Fungal organisms are ubiquitous in nature, and progress of modern medicine is creating an expanding number of severely compromised patients susceptible to a variety of opportunistic fungal infections. These infections are difficult to diagnose and treat, leading to high mortality rates. The limited antifungal arsenal, the toxicity of current antifungal drugs, the development of resistance, and the emergence of new multidrug-resistant fungi, all highlight the urgent need for new antifungal agents.
KEYWORDS: antifungal agents, antifungal drug development, drug repurposing, fungal infections, repositioning
ABSTRACT
Fungal organisms are ubiquitous in nature, and progress of modern medicine is creating an expanding number of severely compromised patients susceptible to a variety of opportunistic fungal infections. These infections are difficult to diagnose and treat, leading to high mortality rates. The limited antifungal arsenal, the toxicity of current antifungal drugs, the development of resistance, and the emergence of new multidrug-resistant fungi, all highlight the urgent need for new antifungal agents. Unfortunately, the development of a novel antifungal is a rather long and expensive proposition, and no new classes of antifungal agents have reached the market in the last 2 decades. Drug repurposing, or finding new indications for old drugs, represents a promising alternative pathway to drug development that is particularly appealing within the academic environment. In the last few years, there has been a growing interest in repurposing approaches in the antifungal arena, with multiple groups of investigators having performed screenings of different repurposing libraries against different pathogenic fungi in search for drugs with previously unrecognized antifungal effects. Overall, these repurposing efforts may lead to the fast deployment of drugs with novel antifungal activity, which can rapidly bring benefits to patients, while at the same time reducing health care costs.
INTRODUCTION
FUNGI, FUNGAL INFECTIONS, AND CURRENT ANTIFUNGAL DRUGS
Fungi are capable of causing a variety of infections ranging from superficial to disseminated invasive infections and do so with increasing frequency in an expanding population of immunocompromised and medically compromised patients. These infections are difficult to treat, and some of them carry unacceptably high levels of morbidity and mortality. The current antifungal arsenal is very limited due to the paucity of selective targets. Clinically used antifungals for the treatment of invasive fungal infections are restricted to the polyenes (i.e., amphotericin B), the azoles (i.e., fluconazole, voriconazole), and the echinocandins (i.e., caspofungin and micafungin) (1). However, problems with toxicity and the emergence of resistance limit the usefulness of current antifungals, and some of the emerging pathogens display intrinsic resistance to all classes of antifungals. Therefore, there is an urgent need for the development of novel antifungal agents, particularly those with new chemical classes and novel mechanisms of activity.
DRUG REPURPOSING VERSUS DE NOVO DRUG DISCOVERY
The process of de novo drug discovery and development, although currently the most common choice for drug development, is time-consuming and expensive, with high attrition rates (2). In general, it can take up to 20 years to bring a drug from its initial discovery to its release on the market after U.S. Food and Drug Administration (FDA) approval, and this development can cost up to $2 billion with only a 5% chance of successful clinical trial completion and therefore successful entrance into the market (3–6). Unfortunately, antifungal research and development has been mostly discontinued at most large pharmaceutical companies, and the development of new antifungals relies heavily on the efforts of a few much smaller biotechnology companies, with a few investigational agents at different stages of the development pipeline (for comprehensive reviews on this topic, readers are referred to references 7 and 8).
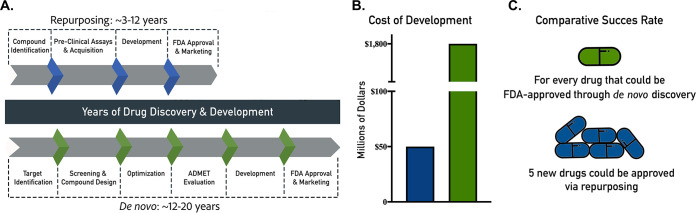
In contrast, drug repurposing (or repositioning), referring to the identification of new uses for established medications and abandoned or failed compounds, has emerged as an expedited alternative approach to find new indications for existing drugs (6, 9). This approach can substantially decrease the time, effort, and cost that it takes to find a new therapeutic indication and bring rapid benefit to the patients (6, 10) (Fig. 1). Repurposing candidates are FDA approved or at the very least have been through several stages of clinical development and therefore have well-known safety and pharmacological profiles. Candidate therapies can be rapidly advanced through the drug development process and be ready for clinical trials quickly, speeding their review by the FDA and, if approved, their integration into health care (11), thereby potentially providing for the quickest possible transition from bench to bedside and potentially saving the lives of thousands of patients. For these reasons, repurposing represents a highly attractive and pragmatic alternative to drug development and has enormous potential for rapid clinical impact, including in the antifungal arena (12, 13). Several FDA-approved drugs have been repurposed from their original indications for treatment of other diseases. For example, thalidomide was originally used to treat morning sickness in pregnant women in 1957; however, after the discovery of its terrible side effects, the drug was banned (6, 14). Despite these problems, it was discovered in 1964 that this drug was effective for treatment of erythema nodosum laprosum (leprae), and it is currently the primary treatment for this disease (14). Besides this use, thalidomide and its analogs have also been approved for the treatment of multiple myeloma (15). Another example of a successfully repurposed drug is sildenafil. It was originally developed as an angina medication, and it is now marketed as Viagra for the treatment of erectile dysfunction (16, 17). Azidothymidine was originally developed as a chemotherapy drug, but it failed; in spite of this failure, it became the first successful drug for the treatment of HIV infection (16, 18). All of these examples show that drug repurposing can be successful in identifying new indications for previously approved drugs.
FIG 1.
Drug repurposing versus de novo drug discovery. This figure shows the time (A), cost (B), and comparative success rates (C) of drug repurposing and de novo discovery.
