Combination antibiotic therapy is highly effective in curing Buruli ulcer (BU) caused by Mycobacterium ulcerans. Treatment failures have been uncommonly reported with the recommended 56 days of antibiotics, and little is known about risk factors for treatment failure. We analyzed treatment failures among BU patients treated with ≥56 days of antibiotics from a prospective observational cohort at Barwon Health, Victoria, from 1 January 1998 to 31 December 2018.
KEYWORDS: Mycobacterium ulcerans, antibiotic, failure, risk factors, treatment
ABSTRACT
Combination antibiotic therapy is highly effective in curing Buruli ulcer (BU) caused by Mycobacterium ulcerans. Treatment failures have been uncommonly reported with the recommended 56 days of antibiotics, and little is known about risk factors for treatment failure. We analyzed treatment failures among BU patients treated with ≥56 days of antibiotics from a prospective observational cohort at Barwon Health, Victoria, from 1 January 1998 to 31 December 2018. Treatment failure was defined as culture-positive recurrence within 12 months of commencing antibiotics under the following conditions: (i) following failure to heal the initial lesion or (ii) a new lesion developing at the original or at a new site. A total of 430 patients received ≥56 days of antibiotic therapy, with a median duration of 56 days (interquartile range [IQR], 56 to 80). Seven (1.6%) patients experienced treatment failure. For six adult patients experiencing treatment failure, all were male, weighed >90 kg, did not have surgery, and received combination rifampin-clarithromycin (median rifampin dose, 5.6 mg per kg of body weight per day; median clarithromycin dose, 8.1 mg/kg/day). When compared to those who did not fail treatment on univariate analysis, treatment failure was significantly associated with a weight of >90 kg (P < 0.001), male gender (P = 0.02), immune suppression (P = 0.04), and a first-line regimen of rifampin-clarithromycin compared to a regimen of rifampin-fluoroquinolone (P = 0.05). There is a low rate of treatment failure in Australian BU patients treated with rifampin-based oral combination antibiotic therapy. Our study raises the possibility that treatment failure risk may be increased in males, those with a body weight of >90 kg, those with immune suppression, and those taking rifampin-clarithromycin antibiotic regimens, but future pharmacokinetic and pharmacodynamics studies are required to determine the validity of these hypotheses.
INTRODUCTION
Mycobacterium ulcerans causes destructive lesions of skin and subcutaneous tissue known as Buruli ulcer (BU), which has been reported from at least 33 countries with the highest burden of disease in Central/West Africa and Australia (1). Once thought to be a surgically treated disease (2), antibiotics are now the recommended first-line treatment for BU (3, 4), although surgery still has as an important adjunctive role (5). The World Health Organization (WHO) currently recommends 56 days of rifampin-based combination antibiotic therapy for all lesions, with recommended rifampin dosing of 10 mg of drug per kg of body weight per day up to a maximum of 600 mg daily and clarithromycin dosing of 15 mg/kg/day up to a maximum of 1,000 mg daily (3). Rifampin is thought to be the most important drug in antibiotic combination regimens used for M. ulcerans treatment due to its highly bactericidal activity against the organism in vitro (6) and in mouse models (7). Its activity against M. ulcerans likely relates to peak concentration levels and area under the concentration-time curve (AUC) (8), meaning that in situations where its serum level is reduced its effectiveness may also be reduced. Furthermore, although rarely reported (9–11), if used at subtherapeutic levels or if not used in combination with another effective antibiotic, there is the potential for M. ulcerans to develop rifampin resistance.
Rifampin-based combination antibiotic therapy has been shown to be highly effective in curing BU, either used alone (12–14) or in combination with surgery (15), with reported success rates ranging from 93% to 100%. Most commonly rifampin is combined with the injectable agent streptomycin (13), oral clarithromycin (12), or oral fluoroquinolones (15). Treatment failures have been uncommonly reported with 56 days of rifampin-based combination antibiotic therapy, and there are no previous reports of associated risk factors. With the increasing use of antibiotic treatment for BU, it is important to monitor the rates of treatment failure and for the potential emergence of drug resistance as well as understand the associated risk factors. This may allow modifications of treatment protocols to minimize these risks.
RESULTS
Between 1 January 1998 and 31 December 2018, 668 BU patients from the Barwon Health cohort were treated with antibiotic therapy. Fifty-six patients received <28 days of antibiotics, of whom 3 (5.3%) failed treatment after 6, 7, and 21 days, respectively. A total of 182 patients received 28 to 55 days of antibiotics, of whom 0 (0.0%) failed treatment. A total of 430 patients received ≥56 days of antibiotics.
