Concerns regarding increased prevalence of daptomycin (DAP)-resistant strains necessitate novel therapies for Enterococcus faecium infections. Obligately lytic bacteriophages are viruses that target, infect, and kill bacterial cells. Limited studies have evaluated phage-antibiotic combinations against E. faecium. After an initial screen of eight E. faecium strains, three strains with varying DAP/phage susceptibilities were selected for further experiments.
KEYWORDS: bacteriophages, bacteriophage-antibiotic combinations, daptomycin, Enterococcus faecium
ABSTRACT
Concerns regarding increased prevalence of daptomycin (DAP)-resistant strains necessitate novel therapies for Enterococcus faecium infections. Obligately lytic bacteriophages are viruses that target, infect, and kill bacterial cells. Limited studies have evaluated phage-antibiotic combinations against E. faecium. After an initial screen of eight E. faecium strains, three strains with varying DAP/phage susceptibilities were selected for further experiments. Phage-to-strain specificity contributed to synergy with antibiotics by time-kill analyses and was associated with lower development of phage resistance.
INTRODUCTION
Vancomycin-resistant enterococcal (VRE) bloodstream infections have been associated with mortality rates of >30% (1, 2). Enterococcus faecium is the most problematic species given that >80% and >90% of strains have been shown to be vancomycin (VAN)- and ampicillin (AMP)-resistant, respectively (3–6). Vancomycin-resistant E. faecium infections are often treated with daptomycin (DAP), because DAP has potent in vitro bactericidal activity against vancomycin-resistant E. faecium isolates (7). Unfortunately, DAP resistance is reported to emerge during treatment of enterococcal infections, and prevalence of this resistance is increasing (8).
Although mechanisms of DAP resistance have not been fully elucidated, relevant genetic pathways in enterococci have been found to involve mutations in liaFSR (three-component regulatory system involved in cellular membrane stress response) (9–11). Emerging data suggest that E. faecium isolates with mutations that increase proclivity for DAP resistance selection (e.g., liaFSR) may not respond to DAP monotherapy, regardless of DAP exposure (12–14). Antibiotic combination therapy, particularly with β-lactams, has been advocated as an alternative strategy to improve patient outcomes and decrease DAP resistance emergence (15, 16). We have demonstrated that DAP–β-lactam combinations can be highly synergistic against E. faecium infection, but this is strain and β-lactam specific (14; unpublished data). Furthermore, DAP–β-lactam combinations have been shown to fail in clinical practice (17). With limited efficacious options, therapeutic alternatives are urgently needed to treat E. faecium infections refractory to DAP–β-lactam combinations.
Obligately lytic bacteriophages (phages) are viruses that target, infect, and kill bacterial cells (18). Multiple positive interactions have been described with phage-antibiotic combinations, including enhanced bacterial killing and alterations in the emergence of antibiotic/phage resistance (19–21). Although few reports have evaluated Enterococcus faecalis, limited to no studies have evaluated phage-antibiotic combinations against E. faecium, the most difficult-to-treat species of enterococci (22–24).
The objective of this study was to evaluate the ability of phage plus DAP alone and in addition to DAP plus various β-lactams to improve bacterial killing and prevent resistance development in E. faecium strains with varying susceptibilities to DAP and phage. Our primary hypothesis was that initial bacterial phage susceptibility would play a role in synergy with antibiotics and prevention of emergence of phage resistance.
E. faecium bacteriophage 113 (ATCC 19950-B1) and propagating organism E. faecium (ATCC 19950) were purchased commercially from ATCC (Manassas, VA). DAP, AMP, and ertapenem (ERT) powder were purchased commercially from Sigma Chemical Company (St. Louis, MO), and ceftaroline (CPT) analytical powder was obtained from Allergan Pharmaceuticals (Parsippany, NJ). Mueller-Hinton broth II (Difco, Detroit, MI) with 50 mg/liter calcium and 12.5 mg/liter magnesium was used for susceptibility testing and time-kill analyses (TKAs). All MICs were performed following CLSI guidelines (25). The susceptibility of phage ATCC 19950-B1 against eight randomly selected E. faecium strains was evaluated as previously described using a modified small-drop agar overlay method (26). After phage quantification, high, medium, and low phage susceptibility were defined as phage counts of >107, between 103 and 107, and <103 PFU/ml, respectively. Phage nonsusceptibility was defined as no visual detection of individual phage plaques and/or no bacterial lawn clearance. Strains fully resistant to the phage were not utilized in further experiments.