There are also important considerations about patenting and commercialization of repurposed drugs that need to be taken into account. For off-patent drugs, a new method-of-use patent can be secured for a new repurposed use of an old generic drug, as long as the novel indication is both new and inventive (i.e., nonobvious) (19). However, because drug repurposing primarily concerns previously known drugs, obtaining patent protection can be challenging, and there are important caveats to be taken into account regarding intellectual property protection and patent submission for repurposed drugs (19, 20). First of all, the U.S. courts have concluded that safe-harbor protections apply to an expanding array of repurposing research activities (21). It is argued that allowing repurposing research, particularly in academia and not-for-profit organizations, serves the broader interests of society by advancing science and the medical practice, particularly in the case of diseases that afflict smaller populations of people or those with limited financial resources (22). Second, in the United States, drug repurposing can employ the 505(b)(2) drug development strategy (23). This is an alternative type of submission to regulatory agencies that can be used to obtain the approval of a new drug if the product in question contains active ingredients similar to those of a previously approved drug, with the data included in the submission relying on safety and effectiveness characteristics related to the existing product (20, 23). Importantly, this alternative pathway can be expedited and requires significantly less resources than the typical regulatory pathway.
REPURPOSING FOR THE IDENTIFICATION OF DRUGS WITH NOVEL ANTIFUNGAL ACTIVITY
Although drug repurposing is a promising option, there have yet to be any medications that have been fully repurposed for new antifungal indications at this time (13). It is worth noting that 5-flucytosine was first developed as a potential anticancer drug. However, since 5-flucytosine was not sufficiently active against tumors, it was eventually investigated and repositioned as an antifungal agent (24).
There are a few notable early examples of attempts by different groups of investigators to repurpose several types of drugs as new antifungals. Clinically used cyclosporine targeting calcineurin, as well rapamycin and its analogues (“rapalogues”) and other targets of rapamycin (TOR) inhibitors, displays antifungal activity and acts synergistically with azole derivatives (25–30). Geldanamycin and other HSP90 inhibitors, originally developed as potential anticancer medications, also potentiate the activity of antifungal drugs when used in combination (31, 32). The activity of sertraline, a well-established antidepressant, against Cryptococcus has been described and improves fluconazole treatment potentially by benefiting from its excellent penetration into the central nervous system (33, 34). Sertraline (see also below) reached phase III clinical trials for treatment of cryptococcal meningitis; however, the drug did not demonstrate superiority compared to current treatment options in preventing morbidity and mortality (35, 36). Other notable reports on repurposing drugs as antifungals include AR-12, a derivative of celecoxib which demonstrated broad-spectrum antifungal activity (37, 38); tamoxifen, an estrogen receptor antagonist used primarily to treat breast cancer (39, 40); and derivatives of the antimalarial drug mefloquine (41).
SCREENING REPURPOSING LIBRARIES TO IDENTIFY COMPOUNDS WITH PREVIOUSLY UNIDENTIFIED ANTIFUNGAL ACTIVITY
Most recently, adopting a common and powerful strategy from the drug discovery field, different academic laboratories have initiated repurposing programs by screening approved compound libraries representing large collections (hundreds to thousands) of existing drugs in search for those with novel antifungal activity. These efforts have been facilitated by the availability of repurposing libraries from different sources, including some that are commercially available, as well as others that can be procured from governmental and not-for-profit entities. More often, these efforts have used phenotypic screens which can identify compounds that demonstrate a relevant effect without the need for prior knowledge of the specific target affected. The majority of screens have been performed to identify drugs that inhibit growth of a given fungal species, although others have focused on the inhibition of a particular trait associated with virulence (i.e., biofilm formation). Regardless, most in vitro phenotypic screens have used some type of cell-based assays in a multiwell format, thereby allowing for the implementation of highly efficient high-throughput screening (HTS) techniques. The following is a summary of such repurposing screening efforts, which are also listed in Table 1.
TABLE 1.
Summary of screening of repurposing libraries in search for compounds with newly described antifungal activity
| Library | No. of compounds | Organism(s) | Phenotype | Main “hits” | Comments | Reference(s) |
|---|---|---|---|---|---|---|
| Johns Hopkins Clinical Compound Library | >1,500 | A. nidulans | Growth inhibition | Polymyxin B, sertraline | Broad spectrum | 33, 42 |
| C. albicans | Growth inhibition | Mycophenolic acid, disulfiram, fluvastatin, octodrine | Octodrine most effective at killing in serum-containing medium | 43 | ||
| Prestwick | 1,200 | C. neoformans | Yeast cell lysis | Amiodarone | Able to cross blood-brain barrier | 45 |
| C. neoformans | Growth inside macrophages | Fendiline hydrochloride | 56 | |||
| C. albicans | Biofilm inhibition | Auranofin | Broad spectrum | 49 | ||
| C. albicans | Growth inhibition of resistant strains | Ribavirin | Activity against other Candida spp. | 51 | ||
| C. auris | Growth inhibition | Ebselen | Broad spectrum | 54 | ||
| C. auris | Growth inhibition | Ebselen, suloctidil | Synergism with current antifungals | 55 | ||
| C. albicans, C. auris, A. fumigatus | Growth inhibition | Alexidine dihydrochloride | Broad spectrum | 57 | ||
| Aspergillus, Fusarium, Scedosporium, Rhizopus, and Lichtheimia spp. | Growth inhibition | Alexidine dihydrochloride | Unique screen against multiple, highly resistant fungi | 58 | ||
| C. albicans, C. neoformans, S. cerevisiae | Synergy with fluconazole | Several hits Sertraline | Emphasis on synergistic effects | 72 | ||
| Pathogen Box (MMV) | 400 | C. albicans | Biofilm inhibition | MMV688768 | Unique activity against biofilm | 59 |
| C. albicans C. neoformans | Growth inhibition under nutrient limited conditions | MMV688271 | Activity under nutrient-limited conditions; targets fungal stress responses | 61 | ||
| C. auris | Growth and biofilm inhibition | Iodoquinol, miltefosine | Active against other Candida spp. | 60 | ||
| Malaria Box (MMV) | 400 | C. neoformans | Growth inhibition | MMV665943 (DM262) | Activity against multidrug-resistant species | 64 |
| Enzo FDA-approved drug library | 640 | C. deuterogattii | Growth inhibition | Nisoldipine | Activity against other yeasts and Aspergillus | 66 |
| Enzo and FIMM | 844 | C. albicans | Growth inhibition | Tosedostat | Activity against multiple Candida spp. | 65 |