Those who had received ≥56 days of antibiotics were included in this study. Of these 430 patients, 56.7% were male, the median age was 58 years, and 13.0% weighed >90 kg. The first-line antibiotic regimen was rifampin-clarithromycin in 62.6% and rifampin-fluoroquinolone (FQ) (either ciprofloxacin or moxifloxacin) in 34.2%. Adjunctive surgery was performed in 32.3% of patients. (Table 1).
TABLE 1.
Comparison of baseline characteristics and treatment modalities for patients who failed treatment compared to patients who did not fail treatment after at least 56 days of antibiotics in the Barwon Health cohort from 1998 to 2018
| Characteristic | Total no. (% of total) | No. of failures (% of treated) | No. of successes (% of treated) | P value (comparing failure vs success) |
|---|---|---|---|---|
| Gender | ||||
| Male | 244 (56.7) | 7 (2.9) | 237 (97.1) | 0.02 |
| Female | 186 (43.3) | 0 (0.0) | 186 (100.0) | |
| Median Age (years [IQR]) | 58 (33–75) | 73 years (43–79) | 58 (33–75) | 0.42 |
| Weight (kg) | ||||
| <60 | 33 (7.7) | 1 (3.0) | 32 (97.0) | <0.001 |
| 60–90 | 150 (34.9) | 0 (0.0) | 150 (100.0) | |
| >90 | 56 (13.0) | 6 (10.7) | 50 (89.3) | |
| Missing | 191 (44.4) | 0 (0.0) | 191 (100.0) | |
| Lesion type | ||||
| Ulcer | 354 (82.3) | 6 (1.7) | 348 (98.3) | 0.44 |
| Nodule | 18 (4.2) | 1 (5.6) | 17 (94.4) | |
| Edema | 53 (12.3) | 0 (0.0) | 53 (100.0) | |
| Plaque | 5 (1.2) | 0 (0.0) | 5 (100.0) | |
| WHO category | ||||
| 1 | 285 (66.3) | 3 (1.1) | 282 (99.0) | 0.35 |
| 2 | 89 (20.7) | 2 (2.3) | 87 (97.8) | |
| 3 | 56 (13.0) | 2 (3.6) | 54 (96.4) | |
| Size (mm2) | ||||
| ≤400 | 60 (20.3) | 0 (0.0) | 60 (100.0) | 0.41 |
| 401–1,600 | 139 (47.0) | 3 (2.2) | 136 (97.8) | |
| >1,600 | 97 (32.8) | 3 (3.1) | 94 (96.9) | |
| Median duration of symptoms (days [IQR]) | 42 (28–67) | 30 (28–60) | 42 (28–70) | 0.60 |
| Lesion location | ||||
| Upper limb | 126 (29.3) | 1 (0.8) | 125 (99.2) | 0.64 |
| Lower limb | 299 (69.5) | 6 (2.0) | 293 (98.0) | |
| Head/trunk | 5 (1.2) | 0 (0.0) | 5 (100.0) | |
| Multiple lesions | ||||
| No | 395 (91.9) | 6 (1.5) | 389 (98.5) | 0.55 |
| Yes | 35 (8.1) | 1 (2.9) | 34 (97.1) | |
| Diabetes | ||||
| No | 390 (90.7) | 7 (1.8) | 383 (98.2) | 0.39 |
| Yes | 40 (9.3) | 0 (0.0) | 40 (100.0) | |
| Immune suppression | ||||
| No | 296 (92.1) | 5 (1.3) | 391 (98.7) | 0.04 |
| Yes | 34 (7.9) | 2 (5.9) | 32 (94.1) | |
| Median eGFR (ml/min [IQR]) | 86 (69,101) | 73 (54,92) | 86 (69,101) | 0.26 |
| Antibiotic regimen | ||||
| Rifampin-clarithromycin | 269 (62.6) | 7 (2.6) | 262 (97.4) | 0.05 |
| Rifampin-fluoroquinolone | 147 (34.2) | 0 (0.0) | 147 (100.0) | |
| Median antibiotic duration (days [IQR]) | 56 (56–80) | 56 (56–70) | 56 (56–81) | 0.46 |
| Paradoxical reaction | ||||
| Yes | 160 (37.2) | 4 (2.5) | 156 (97.5) | 0.27 |
| No | 270 (62.8) | 3 (1.4) | 267 (98.9) | |
| Corticosteroid use | ||||
| Yes | 66 (15.4) | 2 (3.0) | 64 (96.7) | 0.33 |
| No | 364 (84.7) | 5 (1.4) | 359 (98.6) | |
| Surgery | ||||
| Yes | 139 (32.3) | 0 (0.0) | 139 (100.0) | 0.07 |
| No | 291 (67.7) | 7 (2.4) | 284 (97.6) |
Seven (1.6%) patients experienced treatment failure (Table 2). All were male with a median age of 73 years (interquartile range [IQR], 43 to 79 years). For the six adult patients who experienced treatment failure, all weighed more than 90 kg, did not have surgery, and received the antibiotic combination of rifampin and clarithromycin (median dose of rifampin, 5.6 mg/kg/day; median dose of clarithromycin, 8.1 mg/kg/day). Patient 3 had his drug doses reduced to 300 mg rifampin daily and 250 mg clarithromycin twice daily due to liver cirrhosis and concerns of liver toxicity with full drug doses. Patient 4 had his clarithromycin dose reduced to 250 mg twice daily due to a low estimated glomerular filtration rate (eGFR). Patient 1 was a 16-month-old child with nodular disease, who has been previously reported (16). Patient 5 was immune suppressed receiving 25 mg prednisolone daily for ulcerative colitis. No patients had diabetes mellitus or were known to be HIV positive. The diagnosis of BU was confirmed by PCR for 423 (98.4%) patients, 5 (1.2%) patients were diagnosed by histology alone, and 2 (0.5%) were diagnosed by a positive M. ulcerans culture alone.