TKAs were performed as previously described using inocula that were obtained from stationary-phase cultures and with two replicates obtained at each time point (27). All antimicrobials were tested at 0.5× and 0.25× MIC or maximum concentration of free drug in serum (fCmax), whichever was lower. The fCmax concentrations utilized throughout this evaluation were 17 μg/ml for CPT and 15.5 μg/ml for ERT; AMP fCmax did not need to be utilized. Given that phage-induced bacterial killing is strain specific, phage and antibiotic dose optimizations were performed to evaluate the theoretical multiplicity of infection (MOI) (the number of phages added to the well divided by the number of bacteria within the same well) that produced optimal observations of synergy with antibiotic combinations at the two previously listed suboptimal exposures (0.25× and 0.5× MIC) (28). Any samples that contained phage were centrifuged at 12,000 rpm for 2 min with the supernatant removed and replaced with normal saline (0.9% sodium chloride) to reduce the concentration of unadsorbed phages. Synergy was defined as a ≥2-log10-CFU/ml kill compared to the most effective agent (or double-combination regimen) alone at 24 h. Bactericidal activity was defined as a ≥3-log10-CFU/ml reduction from baseline. The emergence of DAP/phage resistance was determined as previously described by using the 24-h TKA liquid sample (14, 26, 29, 30). Phage resistance was scored from the spots as resistant (no visible effect of phage versus phosphate-buffered saline control), intermediate (bacteria present but phage activity visible), and susceptible (<10 CFU in spot) (30). Phage counts were assessed using a modification of the small-drop agar overlay method (to determine whether the presence [or lack] of antibiotics had an impact of phage growth) (26, 29, 30).
Phage activity is well documented to be bacterial strain specific (28). Noting this, we sought to compare the activity of commonly utilized antibiotics for refractory E. faecium infections in combination with phage for strains that exhibited variability susceptibility to phage and DAP. Strains with low (R496; DAP MIC, 32 μg/ml), medium (HOU503; DAP MIC, 2 μg/ml), and high (R497; DAP MIC, 16 μg/ml) phage susceptibility were selected for additional experiments due to the presence of liaFSR mutations and varying DAP/phage susceptibility. Other antibiotic MICs are listed in Table 1.
TABLE 1.
Initial antimicrobial MICs prior to 24-h time-kill analyses
| Antimicrobiala | MIC (μg/ml) for: |
||
|---|---|---|---|
| R497 (high susceptibility to phage) | HOU503 (medium susceptibility to phage) | R496 (low susceptibility to phage) | |
| DAP | 16 | 2 | 32 |
| AMP | 128 | 128 | 64 |
| CPT | >64 | 32 | >64 |
| ERT | >64 | >64 | >64 |
DAP, daptomycin; AMP, ampicillin; CPT, ceftaroline; ERT, ertapenem.
