The altered immune states of aging and HIV infection may affect intracellular metabolism of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC); increased cellular senescence decreases FTC-triphosphate (FTCtp) concentrations. The effects of age and inflammation on the ratio of intracellular metabolites (IMs; tenofovir diphosphate [TFVdp] and FTCtp) to their endogenous nucleotides (ENs; dATP and dCTP), a potential treatment efficacy marker, were assessed among participants of the Women’s Interagency HIV Study (WIHS), who ranged from 25 to 75 years.
KEYWORDS: aging, emtricitabine, human immunodeficiency virus, intracellular drug concentration, nucleoside reverse transcriptase inhibitor, tenofovir
ABSTRACT
The altered immune states of aging and HIV infection may affect intracellular metabolism of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC); increased cellular senescence decreases FTC-triphosphate (FTCtp) concentrations. The effects of age and inflammation on the ratio of intracellular metabolites (IMs; tenofovir diphosphate [TFVdp] and FTCtp) to their endogenous nucleotides (ENs; dATP and dCTP), a potential treatment efficacy marker, were assessed among participants of the Women’s Interagency HIV Study (WIHS), who ranged from 25 to 75 years. Samples from women receiving TDF-FTC with viral loads of <200 copies/ml were dichotomized by age at collection into two groups (≤45 years and ≥60 years). IM/EN concentrations were measured in peripheral blood mononuclear cell (PBMC) pellets; interleukin-6 (IL-6) and sCD163 were measured in plasma; senescent CD8+ T cells were measured in viable PBMCs. The TFVdp:dATP and FTCtp:dCTP ratios had statistically significantly different distributions in older and younger women (log-rank test, P = 0.0023 and P = 0.032, respectively); in general, IM and EN concentrations were higher in the older women. After adjusting for potential confounders, these findings were not significant. In women aged ≤45 years, TFVdp was negatively associated with IL-6 and sCD163, while FTCtp was positively associated with sCD163 and IL-6 in women aged ≥60 years. Body mass index (BMI) was positively associated with IL-6 in both age groups and negatively associated with TFVdp in women aged ≤45 years. After adjustment, age remained significant for sCD163, while black race, BMI, and renal function remained significant for several IMs and ENs, suggesting that factors associated with aging, but not age itself, govern intracellular TDF-FTC pharmacology.
TEXT
Aging causes physiologic changes that may affect drug distribution and clearance, including decreases in total body water; increased adipose tissue; decreased plasma protein concentrations; and reduced gonadal, renal, and liver function (1, 2). In addition, the aging immune system experiences a host of changes, such as telomere shortening, accumulation of terminally differentiated T-cell populations, systemic inflammation, and cellular senescence that contribute to the overall decline in health associated with advancing age (3). Multiple investigators have demonstrated an association between systemic inflammation (as manifested by elevated interleukin-6 [IL-6] concentrations) in persons living with HIV (PLWH) and increased mortality risk (4–6). For example, in a recent investigation in the Multicenter AIDS Cohort Study (MACS), the mortality risk hazard ratio for men in the highest quartile of IL-6 concentrations was 3.54 (95% confidence interval, 2.06 to 6.10) compared with the lower three quartiles, despite viral suppression (7). Similarly, soluble CD163 (sCD163) has been linked to mortality risk (8, 9) and cardiovascular risk (10). Inflammation is also known to affect several enzyme and transporter systems that govern the pharmacokinetics of antiretrovirals (ARVs) (11, 12).
The role of age and its associated inflammatory state on the complex intracellular metabolism of the HIV nucleoside reverse transcriptase inhibitors (NRTIs) (13) has not been specifically explored, although they comprise the backbone of first-line combination ARV therapy (ART) (14–16). The phosphorylated intracellular metabolites (IMs) of these drugs compete with endogenous nucleotides (ENs) for incorporation into and termination of viral reverse transcription; the balance between IMs and ENs in the cell influences efficacy and mitochondrial toxicity (17–19). In clinical studies of combination ARVs in the early 2000s using NRTIs with a high risk of mitochondrial toxicity, older age was identified as a predisposing factor for NRTI toxicity (20, 21). From an inflammation standpoint, increased concentrations of zidovudine triphosphate (IM of zidovudine) have been observed in PLWH upon initiation of ART, when viral loads and inflammation were high, as well as in PLWH with advanced disease, e.g., CD4 T cell count of <100 cells/mm3 (reviewed in reference 13). With ART, which decreases overall inflammation, phosphorylation of zidovudine decreased, suggesting a role of inflammation in the upregulation of enzymes responsible for NRTI metabolism (22). Selvaraj and colleagues found the pool of ENs in PLWH receiving ART was lower than that in healthy volunteers and lower still in PLWH experiencing classic NRTI-mediated adverse effects (23). Taken together, these findings suggest that the IM/EN ratio could be higher with inflammation and with age. However, little is known about the effects of age and inflammation on less toxic, modern NRTIs, such as tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) or their corresponding ENs (dATP and dCTP, respectively). Tenofovir (TFV), the circulating form of TDF, and FTC are both renally eliminated, and as PLWH age and renal function declines, exposure increases (24). We hypothesize that aging may alter TFV and FTC pharmacology beyond renal dysfunction. We have shown that expression of p16INK4a, a tumor suppressor gene associated with cellular senescence (25), is associated with increased tenofovir diphosphate (TFVdp) and emtricitabine triphosphate (FTCtp) intracellular clearance, i.e., lower intracellular concentrations, among HIV-positive men and women ranging in age from 20 to 73 years (26, 27). We did not, however, find associations between p16INK4a expression and a panel of proinflammatory cytokines in our virally suppressed population (28).
In this study of women living with HIV (WLWH) receiving TDF-FTC-based ART, we measured IMs and ENs in peripheral blood mononuclear cells (PBMCs), IL-6 and sCD163 in matching plasma, and the percentage of senescent CD8+ T cells to compare chronologic age, inflammation, cellular senescence, and IM:EN ratios in two age groups. The two age groups were selected to have a clear age gap between them to minimize potentially ambiguous results that would be expected if a specific age (e.g., 50 years) was chosen to categorize WLWH as young versus old. We hypothesized that compared with younger women (defined as ≤45 years), older women (≥60 years) would have a higher IM:EN ratio, with increased concentrations of systemic IL-6 and sCD163; we also hypothesized that these effects might be mitigated by the presence of cellular senescence, as our preliminary data suggested that p16INK4a expression was associated with lower IM and EN exposures.
