 | 8 | ||||||||||||
| Itraconazole | 3 | 1 | 0.12 | 16 | ||||||||||||||
| Amphotericin B | 2 | 2 | 1 | 1 | ||||||||||||||
| Caspofungin | 1 | 3 | 0.5 | 0.5 | ||||||||||||||
| Micafungin | 1 | 3 | 0.5 | 0.5 | ||||||||||||||
| Anidulafungin | 1 | 2 | 1 | 0.12 | 0.25 | |||||||||||||
| C. catenulata, n = 3 | ||||||||||||||||||
| Fluconazole | 1 | 1 | 1 | 2 | 4 | |||||||||||||
| Voriconazole | 1 | 1 | 1 | 0.03 | 0.12 | |||||||||||||
| Posaconazole | 1 | 2 | 0.03 | 0.03 | ||||||||||||||
| Itraconazole | 1 | 2 | 0.03 | 0.03 | ||||||||||||||
| Amphotericin B | 1 | 2 | 0.5 | 0.5 | ||||||||||||||
| Caspofungin | 1 | 1 | 1 | 0.25 | 0.5 | |||||||||||||
| Micafungin | 1 | 2 | 0.03 | 0.03 | ||||||||||||||
| Anidulafungin | 1 | 2 | 0.06 | 0.5 | ||||||||||||||
Isolates represent the first species per patient per infection episode, 2014 to 2018.
Among the rarely encountered Candida spp., elevated fluconazole MICs (>4 μg/ml) were noted for isolates of C. orthopsilosis (n = 1/10), C. pelliculosa (n = 1/8), C. lipolytica (n = 1/4) (Table 3), C. inconspicua (n = 2/2), C. utilis (n = 2/2), C. pararugosa (n = 1/2), C. blankii (n = 1/1), C. bracarensis (n = 1/1), C. ciferrii (n = 1/1), and C. magnoliae (n = 1/1) (data not shown). Voriconazole and posaconazole MICs were typically <1 μg/ml, except for 1 isolate each of C. orthopsilosis (n = 1/10), C. pelliculosa (n = 1/8), C. lipolytica (n = 1/4) (Table 3), C. utilis (n = 1/2), C. blankii (n = 1/1), C. bracarensis (n = 1/1), and C. magnoliae (n = 1/1) (data not shown). MICs of the echinocandins among the rarely encountered Candida spp. were typically quite low (<0.5 μg/ml), except for 2/27 isolates of C. kefyr and 5/10 isolates of C. orthopsilosis (Table 3). Notably, 5 isolates of C. auris were recovered from 2014 to 2018; however, unlike what has been typically reported previously (17), none of the MICs for the 3 main classes of antifungal drugs were elevated. Of note, these isolates all belonged to clade IV (South American) of C. auris as determined by WGS analysis (26).
Among the non-Candida yeasts representing first isolates submitted for AFST per patient per infection episode, MICs of the echinocandins were very high for all isolates of C. neoformans, R. mucilaginosa, and T. asahii, although they remained low (≤0.5 μg/ml) for S. cerevisiae (Tables 1 and 3). Fluconazole MICs were also elevated (>4 μg/ml) among some isolates of C. neoformans (n = 3/77), S. cerevisiae (n = 10/34), and T. asahii (n = 1/6) and most R. mucilaginosa isolates (n = 26/27), although the MICs for voriconazole and posaconazole were typically low (Tables 1 and 3).
When we analyzed all the submitted isolates, we noted examples of 24 sets of sequential isolates of C. albicans (n = 5), C. glabrata (n = 12), C. parapsilosis (n = 2), C. tropicalis (n = 4), and C. krusei (n = 1) from single patients that demonstrated a susceptible-to-nonsusceptible shift in antifungal resistance within a 6-month period (considered to be a single infection episode) (Table 4). Whole-genome sequencing was performed on select sets of these isolates to determine if the isolates were the same strain and in an attempt to identify the molecular mechanisms of resistance. In total, we sequenced 2 pairs of C. albicans (SP0206/SP0369 and SP2512/SP2683) and 1 pair of C. tropicalis (SP4694/SP4785) isolates that demonstrated a shift from azole susceptibility to resistance. We also sequenced 3 pairs of C. albicans (SP1153/SP1274, SP1261/SP1350, and SP4920/SP5012), 5 pairs of C. glabrata (SP1533/SP1643, SP2320/SP2659, SP3417/SP3689, SP3046/SP3439, and SP2983/SP3003), and 1 pair of C. tropicalis (SP4433/SP4501) isolates where the MICs of one or more echinocandins shifted from susceptible to resistant (Table 4). The numbers of days between an initial susceptible isolate and a subsequent resistant isolate ranged from 10 to 127. For 11 of the pairs (SP0206/SP0369, SP2512/SP2683, SP1153/SP1274, SP1261/SP1350, SP4920/SP5012, SP1533/SP1643, SP2320/SP2659, SP3417/SP3689, SP3046/SP3439, SP4694/SP4785, and SP4433/SP4501), multilocus sequence-typing (MLST) analysis and whole-genome single-nucleotide polymorphism (SNP) maximum-parsimony (MP) phylogenetic analysis suggested a high degree of genetic relatedness between the initial susceptible and subsequent resistant isolates from the same patient; the MLST sequence types were the same, and isolate pairs clustered together in the MP trees (Table 4; see Fig. S1 in the supplemental material). For one pair of C. glabrata isolates (SP2982/SP3003), the MLST sequence types were different and the isolates did not cluster together in the whole-genome SNP MP tree (Table 4; see Fig. S1). The specimen source of the initial susceptible isolate of this pair was peritoneal fluid, while the subsequent resistant isolate was derived from blood 10 days later (Table 4).
TABLE 4.