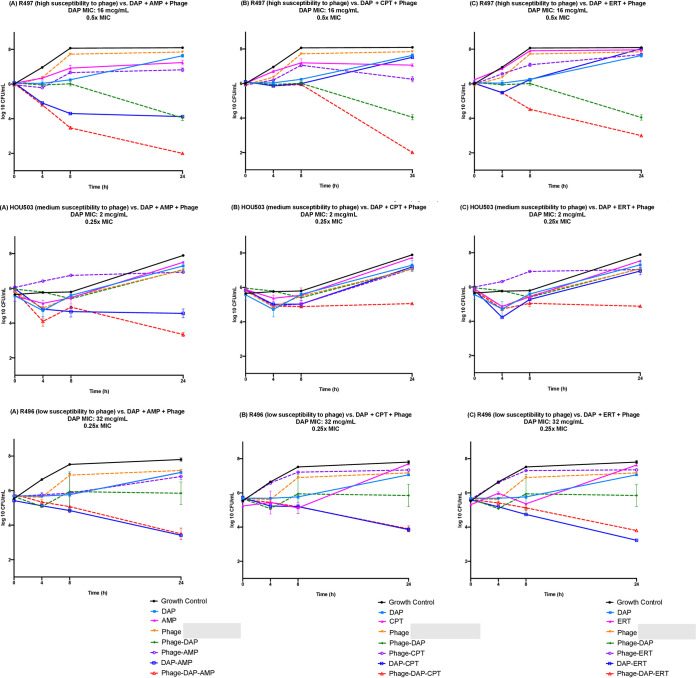
Against DAP-resistant R497 harboring liaFSR substitutions, synergistic/bactericidal effects were seen with DAP-AMP-phage and DAP-CPT-phage, whereas DAP-phage exhibited synergy. Synergistic effects against HOU503 (a DAP-tolerant isolate, also with mutations in liaFSR) were noted with DAP-CPT-phage and DAP-ERT-phage. No enhancement was noted with phage addition to antibiotics against R496 (DAP-resistant isolate with mutations in liaFSR) (Fig. 1). No further DAP resistance emerged in R496 or R497 in any TKA regimens. However, DAP MICs of HOU503 increased from 2 to 32 μg/ml for all regimens except DAP-AMP-phage. We have previously shown in a pharmacokinetic/pharmacodynamic simulated endocardial vegetation (SEV) model that AMP, CPT, and ERT prevented the emergence of DAP resistance in HOU503 at humanized dose exposures (14). We performed TKA at the same humanized exposures, and the emergence of DAP resistance was prevented when β-lactam combination therapy was utilized. The development of phage resistance was less pronounced in strains with higher initial phage susceptibility (Table 2). No meaningful differences were observed in phage quantification. (Supplemental tables may be found at https://www.dropbox.com/s/8ah3ftnocbervb7/E.%20fcm%20-%20Phage%20-%20Supplemental.pdf?dl=0.)
FIG 1.
Time-kill analyses of DAP-AMP, DAP-CPT, and DAP-ERT alone and in combination with bacteriophage against R497 at 0.5× MIC or fCmax (DAP, 8 μg/ml; AMP, 64 μg/ml; CPT, 17 μg/ml; ERT, 15.5 μg/ml) and a theoretical MOI of 0.1, HOU503 at 0.25× MIC or fCmax (DAP, 0.5 μg/ml; AMP, 32 μg/ml; CPT, 8 μg/ml; ERT, 15.5 μg/ml) and a theoretical MOI of 1.0, and R496 at 0.25× MIC or fCmax (DAP, 8 μg/ml; AMP, 16 μg/ml; CPT,17 μg/ml; ERT, 15.5 μg/ml) and a theoretical MOI of 1.0. Synergy was defined as a ≥2-log10-CFU/ml kill compared to the most effective agent (or double-combination regimen) alone at 24 h. Bactericidal activity was defined as a ≥3-log10-CFU/ml reduction from baseline. For R497, bactericidal and synergistic activity was noted with the addition of phage to DAP-AMP (A) and DAP-CPT (B), whereas synergistic activity was noted with the addition of phage to DAP (A to C). For HOU503, synergistic effects were noted with the triple combinations DAP-CPT-phage (B) and DAP-ERT-phage (C). For R496, no enhancement was noted with the addition of phage to DAP-AMP (A), DAP-CPT (B), or DAP-ERT (C). Values are means ± standard deviations.
TABLE 2.
Phage susceptibility after 24-h time-kill analyses
| Regimena | Phage resistanceb for: |
||
|---|---|---|---|
| R497 | HOU503 | R496 | |
| P | I | I | R |
| P-DAP | I | I | R |
| P-AMP | I | I | R |
| P-CPT | I | I | R |
| P-ERT | I | R | R |
| P-DAP-AMP | S | I | R |
| P-DAP-CPT | S | R | R |
| P-DAP-ERT | S | R | R |
DAP, daptomycin; AMP, ampicillin; CPT, ceftaroline; ERT, ertapenem; P, phage.
S, susceptible; I, intermediate; R, resistant.