RESULTS
Demographic and clinical variables used in the data analyses are presented, stratified by age group, in Table 1. Samples from 84 women in the younger group (≤45 years) and 60 women in the older group (≥60 years) were used; 181 unique woman-visits were identified as meeting inclusion criteria, with 144 woman-visits having available PBMC pellets. PBMC concentration observations from 3 women were excluded from the TFVdp:dATP analysis because both TFVdp and dATP measurements were below the limit of quantification (BLQ; ratios with only 1 assay BLQ were retained and accounted for in the analysis; 10 additional samples fell into the latter category) (29). Two women did not have available body mass index (BMI) values at the selected visit or at the previous or subsequent visits and were excluded from the linear regression models. Seventeen IL-6 plasma measurements were BLQ. Qualitatively, more women in the younger group were black (83% versus 53%) and received integrase inhibitors (33% versus 18%), while nonnucleoside reverse transcriptase inhibitors were more common in the older group (48% versus 38%). For all women, the measured viral load was BLQ (ranging from 20 to 80 copies/ml), and 89.6% reported adherence of ≥95% in the preceding 6 months. Twelve women (4 younger and 8 older) reported adherence of 75% to 94%, and 2 younger women reported adherence of <75% in the 6 months prior. Self-reported adherence in the Women’s Interagency HIV Study (WIHS) correlates well with hair concentrations of ARVs and viral suppression (30–32).
TABLE 1.
Demographic characteristics of participants by age group
| Category | Data by age group |
|
|---|---|---|
| ≤45 yrs (n = 84) | ≥60 yrs (n = 60) | |
| Age (yrs) (median [IQRa]) | 35 (32, 37) | 62 (61, 64) |
| BMIb (kg/m2) (median [IQR]) | 34.2 (28.3, 42.2) | 25.1 (22.1, 31.5) |
| CD4+ T cell count/mm3 (median [IQR]) | 690 (533, 880) | 606 (430, 899) |
| CD4+:CD8+ T cell ratio (median [IQR]) | 1.1 (0.75, 1.6) | 0.75 (0.5, 1.2) |
| eGFRc (ml/min/1.73 m2) (median [IQR]) | 109 (96.6, 128.5) | 72.6 (59.5, 90.2) |
| APRId score (median [IQR]) | 0.2 (0.15, 0.25) | 0.27 (0.22, 0.41) |
| Self-reported menopause status (n [%]) | ||
| Not menopausal | 82 (98) | 0 (0) |
| Menopausal | 1 (1) | 46 (77) |
| Hysterectomy | 1 (1) | 14 (23) |
| Hypertension, yes (n [%]) | 24 (29) | 43 (72) |
| Diabetes, yes (n [%]) | 6 (7) | 19 (32) |
| Current smoker (n [%]) | 25 (30) | 22 (37) |
| Race (n [%]) | ||
| White | 6 (7) | 8 (13) |
| Black | 71 (85) | 33 (55) |
| Other | 7 (8) | 19 (32) |
| Background antiretroviral regimen (n [%]) | ||
| Nonnucleoside reverse transcriptase inhibitor | 32 (38) | 29 (48) |
| Atazanavir, lopinavir | 17 (20) | 4 (7) |
| Darunavir | 5 (6) | 9 (15) |
| Integrase inhibitor | 28 (33) | 11 (18) |
| Other | 2 (2) | 7 (12) |
IQR, interquartile range (25th and 75th percentile).
BMI, body mass index. Reported in 59 women in the older group and 83 in the younger group; 3/59 and 1/83 in the older and younger groups, respectively, used imputed values from the WIHS visit immediately before or after the visit of interest.
eGFR, estimated glomerular filtration rate. Calculated by the CKD-EPI equation.
APRI, AST-to-platelet ratio index.
The concentrations of IMs, ENs, IL-6, and sCD163; the percentage of senescent CD8+ T cells; and the IM:EN ratios are presented by age group in Table 2. The IM and EN concentrations are, on average, similar to previously reported values (19, 27). The IL-6 and sCD163 concentrations and percent senescent CD8+ T cells were similar in the age groups and were within the expected range of values (7, 33), given the difficultly of comparing immune biomarkers across laboratories and methods. The TFVdp:dATP and FTCtp:dCTP ratios had statistically significantly different distributions in older and younger women (log-rank test, P = 0.0023 and P = 0.032, respectively); in general, IM and EN concentrations were higher in the older women. The median TFVdp:dATP ratio in women aged ≤45 years was 0.31, compared with 0.54 in women aged ≥60 years. The median FTCtp:dCTP ratio in women aged ≤45 years was 13.4 compared with 17.7 in women aged ≥60 years.
TABLE 2.
Concentrations of endogenous nucleotides, intracellular metabolites, inflammatory markers, and metabolite:endogenous nucleotide ratios by age group
| Biological measurement | Concentrationa by age group |
|
|---|---|---|
| ≤45 yrs | ≥60 yrs | |
| dATP (fmol/106 cells) | 256 (123, 599) | 480 (322, 918) |
| dCTP (fmol/106 cells) | 602 (355, 836) | 566 (330, 809) |
| FTCtp (fmol/106 cells) | 8,002 (4,780, 11,710) | 10,041 (6,696, 14,107) |
| TFVdp (fmol/106 cells) | 89.0 (56.2, 159.0) | 241.0 (120.0, 355.0) |
| TFVdp:dATP ratiob | 0.37 (0.19, 0.60) | 0.54 (0.25, 0.83) |
| FTCtp:dCTP ratioc | 13.5 (8.6, 20.0) | 18.3 (11.0, 32.3) |
| Interleukin-6 (ng/ml) | 2.2 (1.3, 3.5) | 2.0 (0.6, 4.0) |
| Soluble CD163 (ng/ml) | 931 (603, 1,184) | 872 (624, 1,311) |
| % senescent CD8+ T cells (CD28−/CD57+/CD95+/CD8+) | 4.2 (2.3, 8.1) | 5.5 (2.6, 11.2) |
Data are presented as untransformed values, as the median and the interquartile range (IQR; 25th and 75th percentiles).
For comparison between age groups, P = 0.023.
For comparison between age groups, P = 0.032.