Sets of sequential isolates from single patients that demonstrated a susceptible (S)-to-nonsusceptible (I or R) shift in antimicrobial resistance within a 6-month period, representing a single infection episode
| Organism and patient ID | Specimen ID | Specimen source | Interval (no. of days) | FLUa |
VORIa |
CASPa |
MICAa |
ANIa |
STa | Molecular mechanism of resistance | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | Resistance | MIC | Resistance | MIC | Resistance | MIC | Resistance | MIC | Resistance | ||||||
| C. albicans | |||||||||||||||
| PA0188 | SP0206 | Blood | 0 | 0.5 | S | ≤0.008 | S | 918 | Unknown | ||||||
| SP0369 | Pleural fluid | 83 | ≥256 | R | 8 | R | 918 | ||||||||
| PA2062 | SP2512 | Blood | 0 | 2 | S | 0.015 | S | 726 | ERG3: A255T | ||||||
| SP2683 | Blood | 55 | 256 | R | 8 | R | 726 | ||||||||
| PA0909 | SP1153 | Blood | 0 | 0.06 | S | 0.015 | S | ≤0.015 | S | 3568 | GSC1: S645P | ||||
| SP1274 | Blood | 55 | >8 | R | 2 | R | 0.25 | S | 3568 | ||||||
| PA1070 | SP1261 | Blood | 0 | 0.5 | I | 0.06 | S | 0.06 | S | 3570 | GSC1: S645P | ||||
| SP1350 | Peritoneal fluid | 34 | 4 | R | 2 | R | 0.5 | I | 3570 | ||||||
| PA3104 | SP4920 | Blood | 0 | 0.12 | S | 0.015 | S | ≤0.015 | S | 3569 | GSC1: S645P | ||||
| SP5052 | Blood | 39 | 8 | R | 4 | R | 1 | R | 3569 | ||||||
| C. glabrata | |||||||||||||||
| PA1293 | SP1533 | Abscess | 0 | 0.06 | S | 10 | None detected | ||||||||
| SP1643 | Abdomen | 35 | 0.5 | R | 10 | ||||||||||
| PA3825 | SP4776 | Blood | 0 | 0.06 | S | ||||||||||
| SP4784 | Blood | 2 | 0.25 | I | |||||||||||
| PA1823 | SP2186 | Blood | 0 | 0.03 | S | ||||||||||
| SP2198 | Blood | 5 | 0.25 | I | |||||||||||
| PA2095 | SP2554 | Urine | 0 | 0.06 | S | ||||||||||
| SP2585 | Urine | 9 | 0.25 | I | |||||||||||
| PA3539 | SP4419 | Pleural fluid | 0 | 0.06 | S | ||||||||||
| SP4650 | Pleural fluid | 80 | 0.25 | I | |||||||||||
| PA1563 | SP1866 | Peritoneal fluid | 0 | 0.015 | S | ||||||||||
| SP2187 | Blood | 115 | 0.12 | I | |||||||||||
| PA1188 | SP1405 | Blood | 0 | 0.12 | S | 0.12 | S | ||||||||
| SP1475 | Blood | 26 | 0.25 | I | 0.25 | I | |||||||||
| PA1921 | SP2320 | Blood | 0 | 0.12 | S | 0.03 | S | 0.03 | S | 16 | FKS2: S663P | ||||
| SP2659 | Blood | 118 | 8 | R | 2 | R | 2 | R | 16 | ||||||
| PA2117 | SP2583 | Blood | 0 | 0.12 | S | 0.015 | S | 0.03 | S | ||||||
| SP2672 | Blood | 28 | 1 | R | 0.5 | R | 1 | R | |||||||
| PA2320 | SP3417 | Blood | 0 | 0.06 | S | 0.015 | S | 0.03 | S | 3 | FKS2: S663P | ||||
| SP3689 | Blood | 75 | 8 | R | 8 | R | 2 | R | 3 | ||||||
| PA2472 | SP3046 | Blood | 0 | 0.12 | S | 0.015 | S | 0.06 | S | 19 | FKS2: S663P | ||||
| SP3439 | Blood | 127 | 0.25 | I | 0.12 | I | 0.5 | R | 19 | ||||||
| PA2419 | SP2982 | Peritoneal fluid | 0 | 0.06 | S | 0.015 | S | ≤0.015 | S | 17 | |||||
| SP3003 | Blood | 10 | 0.5 | R | 0.12 | I | 0.25 | I | 3 | FKS1: F625I | |||||
| C. parapsilosis | |||||||||||||||
| PA1947 | SP2355 | Wound | 0 | 0.12 | S | ||||||||||
| SP2398 | Other | 17 | 0.25 | I | |||||||||||
| PA2106 | SP2568 | Blood | 0 | 0.06 | S | ||||||||||
| SP2675 | Blood | 35 | 0.25 | I | |||||||||||
| C. tropicalis | |||||||||||||||
| PA3063 | SP3823 | Blood | 0 | 0.12 | S | ||||||||||
| SP3887 | Blood | 25 | 0.25 | I | |||||||||||
| PA3762 | SP4694 | Peritoneal fluid | 0 | 1 | S | 0.12 | S | 975 | Aneuploidy of supercontig 3.8 and increased copy no. of ERG11 and TAC1 | ||||||
| SP4785 | Peritoneal fluid | 28 | 32 | R | 2 | R | 975 | ||||||||
| PA3550 | SP4433 | Blood | 0 | 0.06 | S | 0.015 | S | 0.12 | S | 974 | CTRG_04661: S30P | ||||
| SP4501 | Blood | 22 | 8 | R | 2 | R | 1 | R | 974 | ||||||
| C. krusei | |||||||||||||||
| PA1488 | SP1777 | Blood | 0 | 0.25 | S | ||||||||||
| SP1811 | Blood | 15 | 0.5 | I | |||||||||||
FLU, fluconazole; VORI, voriconazole; CASP, caspofungin; MICA, micafungin; ANI, anidulafungin; ST, sequence type.
Comparison of the pairs of initial susceptible and subsequent resistant isolates of C. albicans where echinocandin resistance was acquired (SP1153/SP1274, SP1261/SP1350, and SP4920/SP5052) revealed that all 3 strains acquired an S645P mutation in hot spot 1 of GSC1 (Table 4). Similar comparisons among initial susceptible and subsequent resistant isolates of C. glabrata revealed 3 strains (SP2320/SP2659, SP3417/SP2689, and SP3046/SP3439) that demonstrated acquisition of S663P in hot spot 1 of FKS2 and 1 resistant isolate (SP3003) with an isoleucine instead of phenylalanine at position 625 of hot spot 1 of FKS1 compared to the C. glabrata reference strain, CBS138. In one set of C. glabrata isolates (SP1533/SP1643), there were no mutations detected in FKS1, FKS2, or FKS3 (Table 4). Comparison of the single pair of susceptible and resistant isolates of C. tropicalis from the same patient revealed the acquisition of an S30P mutation in CTRG_04661 (Table 4), which aligns with amino acid position 663 of the hot spot 1 region of C. albicans GSC1.
Among pairs of isolates demonstrating a shift from azole susceptible to azole resistant, we examined the following genes: ergosterol production enzyme genes ERG3 and ERG11; efflux pump genes CDR1, CDR2, CDR4, CDR5, CDR11, MDR1, FLU1, SNQ2, TPO3, and TOR1; and transcription factor genes MMR1, Ndt80, Stb5, TAC1, and UPC2 (7, 21, 27) (see Table S3 in the supplemental material). In the first pair of C. albicans isolates with acquired azole resistance, no detectable missense mutations in these targets were identified in the resistant isolate SP0369 relative to the initial susceptible isolate SP0206 (see Table S3). Conversely, multiple mutations were noted between the resistant (SP2683) and susceptible (SP2512) isolates of the second pair of C. albicans isolates, most notably a homozygous A255T mutation in ERG3 (see Table S3). Comparison of genome-wide copy number variation between azole-resistant C. tropicalis strain SP4785 and its diploid progenitor, SP4694, suggested that the estimated overall ploidy of supercontig 3.8 was ∼4 (see Table S3 and Fig. S2 in the supplemental material), with the ploidy of the regions containing the ERG11 sterol 14-demethylase gene and the TAC1 transcriptional-activator gene estimated at 6.0 and 6.2, respectively.