TABLE 2.
Characteristics of patients who failed antibiotic treatment after at least 56 days of antibiotics in Barwon Health cohort from 1998 to 2018a
| Patient no. | Age (yrs) | Gender | Wt (kg) | WHO stage | Size (mm2) | Lesion type | IS | Atb duration (days) | Atb regimen | Dose reduced | Rif dose (mg/kg) | Clr dose (mg/kg) | Surgery | Site of relapse | Time to relapse (days) | CS | PR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Male | 15 | 1 | 100 | Nodule | No | 56 | Rif-Clr | No | 10.0 | 13.3 | No | Initial site | 100 | No | No |
| 2 | 62 | Male | 137 | 1 | 700 | Ulcer | No | 56 | Rif-Clr | No | 4.4 | 7.3 | No | Initial site | 175 | No | Yes |
| 3 | 86 | Male | 99 | 3 | 6,630 | Ulcer | Yes | 84 | Rif-Clr | Yes | 3.0 | 5.1 | No | Initial site | 252 | Yes | Yes |
| 4 | 79 | Male | 102 | 2 | 2,500 | Ulcer | No | 56 | Rif-Clr | Yes | 5.9 | 4.9 | No | Local new lesion | 175 | No | No |
| 5 | 74 | Male | 106 | 2 | 2,660 | Ulcer | Yes | 70 | Rif-Clr | No | 5.7 | 9.4 | No | Initial site | 188 | Yes | Yes |
| 6 | 43 | Male | 95 | 1 | 484 | Ulcer | No | 56 | Rif-Clr | No | 6.3 | 10.5 | No | Initial site | 290 | No | No |
| 7 | 73 | Male | 112 | 3 | 572 | Nodule | No | 56 | Rif-Clr | No | 5.4 | 8.9 | No | Initial site | 270 | No | Yes |
IS, immune suppression; Rx, treatment; Atb, antibiotic; Rif, rifampin; Clr, clarithromycin; CS, corticosteroids; PR, paradoxical reaction.
When compared to those who did not fail treatment after at least 56 days of antibiotics, on univariate analysis, failure was significantly associated with the following characteristics (Table 1): weight of >90 kg (P < 0.001), male gender (P = 0.02), immune suppression (P = 0.04), and a first-line antibiotic regimen of rifampin-clarithromycin compared to that of rifampin-FQ (P = 0.05). The association with weight of >90 kg persisted when stratified by gender (P < 0.001 for males). The median duration of antibiotics was shorter for the rifampin-clarithromycin first-line antibiotic regimen than for the rifampin-FQ first-line antibiotic regimen (56 days with an IQR of 56 to 63 compared with 63 days with an IQR of 56 to 86, respectively; P < 0.001). For the 56 patients who weighed >90 kg, 48 (86%) received rifampin-clarithromycin regimens, 8 (14%) received rifampin-FQ regimens, and 6 (11%) were treated with surgery. There were no significant differences in those who failed and did not fail treatment according to median age, median duration of symptoms prior to diagnosis, type, location, WHO category or size of BU lesion, the presence of multiple lesions, diabetes, median eGFR, median duration of antibiotic treatment, the presence of paradoxical reactions, and treatment with corticosteroids.