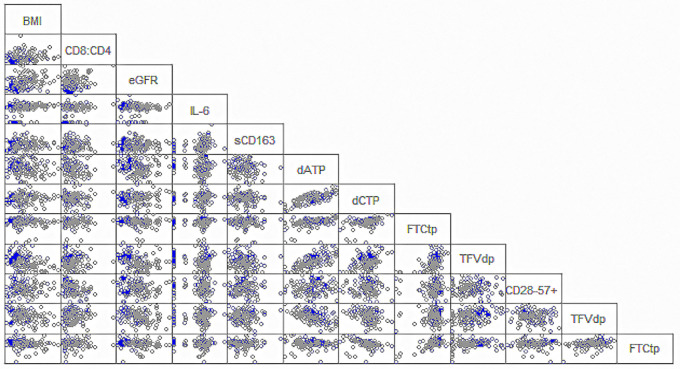
Age-stratified TFVdp concentrations were negatively associated with sCD163 and IL-6 concentrations (Spearman’s rank correlation coefficient [rs] [95% confidence interval] = −0.24 [−0.43, −0.02] and rs= −0.28 [−0.47, −0.007], respectively) in women aged ≤45 years. In women aged ≥60 years, FTCtp concentrations were also associated with sCD163 and IL-6 concentrations but had a positive relationship (rs = 0.29 [0.03, 0.50] and rs = 0.41 [0.17, 0.60], respectively). TFVdp concentrations were negatively associated with eGFR (rs = −0.38 [−0.58, −0.14]) in older women. BMI was positively associated with IL-6 concentrations in both age groups (rs = 0.26 [0.005, 0.48] in older women; rs = 0.42 [0.22,0.58] in younger women) and was negatively associated with TFVdp concentrations in women aged ≤45 years (rs = −0.37 [−0.54, −0.16]). dCTP concentrations were associated with race (P = 0.024; median, 684 women aged ≥60 years fmol/106 cells in black women, 337 fmol/106 cells in white, and 550 fmol/106 cells in other). Race and BMI were not associated in age-stratified analyses; however, in unstratified analysis, the distribution of BMI is not the same in the three race categories, with black women more likely to have higher BMIs. In both age groups, IM and EN concentrations, IL-6 and sCD163 concentrations, and CD4+:CD8+ T cell ratios and percent CD8+ T cells (CD28−CD57+CD95+CD8+) were associated with each other. The correlation matrix for these biologic measurements are presented in Fig. 1. This figure shows the expected close associations among the IMs and ENs, as well as among IL-6, sCD163, and the percent senescent cells by age group. The findings from this analysis are exploratory in nature, as no P value correction was made for the large number of comparisons performed.
FIG 1.
Correlation matrix for concentrations of intracellular metabolites, endogenous nucleotides, inflammation markers, body mass index (BMI), and the ratios of intracellular metabolites to endogenous nucleotides. Women in the younger group (≤45 years) are shown in gray circles; women in the older group (≥60 years) are shown in the blue circles.
Table 3 provides a summary of the results from the multivariable regression models focused on age group and BMI, while adjusting for clinical and demographic factors. Of note, age group was a statistically significant predictor of sCD163 concentrations (mean effect [95% confidence interval] of −0.13 [−0.24, −0.0016] in the young group compared with that of the old group). In the sCD163 model, other race compared with white race (0.16 ([.0097, 0.30]), receipt of darunavir compared with efavirenz (−0.13 [−0.26, −0.0020]), estimated glomerular filtration rate (eGFR) (0.42 [0.012, 0.83]), AST-to-platelet ratio index (APRI) (0.19 [0.063, 0.32]), and BMI (0.39 [0.075, 0.70]) were also significant (see Table S4 in the supplemental material). For the TFVdp:dATP and FTCtp:dCTP ratios, the mean difference estimates in the log10-transformed ratios of the two age groups were attenuated and no longer statistically significant. BMI remained significant in the models for the TFVdp:dATP ratio (−0.73 [−1.27, −0.18]), TFVdp concentrations (−0.80 [−1.39, −0.20]), sCD163, and IL-6 (Table 3) (BMI categorized for IL-6 and quantitative for all others). For the TFVdp:dATP ratio (−0.95 [−1.65, −0.24]) and TFVdp concentrations (−0.80 [−1.39, −0.20]), eGFR remained significant in addition to BMI, while for the FTCtp:dCTP ratio, black race (compared with white race) remained a significant predictor (−0.40 [−0.69, −0.11]) (see Table S2 and S3 in the supplemental material). APRI remained a significant covariate for concentrations of two nucleotides, namely, dCTP (−0.32 [−0.54, −0.098]) and FTCtp (−0.33 [−0.62, −0.033]) (see Table S1 and S2 in the supplemental material). For IL-6, receiving atazanavir or lopinavir compared with efavirenz (0.52 [0.042, 1.00]) was significant in addition to BMI. No significant predictors remained for the percent senescent cells after adjustment for covariates. Complete model results are presented in Table S1, S2, S3, S4, and S5 in the supplemental material.
TABLE 3.
Regression coefficients for age group and BMI from models, adjusting for clinical and demographic covariates
| Biological measurement (log10 scale) | Mean effect of age group with ≥60 yrs as the reference (95% confidence interval)a | P value | Mean effect of log10 (BMI) (95% confidence intervalb | P value |
|---|---|---|---|---|
| dATP (fmol/106 cells) | −0.21 (−0.48, 0.062) | 0.13 | −0.095 (−0.83, 0.65) | 0.80 |
| dCTP (fmol/106 cells) | −0.051 (−0.25, 0.14) | 0.61 | 0.016 (−0.52, 0.55) | 0.95 |
| Emtricitabine triphosphate (fmol/106 cells) | −0.067 (−0.33, 0.20) | 0.62 | −0.17 (−0.89, 0.55) | 0.65 |
| Tenofovir diphosphate (fmol/106 cells) | −0.17 (−0.38, 0.049) | 0.13 | −0.80 (−1.39, −0.20) | 0.0085c |
| sCD163 (ng/ml) | −0.13 (−0.24, −0.016) | 0.025c | 0.39 (0.075, 0.70) | 0.015c |
| % senescent CD8+ T cells (CD28−CD57+CD95+CD8+) | −0.10 (−0.28, 0.074) | 0.25 | 0.40 (−0.092, 0.89) | 0.11 |
| Ratio of tenofovir diphosphate to deoxyadenosine triphosphate | 0.037 (−0.16, 0.24) | 0.72 | −0.73 (−1.27, −0.18) | 0.0086c |
| Ratio of emtricitabine triphosphate to deoxycytidine triphosphate | −0.016 (−0.27, 0.24) | 0.90 | −0.18 (−0.89, 0.52) | 0.61 |
| Interleukin-6 (ng/ml) | 0.10 (−0.40, 0.60) | 0.69 | ||
| Interleukin-6 (ng/ml) with BMI categories | ||||
| <18.5 | −0.55 (−1.39, 0.30) | 0.21 | ||
| 18.5–24.9 | −1.02 (−1.49, −0.54) | <0.0001c | ||
| 25–29.9 | −0.48 (−0.94, −0.013) | 0.044c | ||
| 30–34.9 | −0.034 (−0.48, 0.42) | 0.88 | ||
| >35d |
Full model results are available in Supplemental Materials.
For IL-6, BMI was included in the model as a categorical, rather than continuous, variable to improve model fit (assessed by Akaike’s Information Criteria and residual plots).
Statistically significant P value after covariate adjustment.
Reference.