| NIH/NCI Developmental Therapeutics | 3,000 | Multiple species | Growth inhibition | NSC319726 | Synergy with common antifungals | 67 |
| NIH Clinical Collection | 727 | C. neoformans | Growth inhibition | Mebendazole | Brain penetration | 68 |
| LOPAC | 1,280 | C. albicans | Growth inhibition | CV-3988 | Potent, lacks toxicity | 69 |
| C. neoformans | Killing | 10058-F4 | Screen performed under nutrient limitation | 70 | ||
| TargetMol | 1,068 | C. albicans | Growth inhibition | Robenidine | Activity against other yeasts and Aspergillus | 71 |
| McMaster Bioactives | 3,600 | S. cerevisiae, S. pombe, C. albicans, C. neoformans | Potentiation of 6 different antifungals | Multiple potentiators | Generation of a matrix of antifungal combinations | 73 |
| Pharmakon | 1,600 | C. albicans | Synergism with miconazole against biofilms | Artemisinins | Synergism against highly resistant biofilms | 74 |
In pioneering screening studies, the Lin group screened the Johns Hopkins Clinical Compound Library (JHCCL, v1.0), a collection of over 1,500 FDA- and foreign-approved drugs, in search for those that can inhibit growth of Aspergillus nidulans. This screening identified the antifungal activity of the cationic peptide antibiotic polymyxin B and the antidepressant sertraline, with follow-up studies demonstrating the broad-spectrum activity of both drugs against a variety of medically important fungi, including Candida spp. (33, 42). The same library was screened by another group for drugs with an inhibitory effect on Candida albicans, resulting in the identification of four compounds with no previously known antifungal properties that could be repurposed in the future: mycophenolic acid, disulfiram, fluvastatin, and octodrine, with octodrine demonstrating the most effective killing of serum-grown C. albicans (43).
The Prestwick library, a collection of approximately 1,200 mostly FDA-approved, off-patent drugs with a diverse range of functions, mechanisms of action, and well-characterized pharmacological and toxicological properties, has been screened by multiple groups of investigators to identify drugs that could potentially be repositioned as antifungals. Using a novel HTS assay of yeast cell lysis (44), a pioneering screen of this library by the Krysan group identified 31 drugs/molecules with fungicidal activity against Cryptococcus neoformans, including 15 drugs for which direct antifungal activity had not previously been reported (45). The authors of that study subsequently focused on the drugs capable of crossing the blood-brain barrier and accessing the phagolysosome, which represent two major hallmarks of the pathogenesis of cryptococcosis, and identified amiodarone as their top candidate. Interestingly, this antiarrhythmic had been previously reported to have activity against yeasts, including C. albicans (46).
Since biofilm formation is associated with different manifestations of candidiasis and cells in biofilms display high levels of resistance against most clinically used antifungals (47, 48), Siles et al. screened the Prestwick library in search for inhibitors of C. albicans biofilm formation (49). This screen led to the identification of a total of 38 bioactive drugs capable of inhibiting biofilm formation in this pathogenic fungus. These researchers classified the initial hit compounds as antifungal drugs, general antimicrobials/antiseptics, or miscellaneous drugs, which were considered to be those with no previously described antifungal activity. Among these, Auranofin, a gold thiol compound used to treat rheumatoid arthritis, represented the most attractive drug from a repurposing point of view, with potent activity even against preformed C. albicans biofilms. Interestingly, a follow-up study described its antifungal activity against a variety of medically important fungi, including different yeasts (Candida and Cryptococcus spp.), as well as molds, including some highly resistant emerging pathogens such as those in the genera Scedosporium and Lomentospora (50). Another screen of the Prestwick Chemical Library against two C. albicans strains, one being fluconazole and echinocandin resistant, identified ribavirin as the best option for repurposing (51). Ribavirin was shown to have activity against several Candida species, including those with drug resistance, but further elucidation is needed. The same Prestwick library was screened for efficacy against Candida auris, an emerging and rapidly spreading multidrug-resistant pathogen for which there is an urgent need to develop new antifungal agents (52, 53), leading to the identification of ebselen, a synthetic organoselenium compound that is part of the NIH clinical collection, as the most effective compound inhibiting both planktonic growth and biofilm formation (54). Furthermore, ebselen displayed a broad spectrum of antifungal activity against a variety of medically important fungi, including yeasts (with activity against multiple other Candida species) and molds. Confirming these results, the Zaragoza group also screened the Prestwick library in search of off-patent drugs with antifungal activity against three different strains of C. auris (55), resulting in the identification of ebselen and suloctidil as the most promising repositionable candidates, especially given their synergism with current antifungals (55). A novel cell-based in vitro screen was developed and used to screen the Prestwick library to identify inhibitors of C. neoformans intracellular replication inside macrophages (56). The primary screen identified a total of 19 drugs that could significantly reduce intracellular growth of the pathogen with follow-up studies revealing fendiline hydrochloride as a potential repositionable candidate for future anticryptococcal therapies. A new version of the Prestwick Chemical Library of 1,233 FDA-approved compounds was screened by the Ibrahim and Uppuluri labs against C. albicans, C. auris, and Aspergillus fumigatus (57). These researchers identified six compounds that could inhibit all three organisms, and they chose alexidine dihydrochloride, an antibacterial and antiplaque agent with limited side effects, as the best candidate for further investigation, mostly based on its ability to kill 80% of a mature biofilm at a concentration lower than 10 μM, although thimerosal also met this criteria (57). They also tested alexidine dihydrochloride against several different species of fungi, including filamentous fungi such as Lomentospora corymbifer and Mucorales spp., as well as yeasts such as C. neoformans. These researchers found that this drug was able to inhibit 50% of growth of the yeasts at <1.5 μg/ml and of filamentous fungi at between 1.5 and 3 μg/ml. Alexidine dihydrochloride was also shown to have activity against azole-resistant isolates and displayed relatively low cytotoxicity against human cells (57). These results indicate that this drug may be a good candidate for repurposing in the future. Confirming these results, most recently alexidine dihydrochloride, together with hexachlorophene, clioquinol, and thonzonium bromide, was also identified in a screen of the Prestwick library against six highly resistant filamentous fungi, including Aspergillus, Fusarium, Scedosporium, Rhizopus, and Lichtheimia spp. (58).