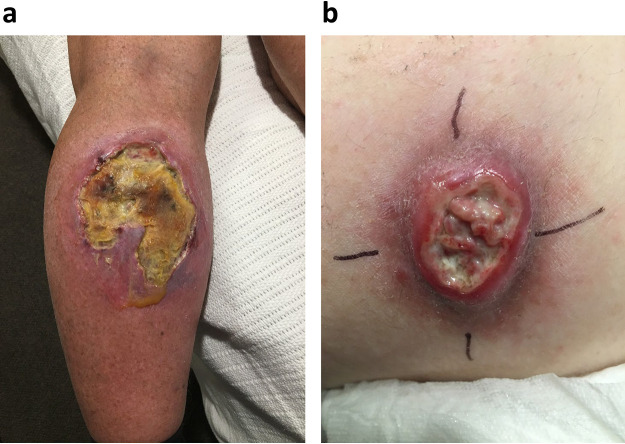
It was noted that BU lesions at the time of relapse differed in clinical appearance from that of BU lesions at the time of paradoxical reactions; relapsed lesions exhibited yellow necrotic tissue with undermining of wound edges, which differed from paradoxical reactions that exhibited areas of pink granulating tissue intermingled with white connective tissue with no undermining of wound edges (Fig. 1).
FIG 1.
(a) Image of patient 5 with relapsed Buruli ulcer at site of initial lesion on upper calf 188 days after commencement of antibiotics. Note large areas of yellow necrotic tissue with undermining of wound edges characteristic of clinically relapsed disease. Patient provided informed consent. (b) Image of a patient with a BU on the inner thigh complicated by paradoxical reaction 164 days after commencement of antibiotics. Note the absence of yellow necrotic tissue, with instead the characteristic findings of a paradoxical reaction showing areas of pink granulating tissue intermingled with white connective tissue with no undermining of wound edges. Patient provided informed consent.
DISCUSSION
In our study, we have described a very low incidence of treatment failure (1.6%) in Australian BU patients treated with at least 56 days of rifampin-based oral combination antibiotic therapy. This is similar to the experience in Benin for all oral antibiotic therapy with rifampin and clarithromycin (no failures from 30 patients) (12) and no failures in 158 patients treated in Ghana using rifampin plus the injectable agent streptomycin (17). Reported failure rates were slightly higher in some other African studies that included rifampin plus the injectable agent streptomycin and/or oral clarithromycin (7% failure rate in 151 patients [13], 7% failure rate in 43 patients [18], and 4% failure rate in 297 patients [14]). However, compared to our study, these studies would have slightly overestimated failure rates, as they classified wounds that failed to heal within 12 months of antibiotic commencement or lesions that increased in size by more than 150% requiring surgery as treatment failures. Considering evidence from Australian BU patients that successfully treated lesions, especially if they are large or affected by paradoxical reactions, can increase in size by more than 150% during treatment and can take longer than 12 months to heal (19) and that surgery plays an important role in BU treatment in improving the rate of wound healing, minimizing antibiotic-associated toxicity, and preventing further tissue loss associated with severe paradoxical reactions (5), we did not include these criteria in our definition of treatment failure.
We found an association of treatment failure with male gender, a body weight of >90 kg, immune suppression, and those who had received the first-line regimen of rifampin-clarithromycin compared with that of rifampin-FQ. Treatment failure related to increased body weight has not been reported previously, but most treatment studies have been reported from African settings where the majority of patients are children, and people with a body weight of >90 kg are few; for example, in a study in Ghana the median BMI was 16.6 to 17.2 (13). Recommended rifampin dosing for BU treatment is weight based (10 mg/kg/day) (3, 4) but limited to 600 mg daily due to assumptions that it is effective at this dose range and fears of toxicity at higher doses (20). However, studies of high-dose rifampin used to treat Mycobacterium tuberculosis have not shown signs of increasing toxicity at doses up to 35 mg/kg/day (21, 22). Once a patient’s weight is greater than 90 kg, 600 mg daily of rifampin equates to less than 6.7 mg/kg/day. In adults within our study, due to the recommended limit of 600 mg daily (3), the median rifampin dose of those that failed treatment was 5.6 mg/kg/day or just over 50% of a recommended 10-mg/kg/day dose.