Enterococcus spp. isolates are leading causes of nosocomial infections internationally, with multidrug-resistant (MDR) strains increasing in prevalence. Daptomycin is a key therapeutic option against these infections. However, the increase in MDR E. faecium strains with reduced susceptibility to VAN, AMP, and DAP coupled with a sparse antibiotic development pipeline for VRE has led to many challenging clinical scenarios. Combination therapy with β-lactams has been advocated as an alternative strategy (16). Although positive interactions have been described, these enhancements have been shown to be strain and β-lactam specific (14, 31–33; unpublished data). This is extremely problematic in the clinical setting, because most practitioners utilize a “best guess” scenario when choosing which β-lactam to add upfront or in recalcitrant infections.
In light of some failures observed with antibiotic combination therapy, the novel addition of phages to antibiotics has been promoted as another alternative strategy. Our report adds to the growing body of literature describing positive interactions with phage-antibiotic combinations (18–21). Not only did we show synergistic activity with phage in addition to two antibiotics, but we also showed synergy with DAP-phage against a DAP-resistant strain (R497) and that phage-to-strain specificity contributed to synergy with antibiotics. Although we did not show that subinhibitory concentrations of antibiotics fully prevented the emergence of phage resistance in all strains tested, there seemed to be a relationship between the initial phage susceptibility of each strain and the subsequent emergence of phage resistance.
Limitations of this study include utilizing monophage therapy in comparison to evaluating phage cocktails. Furthermore, although we performed phage susceptibility testing on eight E. faecium strains, we only conducted further examinations on three strains. Finally, it is important to note that traditional methodology of TKA entails dosing antimicrobials at the beginning of the experiments without repeated administration. Whether repeated phage administrations would enhance bacterial killing or prevent the emergence of resistance in these experiments is yet to be elucidated.
In summary, we analyzed phage specificity as it relates to synergistic activity with antibiotics against varying resistant strains of E. faecium as proof of concept. A phage was used that showed variable susceptibility to the strains of interest, which allowed for the observation of differences in efficacy of bacterial killing and resistance responses. The findings of this study further support that phages with enhanced activity to bacterial strains of interest may be useful in combination with antibiotics in the clinical setting. Further in vitro examinations should be performed to fully understand phage-antibiotic interactions and how these results will translate in vivo, in addition to working to develop tools to optimize the selection of antimicrobial combinations to use upfront in high-risk patients or in recalcitrant infections.
ACKNOWLEDGMENTS
We thank Allergan Pharmaceuticals for providing our laboratory with ceftaroline powder to perform the experiments.
This work was partially supported by NIAID grant R01 AI121400. C.A.A. is partially supported by NIH/NIAID grants R01 AI134637, R21 AI143229, and K24 AI121296, a UTHealth Presidential Award, and a University of Texas System STARS Award.
B.A.D. has received grant support from and consulted on behalf of Ancillia Ltd. and is partially supported by NIAID R01 AI141479. C.A.A. has received grant support from Merck, MemEd Diagnostics, and Entasis Therapeutics. M.J.R. has received research and consulting fees or participated in speaking bureaus for Allergan, Contrafect, Melinta, Merck, Shionogi, and Tetraphase and is partially supported by NIAID grant R01 AI121400.
T.M., R.K., J.C.A.-M., K.C.S., S.M., S.M.L., and G.S.C. have no conflicts of interest to disclose.