Two collections from Medicines for Malaria Ventures (MMV) have been screened for drug-like compounds with antifungal activity. The Pathogen Box, a collection of 400 drug-like compounds, has been screened against C. albicans, C. auris, and C. neoformans (59–61). Vila et al. originally screened this library in search for inhibitors of C. albicans biofilm formation, reporting on the increased anti-biofilm activity of compound MMV688768 compared to its activity against planktonic cultures. This finding points to the fact that this drug-like compound may affect processes with a predominant role during the biofilm mode of growth (59). Almost concomitantly, Mayer and Kronstad screened the Pathogen Box compounds for inhibitors of C. neoformans and C. albicans planktonic growth, leading to the identification of MMV688271, which displays potent fungicidal activity mostly under nutrient-limited conditions, with a novel mechanism targeting the fungal response to stress at the plasma membrane and cell wall (61). Most recently, the Pathogen Box was also screened in search for inhibitors of C. auris, both under planktonic and biofilm growing conditions, with the identification of iodoquinol and miltefosine as the most promising compounds, with follow-up studies indicating antifungal activity against several multidrug-resistant C. auris strains and other Candida spp. (60). We note that the antifungal activity of miltefosine has been described previously (62, 63). A different library from MMV, the Malaria Box containing 400 antimalarial compounds, was also screened by the Casadevall group (64). The best candidate from this screen, MMV665943 (referred to as DM262), showed a 16- to 32-fold increase in potency compared to fluconazole against C. neoformans and also demonstrated potent antifungal activity against C. albicans and other multidrug-resistant fungal species such as Lomentospora prolificans and Cryptococcus gattii.
Different other repurposing libraries have also been screened for compounds with antifungal activity. A report by Stylianou et al. described the screenings of a total of 844 drugs in two compound libraries, the Enzo and the Institute for Molecular Medicine Finland oncology collection libraries, specifically for anti-Candida activity. Besides known antifungals and nonantifungal drugs with known antifungal activity (including auranofin), results identified seven “off-target” drugs with no previously described antifungal activity (65). Of these, the aminopeptidase inhibitor tosedostat, which has undergone clinical trials for anticancer therapy, displayed notable activity against multiple Candida spp. Another screen was done with the Enzo library of 640 FDA-approved compounds against Candida deuterogattii, and L-type calcium channel blockers were identified as having broad-spectrum activity; specifically, nisoldipine showed efficacy against 9 fungal species from 4 genera, including Candida species (66). The Calderone group screened a library of around 3,000 compounds, provided by the Developmental Therapeutics Program of the NIH/NCI, against a panel of multiple fungal species, including Candida (67). These researchers identified NSC319726, an anticancer drug, as an effective growth inhibitor of C. albicans and other fungal species, including A. fumigatus and C. neoformans. Moreover, this drug was shown to display synergy with other commonly used antifungals (67). The Rodrigues laboratory screened the NIH clinical collection of 727 compounds against C. neoformans and, among the initial hits, identified mebendazole as the most effective repositionable drug known to be able to penetrate the brain in animal models, pointing to its potential to be repurposed for the treatment of cryptococcosis (68), and so selected this as the most promising candidate. A total of 1,280 drugs in the Library of Pharmacologically Active Compounds (LOPAC1280) was screened using an antifungal susceptibility test to identify inhibitors of C. albicans growth (69). Initial hits from this screen had fungistatic (26 compounds) or fungicidal (9 compounds) effects. Five main hits were then tested for their inhibitory activity against different Candida species under both planktonic and biofilm growing conditions, and compound CV-3988 emerged as the leading repositionable candidate because of its potency and lack of toxicity (69). The same LOPAC library was screened against C. neoformans by the Williamson group using an HTS technique in order to identify fungicidal compounds under nutrient-deprived conditions, leading to the identification of a chemical scaffold, 10058-F4, with potent fungicidal activity at low micromolar concentrations (70). A total 1,068 compounds in the L4200 chemical library (TargetMol) were screened in search for those displaying antifungal activity against C. albicans in a standard growth inhibition assay (71), leading to the identification of robenidine, an FDA-approved drug for the treatment of coccidian infections in animals, with subsequent experiments indicating that the drug displayed promising broad antifungal activity against other yeasts and Aspergillus.
A complementary approach has been the screening of repurposing libraries in search for compounds that potentiate the activity of current, clinically used antifungal agents. One such screen, using the Prestwick Chemical library, was performed by the Wright group to identify drugs that could be combined with fluconazole to improve its antifungal activity against Candida and Cryptococcus strains and the model yeast Saccharomyces (72). These researchers found that trifluoperazine, terbinafine, sertraline, ketoconazole, and caspofungin had a synergistic effect when combined with fluconazole against C. albicans. The Wright group also found that combining sertraline with terbinafine, trifluoperazine with ketoconazole, and ketoconazole with sertraline resulted in synergistic effects, and they demonstrated that sertraline increased the susceptibility of C. albicans and C. parapsilosis fluconazole-resistant strains to fluconazole (72). The same group screened the McMaster Bioactives collection of around 3,600 compounds derived from commercial sources against Saccharomyces cerevisiae, Schizosaccharomyces pombe, C. albicans, and C. neoformans in search for potentiators of the activity of six different antifungals (fluconazole, caspofungin, amphotericin B, terbinafine, benomyl, and cyprodinil) (73). Altogether, results from this HTS generated a deep reservoir of interactions in the antifungal space termed by these authors as the “antifungal combinations matrix.” For example, the screen identified amiodarone hydrochloride, asiatic acid, clofazimine, and cyclosporine as having synergy with caspofungin for C. albicans, whereas tomatidine was identified as having synergy with fluconazole. After performing further drug combination tests against C. albicans clinical isolates, these researchers identified chlorhexidine, tomatidine, and hypocrellin A as being able to increase the susceptibility of these isolates to fluconazole. In the end, they chose clofazimine, an antimycobacterial drug that had not yet been described as having antifungal activity, as their main hit because of its broad-spectrum antifungal activity when used in combination with fluconazole and caspofungin (73). Another screen was performed by the Thevissen laboratory, using the Pharmakon 1600 repositioning library, to identify compounds that act synergistically with miconazole in the treatment of mature C. albicans biofilms. The screen resulted in the identification of artesunate (belonging to the family of artemisinins, clinically used in the treatment of malaria) as the most promising drug acting as a potentiator of azole activity against highly resistant preformed C. albicans biofilms (74).