DISCUSSION
Surveillance of antifungal susceptibility/resistance among clinical yeast species is an important endeavor given reports of resistance acquisition during treatment, the clinical impact of a variety of uncommon yeasts that are refractory to antifungal agents (9), and the potential for nosocomial spread of resistant strains (28–30). While global surveillance can identify emerging threats, local monitoring is also useful, since it can provide geographically focused information to aid empirical treatment and inform antifungal stewardship work.
The current study describes susceptibility/resistance rates among Candida spp. and non-Candida yeast species received at the provincial reference laboratory in Ontario for antifungal susceptibility testing. As PHO performs the vast majority of AFST in the province and Ontario is Canada’s most populous province, representing almost 40% of the country’s population (22), these data have potential to be relevant nationally. Isolates were submitted from both hospital and community laboratories, with the majority cultured from blood and other sterile sites. Similar to other reports from North America, Europe, and Australia, the majority of the isolates were C. albicans, followed by C. glabrata and C. parapsilosis (8, 20, 31–35). In other geographic regions, including Latin America, Asia, South Africa, the Middle East, and North Africa, C. glabrata is less frequently isolated, with C. parapsilosis and/or C. tropicalis recovered more predominantly in the species distribution (36–40). The proportional species distribution remained fairly constant from 2014 to 2018, except for a significant increase in C. tropicalis. Other studies have also noted a shift toward non-albicans clinical yeast isolates (1, 11, 13, 20). The total number of yeast isolates submitted each year for AFST standardized to population size appears to be increasing, with an annual change of approximately 7.4%. This may signal an increasing prevalence of invasive yeast infections in Ontario, possibly due to an aging population and/or an increasing immunocompromised patient population, as has been noted previously (1), or in the face of reports of increased resistance rates in yeast, it may represent decreased confidence among clinicians to treat empirically, based on species assignment and the desire to have actual susceptibility results or MICs.
Among the set of first isolates per patient per infection episode, C. albicans isolates were largely susceptible to both azoles and echinocandins, as in other studies (8, 20, 21, 33, 36, 39). Resistance rates for C. glabrata to all echinocandins were around 1%, which is lower than those reported for other sets of North American isolates (8, 20, 21). Echinocandin resistance in C. glabrata is more common in North America than in Europe and is quite rare in Asia and Latin America (8, 21, 31, 41). Conversely, azole resistance among C. parapsilosis (9%) and C. tropicalis (12%) isolates was higher here than that observed in other North American studies (8, 20). Higher levels of azole resistance among C. parapsilosis and C. tropicalis have been observed in Europe and Asia, respectively (31, 34, 35, 39, 41, 42). Differences between antifungal susceptibility rates in Ontario and in the other North American studies may be due to a large foreign-born population in Ontario (∼30%) (43), as well as frequent travel to and from a diverse collection of countries of origin compared to other populations studied. Resistance rates have remained stable in Ontario from 2014 to 2018.
In the data set of first isolates per patient per infection episode, the number of yeast isolates other than C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis was notable, with 11.6% identified as other Candida species and 3.1% as non-Candida yeasts (C. neoformans, S. cerevisiae, T. asahii, and R. mucilaginosa). Since many of these species exhibited elevated MICs for one or more azole or echinocandin drugs (9), antifungal susceptibility data for isolates of these rarely encountered species have been included in this study to contribute to the scarce literature. This can help inform treatment and can potentially be combined with similar data sets from other studies to provide a more robust description of MIC profiles (21). Among the non-Candida yeasts, the isolation of 5 C. auris isolates is noteworthy and unusual in the context of the global C. auris crisis (17) in that all 5 appeared to be susceptible to all 3 major classes of antifungal agents. All 5 isolates, though from individual patients over a span of several years, have been determined through WGS analysis to be members of the South American clade (IV) and also to be closely related to one another (26).
Within the data set, 24 pairs of isolates were identified where a patient’s first isolate was susceptible to an antifungal agent while a subsequent isolate within 6 months, defined here as representing a single infection episode, was nonsusceptible. Of these, 12 pairs were investigated by WGS to determine if the isolates represented the same strain and, if so, to attempt to elucidate the potential causal molecular mechanism(s) of acquired resistance. MLST and whole-genome SNP MP phylogenetic analysis demonstrated that for 11 of the 12 pairs (5 C. albicans, 4 C. glabrata, and 2 C. tropicalis), the first susceptible isolate and the subsequent resistant isolate were the same strain, thus confirming that resistance was acquired by the strain via within-host evolution rather than a strain replacement event. In one case (PA2419), a patient had a set of C. glabrata strains isolated from peritoneal fluid (SP2982; susceptible) and blood (SP3003; echinocandin nonsusceptible) 10 days apart; however, the two isolates had different sequence types, indicating that they did not represent within-host evolution of resistance, but instead, the patient harbored 2 different strains of C. glabrata.
In 6 of 7 cases of acquired echinocandin resistance, nonsynonymous mutations resulting in missense variants in a 1,3-beta-glucan synthase protein were noted between the initial susceptible and subsequent resistant isolates. This mechanism of echinocandin resistance is well characterized, and the GSC1 S645P and FKS2 S663P mutations are frequently observed in echinocandin-resistant clinical isolates of C. albicans and C. glabrata, respectively (18, 19, 45, 46). One echinocandin-resistant isolate of C. glabrata (SP3003) had an F625I mutation in FKS1 compared to the C. glabrata reference strain, CBS138. Although in this case (PA2419) we lacked a prior paired susceptible strain (see above) (Table 4), position 625 of FKS1 is within hot spot 1 (19, 45), suggesting that it is the cause of echinocandin resistance in the isolate. For one susceptible-to-resistant C. glabrata isolate pair (SP1533/SP1643), no mutations were detected in the 1,3-beta-glucan synthase gene FKS1, FKS2, or FKS3. This has been noted in other studies, particularly with low-level resistance, as in this case (caspofungin MIC, 0.5 μg/ml), and supports the suggestion of alternate mechanisms of echinocandin resistance yet to be described (47–50). Comparison of the initial susceptible and subsequent echinocandin-resistant isolates of C. tropicalis (SP4433/SP4501) revealed an S30P mutation in CTRG_04661, which aligns with known hot spot 1 position 663 in its ortholog, GSC1, in C. albicans and has previously been reported as a mechanism of echinocandin resistance in this organism (51, 52).