DISCUSSION
Here, we detail the analyses of IM:EN ratios and their component concentrations in women living with HIV receiving TDF-FTC in the WIHS. We observed statistically significant differences in TFVdp:dATP and FTCtp:dCTP ratios between women aged ≤45 years and those aged ≥60 years, with higher ratios in the older women, in unadjusted analyses. These differences were not seen in multivariable analysis after accounting for other potential confounders; the only outcome predicted by age after adjustment was sCD163 concentrations. Renal function and BMI were significant predictors of the TFVdp:dATP ratio, and black race (compared with white race) significantly predicted the FTCtp:dCTP ratio. Our results suggest that factors related to aging and potentially race, but not chronologic age itself, are the influencing factors on the intracellular pharmacology of TDF-FTC in WLWH.
We hypothesized that older women would have higher ratios, due to increased drug phosphorylation with increased inflammation and decreased endogenous nucleotide pools; based on our preliminary data, we thought that cellular senescence may play a role in mitigating these increases, as we had observed negative associations between p16INK4a expression and IM/EN exposure (26, 27). We observed increased ratios in older women for both TFVdp:dATP and FTCtp:dCTP, but the potential reasons for this finding may differ from our hypotheses. Among younger women, who did not have substantially lower cytokine concentrations or decreased cellular senescence, TFVdp was negatively associated with sCD163 and IL-6 concentrations, which was unexpected given that increased inflammation is thought to upregulate drug phosphorylation. Older women demonstrated positive associations of these cytokines with FTCtp concentrations, as hypothesized. Cellular activation state, which we did not measure here, contributes to the phosphorylation rate in vitro, with TDF undergoing metabolism equally well in active and resting cells and lamivudine undergoing metabolism preferentially in resting cells (34, 35). Given the structural similarity between lamivudine and FTC (36), this phenomenon is generally extrapolated to FTC; potential differences in cellular activation between young and old women may partially explain our observations.
We further explored the data set in a regression analysis, controlling for age and several potentially influential covariates, including components of the background ART regimen that may alter TFVdp concentrations (37, 38). Age remained a significant predictor for sCD163 concentrations only; renal function remained significant for TFVdp concentrations and TFVdp:dATP ratios. Of the covariates explored, BMI plays a significant role for both the inflammatory markers (39, 40) and some of the drug markers. Given that obesity is classified as an inflammatory condition, the relationship between obesity with IL-6 and sCD163 in these WLWHs was expected. Patients with high BMIs generally have larger volumes of drug distribution and thus lower drug concentrations, as has been shown for some ARVs (41), although this relationship is less well defined for the intracellular metabolites studied here; BMI remained a significant predictor of TFVdp concentrations and TFVdp:dATP ratios in multivariable analysis.
In the total study sample, race and BMI were related, but were not significantly different by age group, likely due to racial imbalances in the age groups. We noted several extreme BMI values in black women (e.g., 60 to 75 kg/m2, verified by comparing values to those from prior and subsequent visits) in the group ≤45 years; this group also had a higher percentage of black women than the older group (85% versus 55%). While we did observe lower TFVdp concentrations in black women when looking at the whole sample, as has been previously reported by others in concentration analyses of dried blood spots (42, 43), we did not see racial differences within each age group. In the TFVdp multivariable analysis, race and BMI were both included as potential confounders; only BMI remained a significant predictor after adjusting for race. In addition to the race/BMI relationship, several other covariates may be related to both age and drug disposition, as in the case of renal function, and may also be more prevalent in older PLWHs and thus also related to aging (diabetes, hypertension, and BMI). The covariate interrelationships are difficult to disentangle in complex WLWH cases and may have the effect of adjusting the models to the point of obscuring potential effects of aging.
Limitations to our analyses include a smaller sample size of older women than was planned for by statistical power analyses; however, given the increased number of available woman-visits in the younger group, our statistical power was largely unchanged. We did not measure plasma concentrations of parent tenofovir (TFV) and FTC, making it somewhat more difficult to definitively conclude that the BLQ PBMC concentrations of the metabolites resulted from a technical error in specimen processing or storage. However, the intracellular half-lives of the IMs are long, the vast majority of women reported high levels of adherence, and all women were virally suppressed. Thus, nonadherence is not a likely explanation for these BLQ observations.
Given the retrospective nature of this investigation, we were not able to measure p16INK4a expression as in our previous work with IM/EN ratios and cytokine concentrations, although the senescence markers we did measure correspond to differences in p16INK4a expression between young and old HIV-negative Belgian donors (44). More recent work in PLWHs suggests that virologically suppressed patients on ARVs may have p16INK4a expression that correlates with chronologic age in CD4+ T cells, but not in CD8+ T cells (45). Thus, our measure of cellular senescence here compared with that of our previous work may not reflect the same physiologic processes.
Although tenofovir alafenamide (TAF), an alternate prodrug of TFV, has largely replaced TDF in North America and Europe (46, 47), TDF remains in use in Africa and Southeast Asia (16), where the HIV-positive population is also aging. In addition, TAF is designed to increase intracellular TFVdp and decrease TFV plasma concentrations relative to TDF (48), which increases the TFVdp:dATP ratio substantially. The potential long-term consequences of this increase, particularly in aging patients, are unknown. One NRTI-sparing combination product is currently approved in North America and Europe and was recently included in the US Department of Health and Human Services guidelines for initial therapy in patients with viral loads of <500,000 copies/ml who have completed viral resistance testing and do not have hepatitis B infection (46, 47). Other NRTI-sparing two-drug regimens are recommended in certain clinical scenarios (46). While several NRTI-sparing regimens are in development for treating ARV-naive patients, dual NRTI-integrase inhibitor regimens remain the recommended initial treatment with the most clinical evidence to support their use (46).
Chronologic age itself may not be the best predictor of altered physiology, given the diversity in function and immunologic status across patients of similar ages (49–51). Frailty, cellular senescence, and other biomarkers have been suggested as more robust measures of aging (50). WIHS women, who are older, heavier, and more racially and socioeconomically diverse than those typically enrolled in clinical research, are important contributors for understanding pharmacokinetics in aging patients under the conditions of typical use (51). The WIHS, now a part of the MACS/WIHS Combined Cohort Study, routinely performs frailty phenotyping; although it was not completed for all women-visits in this study, these data could be incorporated into future analyses. Our choice to dichotomize age into two groups was influenced by the uncertainty in the underlying relationship of pharmacology with age and the limited amount of testing that we could conduct. Follow-up studies will benefit from greater testing and careful modeling to see whether age effects are linear or display a pattern that might help elucidate associations. Potential sex differences in NRTI pharmacology have been suggested for zidovudine (13, 52) and for inflammation in general (53, 54), and thus subsequent investigations should include men to further test the hypothesis of altered IM:EN ratios in aging patients, as well as to compare men and women.
MATERIALS AND METHODS
Data source and data availability.