FUTURE DIRECTIONS AND CONCLUSIONS
There is no question that we need new antifungal agents. Drug repurposing is less costly, less time-consuming, and more likely to succeed than de novo drug discovery, and this approach can expedite the transition from the bench to the bedside. This review has focused mostly on the physiologically relevant phenotypic screening of different repurposing collections in order to find known drugs with previously undetected antifungal activity. As mentioned above, the different screens conducted to date have led to the identification of several drugs with novel antifungal activity, some of which display a very promising spectrum of antifungal activity. However, we note that most of this work represents the initial steps in repurposing programs started by different groups of investigators. As such, relatively little information on antifungal activity is still available for the majority of hit compounds identified during these screenings, and their repositioning as antifungal agents will require further work to confirm their promise. We anticipate that some of the currently identified repositionable compounds will be subjected to follow-up studies, including further characterization of their in vitro antifungal activities, preclinical studies such as in vivo activity in clinically relevant animal models of fungal infection, and hopefully clinical trials in the not so distant future. Critical questions that will need to be addressed are those related to in vitro/in vivo correlation, for which the efficacy of leading repositionable compounds will need to be tested in clinically relevant models of fungal infections, as well as those addressing key toxicological and pharmacokinetic/pharmacodynamic principles of exposure at the site of action, target binding, and expression of functional pharmacological activity—the so-called “three pillars” of drug development (75). For example, if a medicine is going to be repurposed for the treatment of cryptococcal meningitis, the molecule must be capable of crossing the blood-brain barrier. Also, the new activity identified for a repurposed drug may not be potent enough for the intended clinical new antifungal indication, resulting in lower subinhibitory concentrations that may even lead to the development of resistance. In addition, potentially adverse side effects associated with the original therapeutic indication of the repurposed drug need to be taken into account. Thus, despite the theoretically facilitated pathway for repurposed drugs, it is imperative that the efficacy of the drugs is demonstrated in their newly discovered indications, in this case as antifungals. We also anticipate that more repurposing screens will be performed in the near future, including against different fungal species besides Candida and Cryptococcus, and hopefully expand to those emerging fungi for which current antifungal drugs are not effective. In addition to all these scientific considerations, repurposing represents an ideal milieu for collaboration and for the establishment of new partnerships between academia, nonprofit organizations, governments, and international organizations to fill the existing void in the antifungal drug development space, with emphasis on the accelerated development of antifungals that we so desperately need.
ACKNOWLEDGMENTS
Repurposing work in the laboratory is supported by National Institutes of Health grant R21AI140823 from the National Institute of Allergy and Infectious Diseases to J.L.L.-R. Additional support was provided by the Margaret Batts Tobin Foundation, San Antonio, TX. G.W. was the recipient of a Science, Mathematics, and Research for Transformation (SMART) fellowship provided by the Department of Defense.
REFERENCES
- 1.Odds FC, Brown AJ, Gow NA. 2003. Antifungal agents: mechanisms of action. Trends Microbiol 11:272–279. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 2.Hughes JP, Rees S, Kalindjian SB, Philpott KL. 2011. Principles of early drug discovery. Br J Pharmacol 162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams CP, Brantner VV. 2006. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood) 25:420–428. doi: 10.1377/hlthaff.25.2.420. [DOI] [PubMed] [Google Scholar]
- 4.DiMasi JA, Hansen RW, Grabowski HG. 2003. The price of innovation: new estimates of drug development costs. J Health Econ 22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 5.Boguski MS, Mandl KD, Sukhatme VP. 2009. Drug discovery: repurposing with a difference. Science 324:1394–1395. doi: 10.1126/science.1169920. [DOI] [PubMed] [Google Scholar]
- 6.Ashburn TT, Thor KB. 2004. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 7.Wiederhold NP. 2018. The antifungal arsenal: alternative drugs and future targets. Int J Antimicrob Agents 51:333–339. doi: 10.1016/j.ijantimicag.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Perfect JR. 2017. The antifungal pipeline: a reality check. Nat Rev Drug Discov 16:603–616. doi: 10.1038/nrd.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oprea TI, Mestres J. 2012. Drug repurposing: far beyond new targets for old drugs. AAPS J 14:759–763. doi: 10.1208/s12248-012-9390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue H, Li J, Xie H, Wang Y. 2018. Review of drug repositioning approaches and resources. Int J Biol Sci 14:1232–1244. doi: 10.7150/ijbs.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corsello SM, Bittker JA, Liu Z, Gould J, McCarren P, Hirschman JE, Johnston SE, Vrcic A, Wong B, Khan M, Asiedu J, Narayan R, Mader CC, Subramanian A, Golub TR. 2017. The Drug Repurposing Hub: a next-generation drug library and information resource. Nat Med 23:405–408. doi: 10.1038/nm.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobinick EL. 2009. The value of drug repositioning in the current pharmaceutical market. Drug News Perspect 22:119–125. doi: 10.1358/dnp.2009.22.2.1303818. [DOI] [PubMed] [Google Scholar]