The cause of azole resistance is more difficult to elucidate, since it can be mediated by multiple mechanisms, including activation of a variety of efflux pumps due to mutations in regulatory elements or transcription factors and nonsynonymous mutations or upregulation of one of several genes involved in ergosterol synthesis (7). For 1 strain of C. albicans (SP0206/SP0369) that developed azole resistance, no nonsynonymous mutations were detected in candidate azole resistance genes despite extensive searching. Resistance in this strain is likely mediated by a mechanism not detectable by our analysis, i.e., gene upregulation. Although a variety of mutations were noted in the candidate azole resistance genes for the second pair of C. albicans susceptible-resistant isolates (SP2512/SP2683), the most significant was a homozygous A255T mutation in ERG3. Defective or missing ERG3 renders C. albicans azole resistant (53), and mutations in ERG3 have been noted in azole-resistant clinical isolates (45, 54), making this the likely cause of azole resistance acquired by C. albicans in this case. For C. tropicalis isolate pair SP4694/SP4785, which was investigated for the development of azole resistance, the most significant alteration detected and the likely cause of azole resistance acquisition was the increase in copy number variation of supercontig 3.8. While the exact chromosomal configuration was not determined, YMAP analysis suggested that the entire supercontig 3.8 was duplicated, progressing from diploid to a ploidy of ∼4, with a partial section located on the left side progressing to a ploidy of ∼6, possibly through formation of an isochromosome (55). The ergosterol synthesis gene ERG11 and TAC1, the transcriptional activator of CDR1 and CDR2, are located on the left side of supercontig 3.8, suggesting that aneuploidy in the strain mediated azole resistance by ERG11 and TAC1 gene amplification. Although this is a previously undescribed resistance mechanism in C. tropicalis, it is a known mechanism of azole resistance in C. albicans (55), and point mutations and increased expression of ERG11 have been previously described as mechanisms of azole resistance in C. tropicalis (56, 57).
Although this study examined a fairly large collection of clinical yeast isolates, we acknowledge several limitations of the study. The first limitation is our lack of clinical information on the patients from whom the yeasts were isolated. We do not know the patient setting (e.g., inpatient or outpatient) or treatment history. Knowledge of what antifungal agents the patients received would allow more meaningful analysis of our results but was unavailable to us. A second limitation is the fact that, although we performed much of our analysis using the first isolate submitted to our laboratory, this may not truly represent the patient’s first isolate, as that may not have been forwarded to us for AFST. Similarly, this study does not capture yeast isolates for which no susceptibility testing was requested and the patients were managed empirically. Although we perform the majority of AFST for yeast in the province, some local laboratories do not forward their isolates to us and perform their own testing in house. Finally, no comparison was made between the CLSI broth microdilution and the Sensititre YeastOne methods, although previous studies have demonstrated good agreement between the methods (58–60), and CLSI breakpoints have previously been applied to Sensititre YeastOne MICs (61). Also, ECVs developed for Candida spp. with Sensititre YeastOne are typically within one 2-fold dilution of those determined by the CLSI reference method (24, 25), demonstrating close agreement. Further research is required to establish clinical breakpoints for use with the Sensititre YeastOne panels.
In conclusion, this study describes the species distribution, antifungal susceptibility patterns, and molecular mechanisms of resistance of clinical yeast isolates in Ontario, Canada’s most populous province. Our data suggest that both the species distribution and the rates of resistance remained constant from 2014 to 2018, with the exception of a small but significant increase in the proportion of C. tropicalis among yeast isolates. Rates of resistance to all three classes of antifungal drugs remained relatively low in our population. We also demonstrated the utility of whole-genome sequencing to confirm cases of acquired resistance and to identify the molecular mechanisms associated with resistance. Ongoing testing and surveillance of invasive and recalcitrant yeast infections, as well as public health vigilance, are recommended given the global threat of increasing antifungal resistance (10) and the confirmed presence of C. auris among Ontario patients.
MATERIALS AND METHODS
Organisms and susceptibility testing.
The Public Health Ontario Laboratory (PHOL) is the provincial reference microbiology laboratory for Canada’s most populous province, encompassing 39% of the country’s population (14.5 million of 37.6 million people in 2019) (22). PHOL performs the majority of AFST in Ontario for hospitals and community laboratories. ASFT is performed on request from the treating physician and is typically restricted to invasive isolates from normally sterile sites. Acceptance criteria are relaxed for immunocompromised individuals, patients in intensive care units (ICU), and those failing empirical therapy, in which cases isolates from nonsterile sites may also be tested. Antifungal susceptibility data were retrospectively collected for a total of 5,171 clinical yeast isolates, including Candida spp., C. neoformans, T. asahii, S. cerevisiae, and R. mucilaginosa, submitted to PHOL from 2014 to 2018 for AFST.
Yeast identification was performed by a combination of morphological; biochemical; matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS), using a Bruker MALDI Biotyper (Bruker Daltonics, Billerica, MA); and ITS2 sequence analyses (62–64).
In vitro AFST for fluconazole, voriconazole, posaconazole, itraconazole, amphotericin B, caspofungin, micafungin, and anidulafungin was performed using the BMD-based Sensititre YeastOne Y09 panels (ThermoFisher, Waltham, MA), with MIC results read at 24 h for Candida spp. and up to 72 h for other yeast species, when adequate growth in the positive-control well was observed (65, 66). CLSI breakpoints were applied according to the guidelines of CLSI M60 (23). C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were routinely included as quality control organisms (23), with results within acceptable ranges.
The rate of increase of yeast isolates submitted for AFST from 2014 to 2018 was estimated as the slope of the line of the natural logarithm of the incidence rate per 100,000 population from 2014 to 2018. The Cochran-Armitage test was used to determine the statistical significance (P < 0.05) of temporal trends of proportional changes in species distribution and percent susceptibility. Analyses were performed in R v3.6.2 (67), using the DescTools package (68).
The initial data set of 5,171 clinical yeast isolates was culled to remove duplicate isolates of the same species received from the same patient within a 6-month period, with the assumption that they represented multiple isolates from single infection episodes. Specimen source distribution, species distribution, MIC statistics, susceptible/nonsusceptible percentages, and wild-type/non-wild-type percentages were calculated from the culled data set containing 4,715 isolates using CLSI MIC breakpoints and published Sensititre YeastOne ECVs (23–25).
The initial data set was also examined for sets of sequential isolates from single infection episodes for single patients that demonstrated a susceptible-to-nonsusceptible shift in antimicrobial resistance. These isolates were selected for further analysis, including WGS.
Whole-genome sequencing analysis.
Whole-genome sequencing was performed on select sets of C. albicans, C. glabrata, and C. tropicalis isolates that represented pairs of sequential isolates obtained from the same patient less than 6 months apart, representing single infection episodes, where the AFST profile shifted from susceptible to resistant. Total genomic DNA prepared using a ZymoBiomics DNA Miniprep kit (Zymo Research, Irvine, CA, USA) was used as input for library preparation using a Nextera XT DNA Library Prep kit (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. Sequencing was performed on a MiSeq using a MiSeq reagent kit v3 (Illumina).