REFERENCES
- 1.Kim YJ, Jun YH, Choi HJ, You YK, Kim DG, Choi JY, Yoon SK, Kim SI. 2019. Impact of enterococcal bacteremia in liver transplant recipients. Transplant Proc 51:2766–2770. doi: 10.1016/j.transproceed.2019.02.064. [DOI] [PubMed] [Google Scholar]
- 2.Kramer TS, Remschmidt C, Werner S, Behnke M, Schwab F, Werner G, Gastmeier P, Leistner R. 2018. The importance of adjusting for enterococcus species when assessing the burden of vancomycin resistance: a cohort study including over 1,000 cases of enterococcal bloodstream infections. Antimicrob Resist Infect Control 7:133. doi: 10.1186/s13756-018-0419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Driscoll T, Crank CW. 2015. Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations, and optimal management. Infect Drug Resist 8:217–230. doi: 10.2147/IDR.S54125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathnayake IU, Hargreaves M, Huygens F. 2012. Antibiotic resistance and virulence traits in clinical and environmental Enterococcus faecalis and Enterococcus faecium isolates. Syst Appl Microbiol 35:326–333. doi: 10.1016/j.syapm.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Jabbari Shiadeh SM, Pormohammad A, Hashemi A, Lak P. 2019. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: a systematic review and meta-analysis. Infect Drug Resist 12:2713–2725. doi: 10.2147/IDR.S206084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran TT, Munita JM, Arias CA. 2015. Mechanisms of drug resistance: daptomycin resistance. Ann N Y Acad Sci 1354:32–53. doi: 10.1111/nyas.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamboj M, Cohen N, Gilhuley K, Babady NE, Seo SK, Sepkowitz KA. 2011. Emergence of daptomycin-resistant VRE: experience of a single institution. Infect Control Hosp Epidemiol 32:391–394. doi: 10.1086/659152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz L, Tran TT, Munita JM, Miller WR, Rincon S, Carvajal LP, Wollam A, Reyes J, Panesso D, Rojas NL, Shamoo Y, Murray BE, Weinstock GM, Arias CA. 2014. Whole-genome analyses of Enterococcus faecium isolates with diverse daptomycin MICs. Antimicrob Agents Chemother 58:4527–4534. doi: 10.1128/AAC.02686-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arias CA, Panesso D, McGrath DM, Qin X, Mojica MF, Miller C, Diaz L, Tran TT, Rincon S, Barbu EM, Reyes J, Roh JH, Lobos E, Sodergren E, Pasqualini R, Arap W, Quinn JP, Shamoo Y, Murray BE, Weinstock GM. 2011. Genetic basis for in vivo daptomycin resistance in Enterococci. N Engl J Med 365:892–900. doi: 10.1056/NEJMoa1011138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran TT, Panesso D, Mishra NN, Mileykovskaya E, Guan Z, Munita JM, Reyes J, Diaz L, Weinstock GM, Murray BE, Shamoo Y, Dowhan W, Bayer AS, Arias CA. 2013. Daptomycin-resistant Enterococcus faecalis diverts the antibiotic molecule from the division septum and remodels cell membrane phospholipids. mBio 4:e00281-13. doi: 10.1128/mBio.00281-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steed ME, Werth BJ, Ireland CE, Rybak MJ. 2012. Evaluation of the novel combination of high-dose daptomycin plus trimethoprim-sulfamethoxazole against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus using an in vitro pharmacokinetic/pharmacodynamics model of simulated endocardial vegetations. Antimicrob Agents Chemother 56:5709–5714. doi: 10.1128/AAC.01185-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall AD, Steed ME, Arias CA, Murray BE, Rybak MJ. 2012. Evaluation of standard- and high-dose daptomycin versus linezolid against vancomycin-resistant Enterococcus isolates in an in vitro pharmacokinetic/pharmacodynamics model with simulated endocardial vegetations. Antimicrob Agents Chemother 56:3174–3180. doi: 10.1128/AAC.06439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kebriaei R, Rice SA, Singh KV, Stamper KC, Dinh AQ, Rios R, Diaz L, Murray BE, Munita JM, Tran TT, Arias CA, Rybak MJ. 2018. Influence of inoculum effect on the efficacy of daptomycin monotherapy and in combination with β-lactams against daptomycin-susceptible Enterococcus faecium harboring LiaSR substitutions. Antimicrob Agents Chemother 62:e00315-18. doi: 10.1128/AAC.00315-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arias CE, Contreras GA, Murray BE. 2010. Management of multidrug-resistant enterococcal infections. Clin Microbiol Infect 16:555–562. doi: 10.1111/j.1469-0691.2010.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beganovic M, Luther MK, Rice LB, Arias CE, Rybak MJ, LaPlante KL. 2018. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin Infect Dis 67:303–309. doi: 10.1093/cid/ciy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menon V, Davis R, Shackel N, Espedido BA, Beukers AG, Jensen SO, van Hal SJ. 2018. Failure of daptomycin beta-lactam combination therapy to prevent resistance emergence in Enterococcus faecium. Diagn Microbiol Infect Dis 90:120–122. doi: 10.1016/j.diagmicrobio.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Morrisette T, Kebriaei R, Lev KL, Morales S, Rybak MJ. 2020. Bacteriophage therapeutics: a primer for clinicians on phage-antibiotic combinations. Pharmacotherapy 40:153–168. doi: 10.1002/phar.2358. [DOI] [PubMed] [Google Scholar]
- 19.Comeau AM, Tétart F, Trojet SN, Prére MF, Krisch HM. 2007. Phage-antibiotic synergy (PAS): beta-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One 2:e799. doi: 10.1371/journal.pone.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhry WN, Concepción-Acevedo J, Park T, Andleeb S, Bull JJ, Levin BR. 2017. Synergy and order effects of antibiotics and phages in killing Pseudomonas aeruginosa biofilms. PLoS One 12:e0168615. doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan BK, Sistrom M, Wertz JE, Kortright KE, Narayan D, Turner PE. 2016. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci Rep 6:26717. doi: 10.1038/srep26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelman D, Beyth S, Lerer V, Adler K, Poradosu-Cohen R, Coppenhagen-Glazer S, Hazan R. 2018. Combined bacteriophages and antibiotics as an efficient therapy against VRE Enterococcus faecalis in a mouse model. Res Microbiol 169:531–539. doi: 10.1016/j.resmic.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee A, Johnson CN, Luong P, Hullahalli K, McBride SW, Schubert AM, Palmer KL, Carlson PE Jr, Duerkop BA. 2019. Bacteriophage resistance alters antibiotic-mediated intestinal expansion of enterococci. Infect Immun 87:e00085-19. doi: 10.1128/IAI.00085-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho K, Huo W, Pas S, Dao R, Palmer KL. 2018. Loss-of-function mutations in epaR confer resistance to ϕNPV1 infection in Enterococcus faecalis OG1RF. Antimicrob Agents Chemother 62:e00758-18. doi: 10.1128/AAC.00758-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing; 30th informational supplement. CLSI document MS100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Mazzocco A, Waddell TE, Lingohr E, Johnson RP. 2009. Enumeration of bacteriophages using the small drop plaque assay system. Methods Mol Biol 501:81–85. doi: 10.1007/978-1-60327-164-6_9. [DOI] [PubMed] [Google Scholar]
- 27.Tran KN, Rybak MJ. 2018. β-Lactam combinations with vancomycin show synergistic activity against vancomycin-susceptible Staphylococcus aureus, vancomycin-intermediate S. aureus (VISA), and heterogeneous VISA. Antimicrob Agents Chemother 62:e00157-18. doi: 10.1128/AAC.00157-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber-Dąbrowska B, Jończyk-Matysiak E, Żaczek M, Łobocka M, Łusiak-Szelachowska M, Górski A. 2016. Bacteriophage procurement for therapeutic purposes. Front Microbiol 12:1177. doi: 10.3389/fmicb.2016.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Flynn G, Ross RP, Fitzgerald GF, Coffey A. 2004. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl Environ Microbiol 70:3417–3424. doi: 10.1128/AEM.70.6.3417-3424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehman SM, Mearns G, Rankin D, Cole RA, Smrekar F, Branston SD, Morales S. 2019. Design and preclinical development of a phage product for the treatment of antibiotic-resistant Staphylococcus aureus infections. Viruses 11:88. doi: 10.3390/v11010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. 2012. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 56:838–844. doi: 10.1128/AAC.05551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakoulas G, Rose W, Nonejuie P, Olson J, Pogliano J, Humphries R, Nizet V. 2014. Ceftaroline restores daptomycin activity against daptomycin-nonsusceptible vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 58:1494–1500. doi: 10.1128/AAC.02274-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall Snyder A, Werth BJ, Barber KE, Sakoulas G, Rybak MJ. 2014. Evaluation of the novel combination of daptomycin plus ceftriaxone against vancomycin-resistant enterococci in an in vitro pharmacokinetic/pharmacodynamics simulated endocardial vegetation model. J Antimicrob Chemother 69:2148–2154. doi: 10.1093/jac/dku113. [DOI] [PubMed] [Google Scholar]