- 13.Katragkou A, Roilides E, Walsh TJ. 2016. Can repurposing of existing drugs provide more effective therapies for invasive fungal infections? Expert Opin Pharmacother 17:1179–1182. doi: 10.1080/14656566.2016.1186647. [DOI] [PubMed] [Google Scholar]
- 14.Rehman W, Arfons LM, Lazarus HM. 2011. The rise, fall and subsequent triumph of thalidomide: lessons learned in drug development. Ther Adv Hematol 2:291–308. doi: 10.1177/2040620711413165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schein CH. 2020. Repurposing approved drugs on the pathway to novel therapies. Med Res Rev 40:586–605. doi: 10.1002/med.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nosengo N. 2016. Can you teach old drugs new tricks? Nature 534:314–316. doi: 10.1038/534314a. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein I, Burnett AL, Rosen RC, Park PW, Stecher VJ. 2019. The serendipitous story of sildenafil: an unexpected oral therapy for erectile dysfunction. Sex Med Rev 7:115–128. doi: 10.1016/j.sxmr.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Williams SD. 2010. A turning point in the fight against HIV. ChemMedChem 5:1799–1801. doi: 10.1002/cmdc.201000338. [DOI] [PubMed] [Google Scholar]
- 19.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, Pirmohamed M. 2019. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 20.Talevi A, Bellera CL. 2020. Challenges and opportunities with drug repurposing: finding strategies to find alternative uses of therapeutics. Expert Opin Drug Discov 15:397–401. doi: 10.1080/17460441.2020.1704729. [DOI] [PubMed] [Google Scholar]
- 21.Brown K. 2017. Repurposing old drugs for new uses. DePaul J Art Technol Intellect Prop Law 28:1–35. [Google Scholar]
- 22.Oprea TI, Bauman JE, Bologa CG, Buranda T, Chigaev A, Edwards BS, Jarvik JW, Gresham HD, Haynes MK, Hjelle B, Hromas R, Hudson L, Mackenzie DA, Muller CY, Reed JC, Simons PC, Smagley Y, Strouse J, Surviladze Z, Thompson T, Ursu O, Waller A, Wandinger-Ness A, Winter SS, Wu Y, Young SM, Larson RS, Willman C, Sklar LA. 2011. Drug repurposing from an academic perspective. Drug Discov Today Ther Strateg 8:61–69. doi: 10.1016/j.ddstr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cha Y, Erez T, Reynolds IJ, Kumar D, Ross J, Koytiger G, Kusko R, Zeskind B, Risso S, Kagan E, Papapetropoulos S, Grossman I, Laifenfeld D. 2018. Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol 175:168–180. doi: 10.1111/bph.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grunberg E, Titsworth E, Bennett M. 1963. Chemotherapeutic activity of 5-fluorocytosine. Antimicrob Agents Chemother 161:566–568. [PubMed] [Google Scholar]
- 25.Steinbach WJ, Reedy JL, Cramer RA Jr, Perfect JR, Heitman J. 2007. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol 5:418–430. doi: 10.1038/nrmicro1680. [DOI] [PubMed] [Google Scholar]
- 26.Wong GK, Griffith S, Kojima I, Demain AL. 1998. Antifungal activities of rapamycin and its derivatives, prolylrapamycin, 32-desmethylrapamycin, and 32-desmethoxyrapamycin. J Antibiot 51:487–491. doi: 10.7164/antibiotics.51.487. [DOI] [PubMed] [Google Scholar]
- 27.Bastidas RJ, Shertz CA, Lee SC, Heitman J, Cardenas ME. 2012. Rapamycin exerts antifungal activity in vitro and in vivo against Mucor circinelloides via FKBP12-dependent inhibition of Tor. Eukaryot Cell 11:270–281. doi: 10.1128/EC.05284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz MC, Goldstein AL, Blankenship J, Del Poeta M, Perfect JR, McCusker JH, Bennani YL, Cardenas ME, Heitman J. 2001. Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR. Antimicrob Agents Chemother 45:3162–3170. doi: 10.1128/AAC.45.11.3162-3170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchetti O, Entenza JM, Sanglard D, Bille J, Glauser MP, Moreillon P. 2000. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob Agents Chemother 44:2932–2938. doi: 10.1128/AAC.44.11.2932-2938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Sun S, Guo Q, Ma L, Shi C, Su L, Li H. 2008. In vitro interaction between azoles and cyclosporin A against clinical isolates of Candida albicans determined by the chequerboard method and time-kill curves. J Antimicrob Chemother 61:577–585. doi: 10.1093/jac/dkm493. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Liu W, Tan J, Sun Y, Wan Z, Li R. 2013. Antifungal activity of geldanamycin alone or in combination with fluconazole against Candida species. Mycopathologia 175:273–279. doi: 10.1007/s11046-012-9612-1. [DOI] [PubMed] [Google Scholar]
- 32.Li L, An M, Shen H, Huang X, Yao X, Liu J, Zhu F, Zhang S, Chen S, He L, Zhang J, Zou Z, Jiang Y. 2015. The non-geldanamycin Hsp90 inhibitors enhanced the antifungal activity of fluconazole. Am J Transl Res 7:2589–2602. [PMC free article] [PubMed] [Google Scholar]
- 33.Zhai B, Wu C, Wang L, Sachs MS, Lin X. 2012. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother 56:3758–3766. doi: 10.1128/AAC.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nayak R, Xu J. 2010. Effects of sertraline hydrochloride and fluconazole combinations on Cryptococcus neoformans and Cryptococcus gattii. Mycology 1:99–105. doi: 10.1080/21501203.2010.487054. [DOI] [Google Scholar]
- 35.Villanueva-Lozano H, Treviño-Rangel RdJ, González GM, Hernández-Rodríguez PA, Camacho-Ortiz A, Castillo-Reyna L, Galindo-Alvarado SG, Martínez-Reséndez MF. 2018. Clinical evaluation of the antifungal effect of sertraline in the treatment of cryptococcal meningitis in HIV patients: a single Mexican center experience. Infection 46:25–30. doi: 10.1007/s15010-017-1059-3. [DOI] [PubMed] [Google Scholar]