The Women’s Interagency HIV Study (WIHS) was established in 1993 (55–57). At semiannual study visits, women complete comprehensive interviews that include extensive sociodemographic, behavioral, medication, and medical surveillance and contribute blood and other repository specimens that are available for investigator use (further described at https://statepi.jhsph.edu/wihs/wordpress/). Data and specimens for this investigation were obtained under approved WIHS concept sheet W14101 (J.B.D., lead investigator) and analyzed pursuant to a data use agreement between the WIHS Data Analysis Center at Johns Hopkins University and WIHS investigators at the University of North Carolina Chapel Hill. The University of North Carolina at Chapel Hill Biomedical Institutional Review Board determined that this analysis was an exempt research activity. Data and statistical code are available upon request, with approval of the WIHS under a separate data use agreement.
Sample selection and inclusion/exclusion criteria.
Stored PBMC pellets and viable PBMCs collected during routine WIHS visits were selected for women reporting use of TDF-FTC in their ART regimen at time of their study visit, provided they did not also report the use of additional NRTIs. Among those women who were either ≤45 years old or ≥60 years old at the time of specimen collection, we restricted eligibility for this analysis to those study-visits where HIV RNA concentrations were below 200 copies/ml, as a proxy of medication adherence. One-hundred seventy-one unique woman-visits in April 2015 to March 2016 were identified. Of these visits, we analyzed specimens from 134 unique woman-visits for women who had sufficient sample quantities remaining for analysis; who were not elite controllers, long-term nonprogressors, or recent seroconverters; and whose first use of TDF-FTC occurred before the visit under consideration. All women included in the analysis had received TDF-FTC for at least 6 months. We included 10 additional specimens from women aged ≥60 years meeting these criteria who only contributed specimens obtained between April 2006 and March 2013, as the analytical laboratory had previously analyzed PBMC samples up to 10 years old with no significant loss of analyte (data not shown) and immune biomarkers are largely stable over the long-term (7, 33). These participants may have subsequently changed regimens or been lost to follow-up when the initial query on 2015 data was performed. Their specimens were processed and stored following the same procedures as the newer samples.
A paired blood plasma sample collected at the same time as the PBMC samples was used to measure IL-6 and sCD163 concentrations; both specimens were collected from sodium citrate CPT mononuclear cell preparation tubes (BD Biosciences, San Jose, CA). At the WIHS site, following initial centrifugation, plasma was removed and stored locally at −80°C. PBMCs were isolated and counted using standard methods; pellets of 500,000 cells per women and visit were stored at −80°C, and viable cells were step-frozen in a dimethyl sulfoxide (DMSO)-fetal bovine serum (FBS) mixture and stored in liquid nitrogen. Specimens were batch-shipped from the individual sites to the Division of Acquired Immunodeficiency Syndrome (DAIDS) repository in Frederick, Maryland; chain of custody, shipping conditions, and arrival conditions are strictly documented by Precision for Medicine, LLC (Bethesda, MD) to ensure specimen integrity. The selected specimens, following the same control procedures, were shipped to University of North Carolina (UNC) on dry ice (PBMC pellets and plasma) or liquid nitrogen (viable PBMCs) and were immediately stored upon arrival at −80°C (Dumond laboratory) or in liquid nitrogen (UNC CFAR HIV/STD Laboratory Core). Demographic and clinical data obtained at the same visit as the sample included age, race, smoking status, menopausal status, hypertension diabetes, estimated glomerular filtration rate (eGFR), AST-to-platelet ratio index (APRI) score, concomitant ARVs, CD4+ T cell count, and CD8+ cell count. These variables were defined as follows: self-reported current smoking status (yes/no), self-reported race (white, black, or other), current ART in addition to TDF-FTC (nonnucleoside reverse transcriptase inhibitor, atazanavir or lopinavir, darunavir, integrase inhibitor, or other), any indication of hypertension (yes/no based on systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, self-report, or use of anti-hypertensive medication), any indication of diabetes (yes/no based on confirmation of self-reported diabetes, self-report of anti-diabetic medication, measured fasting glucose ≥126 mg/dl, or measured hemoglobin-A1C of ≥6.5, in nonpregnant women only), eGFR (measured continuously, CKD-EPI [Chronic Kidney Disease Epidemiology Collaboration] formula), and APRI (calculated from laboratory values). The time since last ARV dose at sample collection is captured in the WIHS database; however, given the long half-life of these intracellular moieties that results in fairly constant concentrations over a dosing interval, the time at sample collection since last dose was not incorporated into the statistical analysis. Six of 144 participants did not have BMI measured at the same visit. We imputed BMI values with those measured at the previous or the following visit for 4 participants; if a participant had BMI values at both the previous and following visit, the value from the previous visit was selected. The remaining 2 participants did not contribute to analyses that included BMI (e.g., Table 3).
Analytical methods.
TFVdp (IM), FTCtp (IM), dATP (EN), and dCTP (EN) were measured in PBMC extracts in the UNC Center for AIDS Research (CFAR) Clinical Pharmacology and Analytical Chemistry Core using liquid chromatography/mass spectroscopy (LC/MS-MS) methods as previously described (26). Briefly, the lower limit of quantification is 0.2 ng/ml for all analytes; interrun calibration standards and quality controls were within 15% acceptance criteria for precision and accuracy for all analytes.
IL-6 and sCD163 were measured in blood plasma in duplicate with enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) in the UNC CFAR HIV/STD Laboratory Core according to the manufacturer’s protocols.
Markers of cellular senescence in CD8+ T cells (CD28−CD57+) were measured by flow cytometry as described previously (58) using the following antibodies from Becton, Dickinson (San Jose, CA): CD3, CD4, CD27, CD28, CD57, and CD95. Fluorescence-minus one controls were included for all antibodies. Samples were acquired on an LSRII Fortessa instrument in the UNC Flow Cytometry Core and were analyzed using FlowJo Software, version 10 (Treestar, Ashland, OR). Senescent CD8+ T cells were defined as CD28−CD95+CD57+ and are reported as the percentage of total CD8+ T cells, as determined by Boolean gating.
Statistical methods.
For the analysis comparing TFVdp:dATP and FTCtp:dCTP by age group, sample size calculations were based on data from a previously conducted clinical study in aging men and women living with HIV and receiving TDF-FTC (26). For a total sample size of 140 women, with 70 women aged ≤45 years and 70 women aged ≥60 years, approximately 83% power for TFVdp:dATP and 90% power for FTCtp:dCTP were expected to detect a geometric mean ratio of 1.30 or larger between the age groups.
Comparisons of TFVdp:dATP and FTCtp:dCTP (primary outcomes) by age group were done in two ways. First, log-rank tests were performed to compare the distributions of the ratios. This approach was used as some of the ratios had observations below the limit of quantification (BLQ) (28). To right-censor these observations and allow use of a log-rank test, ratios were subtracted from a value arbitrarily larger than the maximum of the ratios, as suggested by Gillespie et al. (29). Kaplan-Meier estimated medians were used to describe the central tendency of these data. Additionally, Spearman’s rank correlation coefficients were calculated for dATP, dCTP, TFVdp, FTCtp, sCD163, and IL-6 concentrations; CD4+:CD8+ T cell ratio; percent senescent (CD28−CD57+CD95+) CD8+ T cells; eGFR; and BMI, stratified by age group. To check for heterogeneity in the distributions of dATP and dCTP concentration by race (white, black, and other), Kruskal-Wallis tests were used.