Genome assembly and SNP calling were performed according to the GATK Best Practices (79–81). Briefly, paired-end reads of each isolate were mapped against its respective reference genome (C. albicans SC5314 haplotype A version A22, C. glabrata CBS138, or C. tropicalis MYA-3404) downloaded from the Candida Genome Database (69) using the Burrows-Wheeler alignment tool (BWA) version 0.7.17 (70) with the BWA-MEM algorithm and Picard tools v 2.9.0 (http://broadinstitute.github.io/picard). Indel realignment and base recalibration were performed with GATK v 3.8 using known indel and polymorphisms files (71) for C. albicans or indels and polymorphisms obtained from an initial round of HaplotypeCaller (44) for C. glabrata and C. tropicalis. HaplotypeCaller was employed in GVCF mode, followed by joint genotyping of all isolates of each species. SNPs were hard filtered based on the following parameters: QD, <2.0; FS, >60.0; MQ, <40.0; SOR, >3.0; MQRankSum, less than −12.5; and ReadPosRankSum, less than −8.0. The hard-filtering parameters for indels were as follows: QD, <2.0; FS, >200.0; SOR, >10.0; ReadPosRankSum, less than −20.0. For each isolate, SNPs and indels with read depths (DP) of <10 were filtered.
MLST profiles were generated by uploading genomes created using the GATK FastaAlternateReferenceMaker tool (72–74; https://pubmlst.org). Maximum-parsimony phylogenetic analysis of whole-genome SNPs with 500 bootstrap replications was performed in Mega v 10.1.6 (75) following Clustal W (76) alignment. All positions containing gaps and missing data were eliminated (complete deletion option).
Finally, select genes known to be involved in azole or echinocandin resistance were examined to detect genotypic differences between isolate pairs from the same patient that demonstrated a shift from susceptible to resistant. The genes associated with azole resistance and their corresponding locus tags are listed in Table S3. The locus tags of the 1,3-beta-glucan synthase genes associated with echinochandin resistance were as follows: C. albicans GSC1 (CAALFM_C102420CA), GSL2 (CAALFM_CR00850CA), and GSL1 (CAALFM_C105600WA); C. glabrata FKS1 (CAGL0G01034g), FKS2 (CAGL0K04037g), and FKS3 (CAGL0M13827g); and C. tropicalis CTRG-04661, CTRG_04806, and CTRG_00996. SnpEff v 4.3t (77) was used to annotate the SNP differences to identify those that produced missense variants. Finally, genome maps of resistant strains were visualized using YMAP (78), where the corresponding susceptible strain was selected as the parental strain comparator in order to visualize genome-wide ploidy estimates, copy number variation, and loss of heterozygosity events.
This work was reviewed and approved by PHO’s Research Review Board, as well as Research Ethics. A privacy impact assessment was also completed.
Accession number(s).
Raw sequences of the isolates have been deposited in the NCBI BioProject database under accession number PRJNA610214.
Supplementary Material
ACKNOWLEDGMENTS
We thank the clinical mycology laboratory for excellence in performing all the fungal susceptibility testing as part of our routine clinical function. We also thank Michael Li for his assistance with the whole-genome sequencing analysis.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Lamoth F, Lockhart SR, Berkow EL, Calandra T. 2018. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother 73:i4–i13. doi: 10.1093/jac/dkx444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 3.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 4.Trick WE, Fridkin SK, Edwards JR, Hajjeh RA, Gaynes RP, National Nosocomial Infections Surveillance System Hospitals. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin Infect Dis 35:627–630. doi: 10.1086/342300. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, EPIC II Group of Investigators. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 6.Lin SY, Lu PL, Tan BH, Chakrabarti A, Wu UI, Yang JH, Patel AK, Li RY, Watcharananan SP, Liu Z, Chindamporn A, Tan AL, Sun PL, Hsu LY, Chen YC, Asia Fungal Working Group (AFWG). 2019. The epidemiology of non-Candida yeast isolated from blood: the Asia Surveillance Study. Mycoses 62:112–120. doi: 10.1111/myc.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goncalves SS, Souza ACR, Chowdhary A, Meis JF, Colombo AL. 2016. Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses 59:198–219. doi: 10.1111/myc.12469. [DOI] [PubMed] [Google Scholar]
- 8.Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. 2019. Twenty years of the SENTRY antifungal surveillance program: results for Candida species from 1997–2016. Open Forum Infect Dis 6:S79–S94. doi: 10.1093/ofid/ofy358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arendrup MC. 2014. Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect 20(Suppl 6):42–48. doi: 10.1111/1469-0691.12513. [DOI] [PubMed] [Google Scholar]
- 10.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. 2017. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis 17:e383–e392. doi: 10.1016/S1473-3099(17)30316-X. [DOI] [PubMed] [Google Scholar]
- 11.Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, Chiller T. 2012. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol 50:3435–3442. doi: 10.1128/JCM.01283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto E, Boyken L, Tendolkar S, McDanel J, Castanheira M, Pfaller M, Diekema D. 2014. Candidemia surveillance in Iowa: emergence of echinocandin resistance. Diagn Microbiol Infect Dis 79:205–208. doi: 10.1016/j.diagmicrobio.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Hii IM, Chang HL, Lin LC, Lee YL, Liu YM, Liu CE, Chen CH, Cheng YR, Chang CY. 2015. Changing epidemiology of candidemia in a medical center in middle Taiwan. J Microbiol Immunol Infect 48:306–315. doi: 10.1016/j.jmii.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Tan TY, Hsu MM, Alejandria R, Chaiwarith T, Chinniah M, Chayakulkeeree S, Choudhury YH, Chen JH, Shin P, Kiratisin M, Mendoza K, Prabhu K, Supparatpinyo AL, Tan XT, Phan TT, Tran GB, Nguyen MP, Doan VA, Huynh SM, Nguyen TB, Tran H. 2016. Antifungal susceptibility of invasive Candida bloodstream isolates from the Asia-Pacific region. Med Mycol 54:471–477. doi: 10.1093/mmy/myv114. [DOI] [PubMed] [Google Scholar]