- 36.Collaborators, University of Minnesota. 2013. Adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis (ASTRO-CM). U.S. National Library of Medicine, Bethesda, MD: https://clinicaltrials.gov/ct2/show/results/NCT01802385. Accessed 13 January 2020. [Google Scholar]
- 37.Baxter BK, DiDone L, Ogu D, Schor S, Krysan DJ. 2011. Identification, in vitro activity, and mode of action of phosphoinositide-dependent-1 kinase inhibitors as antifungal molecules. ACS Chem Biol 6:502–510. doi: 10.1021/cb100399x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koselny K, Green J, DiDone L, Halterman JP, Fothergill AW, Wiederhold NP, Patterson TF, Cushion MT, Rappelye C, Wellington M, Krysan DJ. 2016. The celecoxib derivative AR-12 has broad-spectrum antifungal activity in vitro and improves the activity of fluconazole in a murine model of cryptococcosis. Antimicrob Agents Chemother 60:7115–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hai TP, Van AD, Ngan NTT, Nhat LTH, Lan NPH, Vinh Chau NV, Thwaites GE, Krysan D, Day JN. 2019. The combination of tamoxifen with amphotericin B, but not with fluconazole, has synergistic activity against the majority of clinical isolates of Cryptococcus neoformans. Mycoses 62:818–825. doi: 10.1111/myc.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolan K, Montgomery S, Buchheit B, Didone L, Wellington M, Krysan DJ. 2009. Antifungal activity of tamoxifen: in vitro and in vivo activities and mechanistic characterization. Antimicrob Agents Chemother 53:3337–3346. doi: 10.1128/AAC.01564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montoya MC, Beattie S, Alden KM, Krysan DJ. 2020. Derivatives of the antimalarial drug mefloquine are broad-spectrum antifungal molecules with activity against drug-resistant clinical isolates. Antimicrob Agents Chemother 64:e02331-19. doi: 10.1128/AAC.02331-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhai B, Zhou H, Yang L, Zhang J, Jung K, Giam CZ, Xiang X, Lin X. 2010. Polymyxin B, in combination with fluconazole, exerts a potent fungicidal effect. J Antimicrob Chemother 65:931–938. doi: 10.1093/jac/dkq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim K, Zilbermintz L, Martchenko M. 2015. Repurposing FDA approved drugs against the human fungal pathogen, Candida albicans. Ann Clin Microbiol Antimicrob 14:32. doi: 10.1186/s12941-015-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiDone L, Scrimale T, Baxter BK, Krysan DJ. 2010. A high-throughput assay of yeast cell lysis for drug discovery and genetic analysis. Nat Protoc 5:1107–1114. doi: 10.1038/nprot.2010.47. [DOI] [PubMed] [Google Scholar]
- 45.Butts A, DiDone L, Koselny K, Baxter BK, Chabrier-Rosello Y, Wellington M, Krysan DJ. 2013. A repurposing approach identifies off-patent drugs with fungicidal cryptococcal activity, a common structural chemotype, and pharmacological properties relevant to the treatment of cryptococcosis. Eukaryot Cell 12:278–287. doi: 10.1128/EC.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Courchesne WE. 2002. Characterization of a novel, broad-based fungicidal activity for the antiarrhythmic drug amiodarone. J Pharmacol Exp Ther 300:195–199. doi: 10.1124/jpet.300.1.195. [DOI] [PubMed] [Google Scholar]
- 47.Wall G, Montelongo-Jauregui D, Vidal Bonifacio B, Lopez-Ribot JL, Uppuluri P. 2019. Candida albicans biofilm growth and dispersal: contributions to pathogenesis. Curr Opin Microbiol 52:1–6. doi: 10.1016/j.mib.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. 2009. Our current understanding of fungal biofilms. Crit Rev Microbiol 35:340–355. doi: 10.3109/10408410903241436. [DOI] [PubMed] [Google Scholar]
- 49.Siles SA, Srinivasan A, Pierce CG, Lopez-Ribot JL, Ramasubramanian AK. 2013. High-throughput screening of a collection of known pharmacologically active small compounds for identification of Candida albicans biofilm inhibitors. Antimicrob Agents Chemother 57:3681–3687. doi: 10.1128/AAC.00680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiederhold NP, Patterson TF, Srinivasan A, Chaturvedi AK, Fothergill AW, Wormley FL, Ramasubramanian AK, Lopez-Ribot JL. 2017. Repurposing auranofin as an antifungal: in vitro activity against a variety of medically important fungi. Virulence 8:138–142. doi: 10.1080/21505594.2016.1196301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yousfi H, Cassagne C, Ranque S, Rolain JM, Bittar F. 2019. Repurposing of ribavirin as an adjunct therapy against invasive Candida strains in an in vitro study. Antimicrob Agents Chemother 63:e00263-19. doi: 10.1128/AAC.00263-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ostrowsky B, Greenko J, Adams E, Quinn M, O’Brien B, Chaturvedi V, Berkow E, Vallabhaneni S, Forsberg K, Chaturvedi S, Lutterloh E, Blog D, C. auris Investigation Work Group. 2020. Candida auris isolates resistant to three classes of antifungal medications: New York, 2019. MMWR Morb Mortal Wkly Rep 69:6–9. doi: 10.15585/mmwr.mm6901a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lone SA, Ahmad A. 2019. Candida auris: the growing menace to global health. Mycoses 62:620–637. doi: 10.1111/myc.12904. [DOI] [PubMed] [Google Scholar]