Second, the relationships between age group (predictor) and dATP, dCTP, TFVdp, FTCtp, IL-6, and sCD163 concentrations; TFVdp:dATP ratio; FTCtp:dCTP ratio; and percent senescent (CD28−CD57+CD95+) CD8+ T cells (responses) were assessed using censored multivariable linear regression models in order to account for responses that were BLQ. All responses were log10-transformed to stabilize the variance of and normalize model residuals.
Each model was adjusted for current smoking status (binary), race (categorical), current ART (categorical), hypertension (binary), diabetes (binary), eGFR (categorical), BMI (categorical for IL-6; continuous for all others), and APRI score (continuous), as we hypothesized that these factors may influence drug concentrations and/or inflammatory status. Background regimen was divided by class, and protease inhibitors were further categorized to account for potentially increased TFVdp concentrations with specific protease inhibitors (atazanavir-ritonavir and lopinavir-ritonavir, compared with darunavir-ritonavir) (37, 38). Race was included based on both literature from analyses of dried blood spots (42, 43) and differences in the racial composition of the age groups. For continuous covariates, we used Akaike’s Information Criterion and diagnostics of model fit to guide the choice of proper functional forms. All statistical testing was performed using two-sided tests, and a P value of <0.05 denoted statistical significance without adjustment for multiple comparisons. All analyses were conducted in SAS 9.4 (SAS Institute Inc., Cary, NC).
Supplementary Material
ACKNOWLEDGMENTS
We thank the WIHS participants for their continued contributions to the cohort; Catalina Ramirez at the UNC WIHS for assistance with WIHS database queries, variable interpretation, and repository sample selection; Eryka Wentz and Jess Donahue at WDMAC for assistance with obtaining samples from the WIHS repository; Dana Lapple, Paul Alabanza, Hannah Munro, and Takesha McMillion at the UNC CFAR HIV/STD Laboratory Core for performing the flow cytometry assays. We also acknowledge the contributions of Yen Chang, Katie Mollan, and Michael Hudgens for their statistical assistance in responding to reviewer comments.
This work was supported by the UNC WIHS (U01 AI103390) and the WIHS Data Management and Analysis Center (U01 AI042590), as well as by R21 AG058490 (J.B.D., C.P.B., and A.D.). The UNC Center for AIDS Research (CFAR) Clinical Pharmacology and Analytical Chemistry Core (C.S.), HIV/STD Laboratory Core (J.A.E.N. and K.D.P.), and Biostatistics Core (C.P.B. and A.D.) are supported by P30 AI50410. The UNC Flow Cytometry Core is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center.
Data in the manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
The WIHS sites and principal investigators are as follows: UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Brooklyn WIHS (Deborah Gustafson and Tracey Wilson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye and Daniel Merenstein), U01-AI-034994; Miami WIHS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Bradley Aouizerat and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; and Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I to WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Butler JM, Begg EJ. 2008. Free drug metabolic clearance in elderly people. Clin Pharmacokinet 47:297–321. doi: 10.2165/00003088-200847050-00002. [DOI] [PubMed] [Google Scholar]
- 2.Klotz U. 2009. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 41:67–76. doi: 10.1080/03602530902722679. [DOI] [PubMed] [Google Scholar]
- 3.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, Effros RB, Gebo K, Goronzy JJ, Justice AC, Landay A, Levin J, Miotti PG, Munk RJ, Nass H, Rinaldo CR, Shlipak MG, Tracy R, Valcour V, Vance DE, Walston JD, Volberding P, OAR Working Group on HIV and Aging. 2012. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 60:S1–S18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French MA, Cozzi-Lepri A, Arduino RC, Johnson M, Achhra AC, Landay A, INSIGHT SMART Study Group. 2015. Plasma levels of cytokines and chemokines and the risk of mortality in HIV-infected individuals: a case-control analysis nested in a large clinical trial. AIDS 29:847–851. doi: 10.1097/QAD.0000000000000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu DC, Ma YF, Hur S, Li D, Rupert A, Scherzer R, Kalapus SC, Deeks S, Sereti I, Hsue PY. 2016. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive antiretroviral therapy. AIDS 30:2065–2074. doi: 10.1097/QAD.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald B, Moyo S, Gabaitiri L, Gaseitsiwe S, Bussmann H, Koethe JR, Musonda R, Makhema J, Novitsky V, Marlink RG, Wester CW, Essex M. 2013. Persistently elevated serum interleukin-6 predicts mortality among adults receiving combination antiretroviral therapy in Botswana: results from a clinical trial. AIDS Res Hum Retroviruses 29:993–999. doi: 10.1089/aid.2012.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada NI, Bream JH, Martinez-Maza O, Macatangay B, Galvin SR, Margolick JB, Jacobson LP. 2016. Inflammatory biomarkers and mortality risk among HIV-suppressed men: a multisite prospective cohort study. Clin Infect Dis 63:984–990. doi: 10.1093/cid/ciw409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudsen TB, Ertner G, Petersen J, Moller HJ, Moestrup SK, Eugen-Olsen J, Kronborg G, Benfield T. 2016. Plasma soluble CD163 level independently predicts all-cause mortality in HIV-1-infected individuals. J Infect Dis 214:1198–1204. doi: 10.1093/infdis/jiw263. [DOI] [PubMed] [Google Scholar]
- 9.Williams B, Livak B, Bahk M, Keating SM, Adeyemi OM. 2016. Short communication: SCD14 and SCD163 levels are correlated with VACS index scores: initial data from the blunted immune recovery in CORE Patients with HIV (BIRCH) cohort. AIDS Res Hum Retroviruses 32:144–147. doi: 10.1089/aid.2015.0012. [DOI] [PubMed] [Google Scholar]
- 10.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ Jr., Kingsley LA, Witt MD, George RT, Jacobson LP, Budoff M, Tracy RP, Brown TT, Post WS. 2015. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis 211:1219–1228. doi: 10.1093/infdis/jiu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller DS, Bauer B, Hartz AM. 2008. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev 60:196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah RR, Smith RL. 2015. Inflammation-induced phenoconversion of polymorphic drug metabolizing enzymes: hypothesis with implications for personalized medicine. Drug Metab Dispos 43:400–410. doi: 10.1124/dmd.114.061093. [DOI] [PubMed] [Google Scholar]