- 15.Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. 2012. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol 50:1199–1203. doi: 10.1128/JCM.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spivak ES, Hanson KE. 2018. Candida auris: an emerging fungal pathogen. J Clin Microbiol 56:e01588-17. doi: 10.1128/JCM.01588-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niimi K, Monk BC, Hirai A, Hatakenaka K, Umeyama T, Lamping E, Maki K, Tanabe K, Kamimura T, Ikeda F, Uehara Y, Kano R, Hasegawa A, Cannon RD, Niimi M. 2010. Clinically significant micafungin resistance in Candida albicans involves modification of a glucan synthase catalytic subunit GSC1 (FKS1) allele followed by loss of heterozygosity. J Antimicrob Chemother 65:842–852. doi: 10.1093/jac/dkq073. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-D-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother 53:3690–3699. doi: 10.1128/AAC.00443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller J, Dingle TC, Bull A, Shokoples S, Laverdière M, Baxter MR, Adam HJ, Karlowsky JA, Zhanel GG, Hoban DJ, Adam HJ, Baxter MR, Nichol KA, Lagacé-Wiens PRS, Walkty A, Blondeau J, Slinger R, Davidson R, Delport J, Ellis C, Loo V, Poutanen S, Fuller J, Roscoe D, Desjardins M, Matukas L, Goyette M, Lee C, Carignan A, Bergevin M, Pelletier R, Canadian Antimicrobial Resistance Alliance (CARA), CANWARD. 2019. Species distribution and antifungal susceptibility of invasive Candida isolates from Canadian hospitals: results of the CANWARD 2011–16 study. J Antimicrob Chemother 74:iv48–iv54. doi: 10.1093/jac/dkz287. [DOI] [PubMed] [Google Scholar]
- 21.Castanheira M, Deshpande LM, Davis AP, Rhomberg PR, Pfaller MA. 2017. Monitoring antifungal resistance in a global collection of invasive yeasts and molds: application of CLSI epidemiological cutoff values and whole-genome sequencing analysis for detection of azole resistance in Candida albicans. Antimicrob Agents Chemother 61:e00906-17. doi: 10.1128/AAC.00906-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Statistics Canada. 2019. Table 17-10-0005–01. Population estimates on July 1st, by age and sex. doi: 10.25318/1710000501-eng. [DOI]
- 23.CLSI. 2017. Performance standards for antifungal susceptibility testing of yeasts, 1st ed CLSI supplement M60 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.Espinel-Ingroff A, Alvarez-Fernandez M, Cantón E, Carver PL, Chen SC-A, Eschenauer G, Getsinger DL, Gonzalez GM, Govender NP, Grancini A, Hanson KE, Kidd SE, Klinker K, Kubin CJ, Kus JV, Lockhart SR, Meletiadis J, Morris AJ, Pelaez T, Quindós G, Rodriguez-Iglesias M, Sánchez-Reus F, Shoham S, Wengenack NL, Borrell Solé N, Echeverria J, Esperalba J, Gómez de la Pedrosa E, García I, Linares MJ, Marco F, Merino P, Pemán J, Pérez Del Molino L, Roselló Mayans E, Rubio Calvo C, Ruiz Pérez de Pipaon M, Yagüe G, Garcia-Effron G, Guinea J, Perlin DS, Sanguinetti M, Shields R, Turnidge J. 2015. Multicenter study of epidemiological cutoff values and detection of resistance in Candida spp. to anidulafungin, caspofungin, and micafungin using the Sensititre YeastOne colorimetric method. Antimicrob Agents Chemother 59:6725–6732. doi: 10.1128/AAC.01250-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espinel-Ingroff A, Turnidge J, Alastruey-Izquierdo A, Botterel F, Canton E, Castro C, Chen YC, Chen Y, Chryssanthou E, Dannaoui E, Garcia-Effron G, Gonzalez GM, Govender NP, Guinea J, Kidd S, Lackner M, Lass-Florl C, Linares-Sicilia MJ, Lopez-Soria L, Magobo R, Pelaez T, Quindos G, Rodriguez-Iglesia MA, Ruiz MA, Sanchez-Reus F, Sanguinetti M, Shields R, Szweda P, Tortorano A, Wengenack NL, Bramati S, Cavanna C, DeLuca C, Gelmi M, Grancini A, Lombardi G, Meletiadis J, Negri CE, Passera M, Peman J, Prigitano A, Sala E, Tejada M. 2018. Method-dependent epidemiological cutoff values for detection of triazole resistance in Candida and Aspergillus species for the Sensititre YeastOne colorimetric broth and Etest agar diffusion methods. Antimicrob Agents Chemother 63:e01651-18. doi: 10.1128/AAC.01651-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bharat A, Alexander DC, Dingle TC, Dufresne PJ, Hoang LM, Kus JV, Gade L, Litvintseva AP, McGeer A, Mulvey MR. 2019. Candida auris cases in Canada, 2012–2018. J Assoc Med Microbiol Infect Dis Canada 4(Suppl 1):P81. [Google Scholar]
- 27.Munoz JF, Gade L, Chow NA, Loparev VN, Juieng P, Berkow EL, Farrer RA, Litvintseva AP, Cuomo CA. 2018. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun 9:5346. doi: 10.1038/s41467-018-07779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomaz DY, de Almeida JN Jr, Lima GME, Nunes MO, Camargo CH, Grenfell RC, Benard G, Del Negro GMB. 2018. An azole-resistant Candida parapsilosis outbreak: clonal persistence in the intensive care unit of a Brazilian teaching hospital. Front Microbiol 9:2997. doi: 10.3389/fmicb.2018.02997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinhati HM, Casulari LA, Souza AC, Siqueira RA, Damasceno CM, Colombo AL. 2016. Outbreak of candidemia caused by fluconazole resistant Candida parapsilosis strains in an intensive care unit. BMC Infect Dis 16:433. doi: 10.1186/s12879-016-1767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhodes J, Fisher MC. 2019. Global epidemiology of emerging Candida auris. Curr Opin Microbiol 52:84–89. doi: 10.1016/j.mib.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Chapman B, Slavin M, Marriott D, Halliday C, Kidd S, Arthur I, Bak N, Heath CH, Kennedy K, Morrissey CO, Sorrell TC, van Hal S, Keighley C, Goeman E, Underwood N, Hajkowicz K, Hofmeyr A, Leung M, Macesic N, Botes J, Blyth C, Cooley L, George CR, Kalukottege P, Kesson A, McMullan B, Baird R, Robson J, Korman TM, Pendle S, Weeks K, Liu E, Cheong E, Chen S, Australian and New Zealand Mycoses Interest Group. 2017. Changing epidemiology of candidaemia in Australia. J Antimicrob Chemother 72:1103–1108. doi: 10.1093/jac/dkw422. [DOI] [PubMed] [Google Scholar]
- 32.Cleveland AA, Harrison LH, Farley MM, Hollick R, Stein B, Chiller TM, Lockhart SR, Park BJ. 2015. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008–2013: results from population-based surveillance. PLoS One 10:e0120452. doi: 10.1371/journal.pone.0120452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hesstvedt L, Gaustad P, Andersen CT, Haarr E, Hannula R, Haukland HH, Hermansen NO, Larssen KW, Mylvaganam H, Ranheim TE, Sandven P, Nordoy I, Kanestrom A, Grub C, Onken A, Thielsen C, Skaare D, Tofteland S, Sonsteby LJ, Hjetland R, Hide R, Vik E, Kummel A, Asheim S, Norwegian Yeast Study Group. 2015. Twenty-two years of candidaemia surveillance: results from a Norwegian national study. Clin Microbiol Infect 21:938–945. doi: 10.1016/j.cmi.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Beyer R, Spettel K, Zeller I, Lass-Flörl C, Achleitner D, Krause R, Apfalter P, Buzina W, Strauss J, Gregori C, Schüller C, Willinger B. 2019. Antifungal susceptibility of yeast bloodstream isolates collected during a 10-year period in Austria. Mycoses 62:357–367. doi: 10.1111/myc.12892. [DOI] [PubMed] [Google Scholar]