- 54.Wall G, Chaturvedi AK, Wormley FL Jr, Wiederhold NP, Patterson HP, Patterson TF, Lopez-Ribot JL. 2018. Screening a repurposing library for inhibitors of multidrug-resistant Candida auris identifies ebselen as a repositionable candidate for antifungal drug development. Antimicrob Agents Chemother 62:e01084-18. doi: 10.1128/AAC.01084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Oliveira HC, Monteiro MC, Rossi SA, Peman J, Ruiz-Gaitan A, Mendes-Giannini MJS, Mellado E, Zaragoza O. 2019. Identification of off-patent compounds that present antifungal activity against the emerging fungal pathogen Candida auris. Front Cell Infect Microbiol 9:83. doi: 10.3389/fcimb.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samantaray S, Correia JN, Garelnabi M, Voelz K, May RC, Hall RA. 2016. Novel cell-based in vitro screen to identify small-molecule inhibitors against intracellular replication of Cryptococcus neoformans in macrophages. Int J Antimicrob Agents 48:69–77. doi: 10.1016/j.ijantimicag.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mamouei Z, Alqarihi A, Singh S, Xu S, Mansour MK, Ibrahim AS, Uppuluri P. 2018. Alexidine dihydrochloride has broad-spectrum activities against diverse fungal pathogens. mSphere 3:e00539-18. doi: 10.1128/mSphere.00539-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yousfi H, Ranque S, Cassagne C, Rolain JM, Bittar F. 2020. Identification of repositionable drugs with novel antimycotic activity by screening Prestwick Chemical Library against emerging invasive molds. J Glob Antimicrob Resist 21:314–317. doi: 10.1016/j.jgar.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Vila T, Lopez-Ribot JL. 2017. Screening the pathogen box for identification of Candida albicans biofilm inhibitors. Antimicrob Agents Chemother 61:e02006-16. doi: 10.1128/AAC.02006-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wall G, Herrera N, Lopez-Ribot JL. 2019. Repositionable compounds with antifungal activity against multidrug-resistant Candida auris identified in the Medicines for Malaria Ventures Pathogen Box. J Fungi 5:92. doi: 10.3390/jof5040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayer FL, Kronstad JW. 2017. Discovery of a novel antifungal agent in the Pathogen Box. mSphere 2:e00120-17. doi: 10.1128/mSphere.00120-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vila T, Ishida K, Seabra SH, Rozental S. 2016. Miltefosine inhibits Candida albicans and non-albicans Candida spp. biofilms and impairs the dispersion of infectious cells. Int J Antimicrob Agents 48:512–520. doi: 10.1016/j.ijantimicag.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 63.Vila TV, Chaturvedi AK, Rozental S, Lopez-Ribot JL. 2015. In vitro activity of miltefosine against Candida albicans under planktonic and biofilm growth conditions and in vivo efficacy in a murine model of oral candidiasis. Antimicrob Agents Chemother 59:7611–7620. doi: 10.1128/AAC.01890-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jung EH, Meyers DJ, Bosch J, Casadevall A. 2018. Novel antifungal compounds discovered in Medicines for Malaria Ventures Malaria Box. mSphere 3:e00537-17. doi: 10.1128/mSphere.00537-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stylianou M, Kulesskiy E, Lopes JP, Granlund M, Wennerberg K, Urban CF. 2014. Antifungal application of nonantifungal drugs. Antimicrob Agents Chemother 58:1055–1062. doi: 10.1128/AAC.01087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Truong M, Monahan LG, Carter DA, Charles IG. 2018. Repurposing drugs to fast-track therapeutic agents for the treatment of cryptococcosis. PeerJ 6:e4761. doi: 10.7717/peerj.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun N, Li D, Zhang Y, Killeen K, Groutas W, Calderone R. 2017. Repurposing an inhibitor of ribosomal biogenesis with broad anti-fungal activity. Sci Rep 7:17014. doi: 10.1038/s41598-017-17147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joffe LS, Schneider R, Lopes W, Azevedo R, Staats CC, Kmetzsch L, Schrank A, Del Poeta M, Vainstein MH, Rodrigues ML. 2017. The anti-helminthic compound mebendazole has multiple antifungal effects against Cryptococcus neoformans. Front Microbiol 8:535. doi: 10.3389/fmicb.2017.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watamoto T, Egusa H, Sawase T, Yatani H. 2015. Screening of pharmacologically active small molecule compounds identifies antifungal agents against Candida biofilms. Front Microbiol 6:1453. doi: 10.3389/fmicb.2015.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rabjohns JLA, Park YD, Dehdashti J, Henderson C, Zelazny A, Metallo SJ, Zheng W, Williamson PR. 2014. A high-throughput screening assay for fungicidal compounds against Cryptococcus neoformans. J Biomol Screen 19:270–277. doi: 10.1177/1087057113496847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mei Y, Jiang T, Zou Y, Wang Y, Zhou J, Li J, Liu L, Tan J, Wei L, Li J, Dai H, Peng Y, Zhang L, Lopez-Ribot JL, Shapiro RS, Chen C, Liu N-N, Wang H. 2020. FDA-approved drug library screening identifies robenidine as a repositionable antifungal. Front Microbiol 11:996. doi: 10.3389/fmicb.2020.00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Ejim L, Rossi L, De Pascale G, Curak J, Brown E, Tyers M, Wright GD. 2011. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol 7:499. doi: 10.1038/msb.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robbins N, Spitzer M, Yu T, Cerone RP, Averette AK, Bahn YS, Heitman J, Sheppard DC, Tyers M, Wright GD. 2015. An antifungal combination matrix identifies a rich pool of adjuvant molecules that enhance drug activity against diverse fungal pathogens. Cell Rep 13:1481–1492. doi: 10.1016/j.celrep.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Cremer K, Lanckacker E, Cools TL, Bax M, De Brucker K, Cos P, Cammue BP, Thevissen K. 2015. Artemisinins, new miconazole potentiators resulting in increased activity against Candida albicans biofilms. Antimicrob Agents Chemother 59:421–426. doi: 10.1128/AAC.04229-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morgan P, Van Der Graaf PH, Arrowsmith J, Feltner DE, Drummond KS, Wegner CD, Street S. 2012. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug Discov Today 17:419–424. doi: 10.1016/j.drudis.2011.12.020. [DOI] [PubMed] [Google Scholar]