- 13.Anderson PL, Kakuda TN, Lichtenstein KA. 2004. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin Infect Dis 38:743–753. doi: 10.1086/381678. [DOI] [PubMed] [Google Scholar]
- 14.Panel on Antiretroviral Guidelines for Adults and Adolescents. 2019. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services, Washington, DC: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 15.European AIDS Clinical Society. 2019. Guidelines version 10.0. European AIDS Clinical Society, Brussels, Belgium: https://www.eacsociety.org/files/guidelines-10.0_final_2_2.pdf. [Google Scholar]
- 16.World Health Organization. 2019. Update of recommendations on first- and second-line antiretroviral regimens. WHO/CDS/HIV/19.15 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 17.Margolis DM, Kewn S, Coull JJ, Ylisastigui L, Turner D, Wise H, Hossain MM, Lanier ER, Shaw LM, Back D. 2002. The addition of mycophenolate mofetil to antiretroviral therapy including abacavir is associated with depletion of intracellular deoxyguanosine triphosphate and a decrease in plasma HIV-1 RNA. J Acquir Immune Defic Syndr 31:45–49. doi: 10.1097/00126334-200209010-00006. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Lerma JG, Aung W, Cong ME, Zheng Q, Youngpairoj AS, Mitchell J, Holder A, Martin A, Kuklenyik S, Luo W, Lin CY, Hanson DL, Kersh E, Pau CP, Ray AS, Rooney JF, Lee WA, Heneine W. 2011. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol 85:6610–6617. doi: 10.1128/JVI.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cottrell ML, Yang KH, Prince HM, Sykes C, White N, Malone S, Dellon ES, Madanick RD, Shaheen NJ, Hudgens MG, Wulff J, Patterson KB, Nelson JA, Kashuba AD. 2016. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 214:55–64. doi: 10.1093/infdis/jiw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtenstein KA, Delaney KM, Armon C, Ward DJ, Moorman AC, Wood KC, Holmberg SD, HIV Outpatient Study Investigators. 2003. Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1-infected patients. J Acquir Immune Defic Syndr 32:48–56. doi: 10.1097/00126334-200301010-00007. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein KA, Ward DJ, Moorman AC, Delaney KM, Young B, Palella FJ Jr, Rhodes PH, Wood KC, Holmberg SD, HIV Outpatient Study Investigators. 2001. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS 15:1389–1398. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 22.Hoggard PG, Lloyd J, Khoo SH, Barry MG, Dann L, Gibbons SE, Wilkins EG, Loveday C, Back DJ. 2001. Zidovudine phosphorylation determined sequentially over 12 months in human immunodeficiency virus-infected patients with or without previous exposure to antiretroviral agents. Antimicrob Agents Chemother 45:976–980. doi: 10.1128/AAC.35.3.976-980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvaraj S, Ghebremichael M, Li M, Foli Y, Langs-Barlow A, Ogbuagu A, Barakat L, Tubridy E, Edifor R, Lam W, Cheng YC, Paintsil E. 2014. Antiretroviral therapy-induced mitochondrial toxicity: potential mechanisms beyond polymerase-[gamma] inhibition. Clin Pharmacol Ther 96:110–120. doi: 10.1038/clpt.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baxi SM, Scherzer R, Greenblatt RM, Minkoff H, Sharma A, Cohen M, Young MA, Abraham AG, Shlipak AG, Michael G. for the Women’s Interagency HIV study (WIHS). 2016. Higher tenofovir exposure is associated with longitudinal declines in kidney function in women living with HIV. AIDS 30:609–618. doi: 10.1097/QAD.0000000000000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson JA, Krishnamurthy J, Menezes P, Liu Y, Hudgens MG, Sharpless NE, Eron JJ Jr.. 2012. Expression of p16(INK4a) as a biomarker of T-cell aging in HIV-infected patients prior to and during antiretroviral therapy. Aging Cell 11:916–918. doi: 10.1111/j.1474-9726.2012.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumond JB, Collins JW, Cottrell ML, Trezza CR, Prince H, Sykes C, Torrice C, White N, Malone S, Wang R, Patterson KB, Sharpless NE, Forrest A. 2017. p16INK4a, a senescence marker, influences tenofovir/emtricitabine metabolite disposition in HIV-infected subjects. CPT Pharmacometrics Syst Pharmacol 6:120–127. doi: 10.1002/psp4.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumond JB, Francis O, Cottrell M, Trezza C, Prince HM, Mollan K, Sykes C, Torrice C, White N, Malone S, Wang R, Van Dam C, Patterson KB, Hudgens MG, Sharpless NE, Forrest A. 2016. Tenofovir/emtricitabine metabolites and endogenous nucleotide exposures are associated with p16(INK4a) expression in subjects on combination therapy. Antivir Ther 21:441–445. doi: 10.3851/IMP3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maas BM, Francis O, Mollan KR, Lee C, Cottrell ML, Prince HM, Sykes C, Trezza C, Torrice C, White N, Malone S, Hudgens MG, Sharpless NE, Dumond JB. 2016. Concentrations of pro-inflammatory cytokines are not associated with senescence marker p16INK4a or predictive of intracellular emtricitabine/tenofovir metabolite and endogenous nucleotide exposures in adults with HIV infection. PLoS One 11:e0168709. doi: 10.1371/journal.pone.0168709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillespie BW, Chen Q, Reichert H, Franzblau A, Hedgeman E, Lepkowski J, Adriaens P, Demond A, Luksemburg W, Garabrant DH. 2010. Estimating population distributions when some data are below a limit of detection by using a reverse Kaplan-Meier estimator. Epidemiology 21:S64–S70. doi: 10.1097/EDE.0b013e3181ce9f08. [DOI] [PubMed] [Google Scholar]
- 30.Baxi SM, Greenblatt RM, Bacchetti P, Jin C, French AL, Keller MJ, Augenbraun MH, Gange SJ, Liu C, Mack WJ, Gandhi M, Women’s Interagency HIV Study (WIHS). 2015. Nevirapine concentration in hair samples is a strong predictor of virologic suppression in a prospective cohort of HIV-infected patients. PLoS One 10:e0129100. doi: 10.1371/journal.pone.0129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H, Young M, Milam J, Cohen MH, Sharp GB, Huang Y, Greenblatt RM. 2011. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis 52:1267–1275. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gandhi M, Ameli N, Bacchetti P, Gange SJ, Anastos K, Levine A, Hyman CL, Cohen M, Young M, Huang Y, Greenblatt RM. 2009. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS 23:471–478. doi: 10.1097/QAD.0b013e328325a4a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, Martinez-Maza O, Bream JH. 2015. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao W, Agbaria R, Driscoll J, Mitsuya H. 1994. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2',3'-dideoxynucleoside analogs in resting and activated human cells. J Biol Chem 269:12633–12638. [PubMed] [Google Scholar]