- 35.Bailly S, Maubon D, Fournier P, Pelloux H, Schwebel C, Chapuis C, Foroni L, Cornet M, Timsit JF. 2016. Impact of antifungal prescription on relative distribution and susceptibility of Candida spp.: trends over 10 years. J Infect 72:103–111. doi: 10.1016/j.jinf.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 36.da Matta DA, Souza ACR, Colombo AL. 2017. Revisiting species distribution and antifungal susceptibility of Candida bloodstream isolates from Latin American medical centers. J Fungi 3:24. doi: 10.3390/jof3020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Schalkwyk E, Mpembe RS, Thomas J, Shuping L, Ismail H, Lowman W, Karstaedt AS, Chibabhai V, Wadula J, Avenant T, Messina A, Govind CN, Moodley K, Dawood H, Ramjathan P, Govender NP, GERMS-SA. 2019. Epidemiologic shift in candidemia driven by Candida auris, South Africa, 2016–2017. Emerg Infect Dis 25:1698–1707. doi: 10.3201/eid2509.190040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan BH, Chakrabarti A, Li RY, Patel AK, Watcharananan SP, Liu Z, Chindamporn A, Tan AL, Sun PL, Wu UI, Chen YC, Asia Fungal Working Group. 2015. Incidence and species distribution of candidaemia in Asia: a laboratory-based surveillance study. Clin Microbiol Infect 21:946–953. doi: 10.1016/j.cmi.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Xiao M, Sun ZY, Kang M, Guo DW, Liao K, Chen SC, Kong F, Fan X, Cheng JW, Hou X, Zhou ML, Li Y, Yu SY, Huang JJ, Wang H, Xu YC, China Hospital Invasive Fungal Surveillance Net (CHIF-NET) Study Group. 2018. Five-year national surveillance of invasive candidiasis: species distribution and azole susceptibility from the China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study. J Clin Microbiol 56:e00577-18. doi: 10.1128/JCM.00577-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghazi S, Rafei R, Osman M, Safadi DE, Mallat H, Papon N, Dabboussi F, Bouchara JP, Hamze M. 2019. The epidemiology of Candida species in the Middle East and North Africa. J Mycol Med 29:245–252. doi: 10.1016/j.mycmed.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Xiao M, Fan X, Chen SC, Wang H, Sun ZY, Liao K, Chen SL, Yan Y, Kang M, Hu ZD, Chu YZ, Hu TS, Ni YX, Zou GL, Kong F, Xu YC. 2015. Antifungal susceptibilities of Candida glabrata species complex, Candida krusei, Candida parapsilosis species complex and Candida tropicalis causing invasive candidiasis in China: 3 year national surveillance. J Antimicrob Chemother 70:802–810. doi: 10.1093/jac/dku460. [DOI] [PubMed] [Google Scholar]
- 42.Astvad KMT, Johansen HK, Roder BL, Rosenvinge FS, Knudsen JD, Lemming L, Schonheyder HC, Hare RK, Kristensen L, Nielsen L, Gertsen JB, Dzajic E, Pedersen M, Ostergard C, Olesen B, Sondergaard TS, Arendrup MC. 2018. Update from a 12-year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. J Clin Microbiol 56:e01564-17. doi: 10.1128/JCM.01564-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Statistics Canada. 2017. Ontario [province] and Canada [country] (table). Census profile. 2016 census. Statistics Canada catalogue no. 98–316-X2016001. Ottawa. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E.
- 44.Poplin R, Ruano-Rubio V, DePristo MA, Fennell TJ, Carneiro MO, Van der Auwera GA, Kling DE, Gauthier LD, Levy-Moonshine A, Roazen D, Shakir K, Thibault J, Chandran S, Whelan C, Lek M, Gabriel S, Daly MJ, Neale B, MacArthur DG, Banks E. 2017. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv. doi: 10.1101/201178. [DOI]
- 45.Spettel K, Barousch W, Makristathis A, Zeller I, Nehr M, Selitsch B, Lackner M, Rath PM, Steinmann J, Willinger B. 2019. Analysis of antifungal resistance genes in Candida albicans and Candida glabrata using next generation sequencing. PLoS One 14:e0210397. doi: 10.1371/journal.pone.0210397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivero-Menendez O, Navarro-Rodriguez P, Bernal-Martinez L, Martin-Cano G, Lopez-Perez L, Sanchez-Romero I, Perez-Ayala A, Capilla J, Zaragoza O, Alastruey-Izquierdo A. 2019. Clinical and laboratory development of echinocandin resistance in Candida glabrata: molecular characterization. Front Microbiol 10:1585. doi: 10.3389/fmicb.2019.01585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vallabhaneni S, Cleveland AA, Farley MM, Harrison LH, Schaffner W, Beldavs ZG, Derado G, Pham CD, Lockhart SR, Smith RM. 2015. Epidemiology and risk factors for echinocandin nonsusceptible Candida glabrata bloodstream infections: data from a large multisite population-based candidemia surveillance program, 2008–2014. Open Forum Infect Dis 2:ofv163. doi: 10.1093/ofid/ofv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biswas C, Marcelino VR, Van Hal S, Halliday C, Martinez E, Wang Q, Kidd S, Kennedy K, Marriott D, Morrissey CO, Arthur I, Weeks K, Slavin MA, Sorrell TC, Sintchenko V, Meyer W, Chen SC. 2018. Whole genome sequencing of Australian Candida glabrata isolates reveals genetic diversity and novel sequence types. Front Microbiol 9:2946. doi: 10.3389/fmicb.2018.02946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fekkar A, Dannaoui E, Meyer I, Imbert S, Brossas JY, Uzunov M, Mellon G, Nguyen S, Guiller E, Caumes E, Leblond V, Mazier D, Fievet MH, Datry A. 2014. Emergence of echinocandin-resistant Candida spp. in a hospital setting: a consequence of 10 years of increasing use of antifungal therapy? Eur J Clin Microbiol Infect Dis 33:1489–1496. doi: 10.1007/s10096-014-2096-9. [DOI] [PubMed] [Google Scholar]
- 50.Barber AE, Weber M, Kaerger K, Linde J, Golz H, Duerschmied D, Markert A, Guthke R, Walther G, Kurzai O. 2019. Comparative genomics of serial Candida glabrata isolates and the rapid acquisition of echinocandin resistance during therapy. Antimicrob Agents Chemother 63:e01628-18. doi: 10.1128/AAC.01628-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Effron G, Chua DJ, Tomada JR, DiPersio J, Perlin DS, Ghannoum M, Bonilla H. 2010. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrob Agents Chemother 54:2225–2227. doi: 10.1128/AAC.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chew KL, Octavia S, Lin RTP, Yan GZ, Teo JWP. 2019. Delay in effective therapy in anidulafungin-resistant Candida tropicalis fungaemia: potential for rapid prediction of antifungal resistance with whole-genome-sequencing. J Glob Antimicrob Resist 16:105–107. doi: 10.1016/j.jgar.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Sanglard D, Ischer F, Parkinson T, Falconer D, Bille J. 2003. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob Agents Chemother 47:2404–2412. doi: 10.1128/aac.47.8.2404-2412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y, Li C, Wang Z, Gao J, Tang Z, Chen H, Ying C. 2018. Clonal spread and azole-resistant mechanisms of non-susceptible Candida albicans isolates from vulvovaginal candidiasis patients in three Shanghai maternity hospitals. Med Mycol 56:687–694. doi: 10.1093/mmy/myx099. [DOI] [PubMed] [Google Scholar]
- 55.Selmecki A, Gerami-Nejad M, Paulson C, Forche A, Berman J. 2008. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol 68:624–641. doi: 10.1111/j.1365-2958.2008.06176.x. [DOI] [PubMed] [Google Scholar]
- 56.Fan X, Xiao M, Zhang D, Huang JJ, Wang H, Hou X, Zhang L, Kong F, Chen SC, Tong ZH, Xu YC. 2019. Molecular mechanisms of azole resistance in Candida tropicalis isolates causing invasive candidiasis in China. Clin Microbiol Infect 25:885–891. doi: 10.1016/j.cmi.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Tan J, Zhang J, Chen W, Sun Y, Wan Z, Li R, Liu W. 2015. The A395T mutation in ERG11 gene confers fluconazole resistance in Candida tropicalis causing candidemia. Mycopathologia 179:213–218. doi: 10.1007/s11046-014-9831-8. [DOI] [PubMed] [Google Scholar]
- 58.Cuenca-Estrella M, Gomez-Lopez A, Alastruey-Izquierdo A, Bernal-Martinez L, Cuesta I, Buitrago MJ, Rodriguez-Tudela JL. 2010. Comparison of the Vitek 2 antifungal susceptibility system with the clinical and laboratory standards institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) broth microdilution reference methods and with the Sensititre YeastOne and Etest techniques for in vitro detection of antifungal resistance in yeast isolates. J Clin Microbiol 48:1782–1786. doi: 10.1128/JCM.02316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfaller MA, Chaturvedi V, Diekema DJ, Ghannoum MA, Holliday NM, Killian SB, Knapp CC, Messer SA, Miskou A, Ramani R. 2012. Comparison of the Sensititre YeastOne colorimetric antifungal panel with CLSI microdilution for antifungal susceptibility testing of the echinocandins against Candida spp., using new clinical breakpoints and epidemiological cutoff values. Diagn Microbiol Infect Dis 73:365–368. doi: 10.1016/j.diagmicrobio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 60.Aigner M, Erbeznik T, Gschwentner M, Lass-Florl C. 2017. Etest and Sensititre YeastOne susceptibility testing of echinocandins against Candida species from a single center in Austria. Antimicrob Agents Chemother 61:e00512-17. doi: 10.1128/AAC.00512-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Posteraro B, Spanu T, Fiori B, De Maio F, De Carolis E, Giaquinto A, Prete V, De Angelis G, Torelli R, D'Inzeo T, Vella A, De Luca A, Tumbarello M, Ricciardi W, Sanguinetti M. 2015. Antifungal susceptibility profiles of bloodstream yeast isolates by Sensititre YeastOne over nine years at a large Italian teaching hospital. Antimicrob Agents Chemother 59:3944–3955. doi: 10.1128/AAC.00285-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.CLSI. 2008. Abbreviated identification of bacteria and yeast. Approved guideline, 2nd ed. CLSI document M35-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 63.CLSI. 2017. Methods for the identification of cultured microorganisms using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, 1st ed CLSI guideline M58 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 64.CLSI. 2018. Interpretive criteria for identification of bacteria and fungi by targeted DNA sequencing, 2nd ed CLSI guideline MM18 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 65.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3rd ed CLSI document M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 66.CLSI. 2017. Reference method for broth dilution antifungal susceptibility testing of yeasts, 4th ed CLSI standard M27 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 67.R Core Team. 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- 68.Signorell A. 2019. DescTools: tools for descriptive statistics. R package version 0.99.31. https://cran.r-project.org/package=DescTools.
- 69.Skrzypek MS, Binkley J, Binkley G, Miyasato SR, Simison M, Sherlock G. 2017. The Candida Genome Database (CGD): incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data. Nucleic Acids Res 45:D592–D596. doi: 10.1093/nar/gkw924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones T, Federspiel NA, Chibana H, Dungan J, Kalman S, Magee BB, Newport G, Thorstenson YR, Agabian N, Magee PT, Davis RW, Scherer S. 2004. The diploid genome sequence of Candida albicans. Proc Natl Acad Sci U S A 101:7329–7334. doi: 10.1073/pnas.0401648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bougnoux M-E, Tavanti A, Bouchier C, Gow NAR, Magnier A, Davidson AD, Maiden MCJ, D'Enfert C, Odds FC. 2003. Collaborative consensus for optimized multilocus sequence typing of Candida albicans. J Clin Microbiol 41:5265–5266. doi: 10.1128/jcm.41.11.5265-5266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dodgson AR, Pujol C, Denning DW, Soll DR, Fox AJ. 2003. Multilocus sequence typing of Candida glabrata reveals geographically enriched clades. J Clin Microbiol 41:5709–5717. doi: 10.1128/jcm.41.12.5709-5717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jolley KA, Maiden MC. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 77.Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2;iso-3. Fly 6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abbey DA, Funt J, Lurie-Weinberger MN, Thompson DA, Regev A, Myers CL, Berman J. 2014. YMAP: a pipeline for visualization of copy number variation and loss of heterozygosity in eukaryotic pathogens. Genome Med 6:100. doi: 10.1186/s13073-014-0100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. 2010. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DePristo M, Banks E, Poplin R, Garimella K, Maguire J, Hartl C, Philippakis A, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell T, Kernytsky A, Sivachenko A, Cibulskis K, Gabriel S, Altshuler D, Daly M. 2011. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van der Auwera GA, Carneiro M, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J, Banks E, Garimella K, Altshuler D, Gabriel S, DePristo M. 2013. From FastQ data to high-confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.