- 35.Gao WY, Shirasaka T, Johns DG, Broder S, Mitsuya H. 1993. Differential phosphorylation of azidothymidine, dideoxycytidine, and dideoxyinosine in resting and activated peripheral blood mononuclear cells. J Clin Invest 91:2326–2333. doi: 10.1172/JCI116463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rousseau FS, Kahn JO, Thompson M, Mildvan D, Shepp D, Sommadossi J-P, Delehanty J, Simpson JN, Wang LH, Quinn JB, Wakeford C, van der Horst C. 2001. Prototype trial design for rapid dose selection of antiretroviral drugs: an example using emtricitabine (Coviracil). J Antimicrob Chemother 48:507–513. doi: 10.1093/jac/48.4.507. [DOI] [PubMed] [Google Scholar]
- 37.Lahiri CD, Tao S, Jiang Y, Sheth AN, Acosta EP, Marconi VC, Armstrong WS, Schinazi RF, Vunnava A, Sanford S, Ofotokun I. 2015. Impact of protease inhibitors on intracellular concentration of tenofovir-diphosphate among HIV-1 infected patients. AIDS 29:1113–1115. doi: 10.1097/QAD.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pruvost A, Negredo E, Théodoro F, Puig J, Levi M, Ayen R, Grassi J, Clotet B. 2009. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob Agents Chemother 53:1937–1943. doi: 10.1128/AAC.01064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koethe JR, Jenkins CA, Lau B, Shepherd BE, Silverberg MJ, Brown TT, Blashill AJ, Anema A, Willig A, Stinnette S, Napravnik S, Gill J, Crane HM, Sterling TR, North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). 2015. Body mass index and early CD4 T-cell recovery among adults initiating antiretroviral therapy in North America, 1998–2010. HIV Med 16:572–577. doi: 10.1111/hiv.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palermo B, Bosch RJ, Bennett K, Jacobson JM. 2011. Body mass index and CD4+ T-lymphocyte recovery in HIV-infected men with viral suppression on antiretroviral therapy. HIV Clin Trials 12:222–227. doi: 10.1310/hct1204-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madelain V, Le MP, Champenois K, Charpentier C, Landman R, Joly V, Yeni P, Descamps D, Yazdanpanah Y, Peytavin G. 2017. Impact of obesity on antiretroviral pharmacokinetics and immuno-virological response in HIV-infected patients: a case-control study. J Antimicrob Chemother 72:1137–1146. doi: 10.1093/jac/dkw527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castillo-Mancilla JR, Morrow M, Coyle RP, Coleman SS, Gardner EM, Zheng JH, Ellison L, Bushman LR, Kiser JJ, MaWhinney S, Anderson PL. 2019. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with HIV infection. Clin Infect Dis 68:1335–1342. doi: 10.1093/cid/ciy708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seifert SM, Castillo-Mancilla JR, Erlandson K, Morrow M, Gandhi M, Kuncze K, Horng H, Zheng JH, Bushman LR, Kiser JJ, MaWhinney S, Anderson PL. 2018. Brief report: adherence biomarker measurements in older and younger HIV-infected adults receiving tenofovir-based therapy. J Acquir Immune Defic Syndr 77:295–298. doi: 10.1097/QAI.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onyema OO, Njemini R, Bautmans I, Renmans W, De Waele M, Mets T. 2012. Cellular aging and senescence characteristics of human T-lymphocytes. Biogerontology 13:169–181. doi: 10.1007/s10522-011-9366-z. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro SP, Milush JM, Cunha-Neto E, Kallas EG, Kalil J, Passero LFD, Hunt PW, Deeks SG, Nixon DF, SenGupta D. 2016. p16INK4a expression and immunologic aging in chronic HIV infection. PLoS One 11:e0166759. doi: 10.1371/journal.pone.0166759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anonymous. 2019. Panel on Antiretroviral Guidelines for Adults and Adolescents. In Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC. [Google Scholar]
- 47.European AIDS Clinical Society. 2017. European guidelines for the treatment of HIV, version 9.0 European AIDS Clinical Society, Brussels, Belgium. [Google Scholar]
- 48.Ray AS, Fordyce MW, Hitchcock MJ. 2016. Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res 125:63–70. doi: 10.1016/j.antiviral.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lagathu C, Cossarizza A, Bereziat V, Nasi M, Capeau J, Pinti M. 2017. Basic science and pathogenesis of ageing with HIV: potential mechanisms and biomarkers. AIDS 31:S105–S119. doi: 10.1097/QAD.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 51.Gandhi M, Ameli N, Bacchetti P, Sharp GB, French AL, Young M, Gange SJ, Anastos K, Holman S, Levine A, Greenblatt RM. 2005. Eligibility criteria for HIV clinical trials and generalizability of results: the gap between published reports and study protocols. AIDS 19:1885–1896. doi: 10.1097/01.aids.0000189866.67182.f7. [DOI] [PubMed] [Google Scholar]
- 52.Rower JE, Meditz A, Gardner EM, Lichtenstein K, Predhomme J, Bushman LR, Klein B, Zheng JH, Mawhinney S, Anderson PL. 2012. Effect of HIV-1 infection and sex on the cellular pharmacology of the antiretroviral drugs zidovudine and lamivudine. Antimicrob Agents Chemother 56:3011–3019. doi: 10.1128/AAC.06337-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, Cheng W-J, Paukovics G, Palmer CS, Novak RM, Jaworowski A, Landay AL, Crowe SM. 2013. Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One 8:e55279. doi: 10.1371/journal.pone.0055279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathad JS, Gupte N, Balagopal A, Asmuth D, Hakim J, Santos B, Riviere C, Hosseinipour M, Sugandhavesa P, Infante R, Pillay S, Cardoso SW, Mwelase N, Pawar J, Berendes S, Kumarasamy N, Andrade BB, Campbell TB, Currier JS, Cohn SE, Gupta A, New Work Concept Sheet 319 and AIDS Clinical Trials Group A5175 (PEARLS) Study Teams. 2016. Sex-related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J Acquir Immune Defic Syndr 73:123–129. doi: 10.1097/QAI.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J. 1998. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 9:117–125. doi: 10.1097/00001648-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA. 2005. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adimora AA, Ramirez C, Benning L, Greenblatt RM, Kempf MC, Tien PC, Kassaye SG, Anastos K, Cohen M, Minkoff H, Wingood G, Ofotokun I, Fischl MA, Gange S. 2018. Cohort profile: the Women's Interagency HIV Study (WIHS). Int J Epidemiol 47:393–394i. doi: 10.1093/ije/dyy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rahangdale L, De Paris K, Kashuba AD, Nelson JA, Cottrell M, Sykes C, Emerson C, Young SL, Stevens T, Patterson KB, Cohen MS. 2015. Immunologic, virologic, and pharmacologic characterization of the female upper genital tract in HIV-infected women. J Acquir Immune Defic Syndr 68:420–424. doi: 10.1097/QAI.0000000000000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.