Rifampin activity against tuberculosis relates to peak concentration levels and area under the concentration time curve (AUC) (8), and increasing the rifampin dose results in a more than proportional increase in the level of exposure to rifampin in plasma (23). Furthermore, there is in vitro evidence that higher levels of exposure to rifampin kill mycobacteria more rapidly and prevent the emergence of resistance (24). Mouse footpad models of BU have also shown that high-dose rifampin (up to 40 mg/kg) combined with clofazimine (25) or clarithromycin (26) can increase the bactericidal activity of antibiotic regimens and allow shorter durations of treatment without increasing the rates of treatment failure. In treatment of M. tuberculosis, there have been concerns that current dosing regimens limited to 600 mg daily are suboptimal and at the lower end of the dose-response curve, potentially leading to treatment failure and the development of antibiotic resistance (8, 22, 27). Our finding of an association of increased treatment failure rates in the heaviest patients with expected lowest levels of rifampin raises the possibility that this may also be the case in the treatment of BU. It is possible that in these higher-weight-range populations, higher doses of rifampin may be needed to prevent treatment failure.
The companion drug to rifampin in all of our failed treatment cases was clarithromycin, which also has a weight based dose recommendation (15 mg/kg/day), limited at 1,000 mg daily (3). Once again, for patients weighing more than 90 kg, daily clarithromycin dosing limited at 1,000 mg daily results in doses equivalent to less than 11.1 mg/kg/day. In our study, due to the recommended limit of 1,000 mg daily (3), the median clarithromycin dose in adults that failed treatment was 8.1 mg/kg/day or about 54% of the recommended 15-mg/kg/day dose. Serum levels of clarithromycin are further significantly reduced by up to 90% due to an interaction with rifampin (28), and a previous study examining 5 African patients with BU treated with rifampin and clarithromycin, albeit at a reduced dose of clarithromycin (7.5 mg/kg daily), found potentially subtherapeutic clarithromycin levels (29). Thus, there would be significant concerns that in patients with a weight of >90 kg, the dosing regimen limit of 1,000 mg daily would be inadequate to reach therapeutic levels and may have contributed to the cases of treatment failure seen in our study.
Fluoroquinolones may be more suitable companion drugs to rifampin in those with increased weight, as serum levels are not significantly reduced by rifampin (approximately 10%) (30), they have high oral bioavailability (31), and they are concentrated in tissues where the infection exists (32). They have also been shown to have good in vitro effectiveness against M. ulcerans (6) and, combined with rifampin, have been successfully used to treat BU in humans (15). It is interesting that in our study there were no treatment failures in patients treated with a first-line regimen of rifampin and a fluoroquinolone (ciprofloxacin or moxifloxacin) despite 34% of patients receiving it. However, this may have been influenced by the fact that these combinations were given for a median of 9 days longer than the rifampin-clarithromycin combinations, and only 14% (8 patients) of those weighing >90 kg received the rifampin-FQ regimen.
The concern about inadequate drug dosing not only relates to the risk of treatment failure but, more importantly, the risk of developing antibacterial resistance. This is common in other mycobacterial species, especially tuberculosis (33) and leprosy (34), but at this stage has been infrequently reported in M. ulcerans. Marsollier et al. reported in 2003 the development of rpoB resistance mutations in three mice that had been exposed to rifampin monotherapy (9), and Beissner reported in 2010 a mutation in the rpoB gene of one patient who had been treated with surgery and antibiotics, though the combination and duration of antibiotics were unknown, and the isolate could not be subcultured to test for phenotypic resistance (10). Owusu et al., in 2016, reported the susceptibility profiles of M. ulcerans isolates to rifampin and streptomycin following swabs from relapsed or prolonged human disease cases (11). Of 70 isolates, 12 showed resistance to rifampin disks in culture, and two were resistant to streptomycin, though no isolates were found to be resistant to both drugs. This study, however, did not examine the genomic profile of the resistance. Thus, there is the potential for drug resistance to develop with monotherapy, subtherapeutic drug levels related to either inadequate dosing or suboptimal adherence, or the long duration of recommended treatment (8 weeks).
There was only one failure reported in children in our study. It is possible in this patient that due to their very young age (17 months), factors such as the liquid preparation may have affected adherence and bioavailability and dosing recommendations may be suboptimal in young children (27).
Our research raises the possibility that there may be an increased risk of treatment failure in males. This may relate to potentially lower serum levels of rifampin in men than women, as this has been shown to be the case in tuberculosis (TB) and Mycobacterium avium complex patients where serum rifampin levels were 19 to 48% lower in men (30, 35, 36). Serum levels of clarithromycin and FQs do not appear to be influenced by gender (30).
All adult patients who failed treatment had medical therapy alone without surgical intervention, and the absence of surgery showed a trend to be associated with treatment failure (P = 0.07). We have previously demonstrated that surgery can allow a reduction in duration of antibiotics required to cure lesions (37), likely by reducing the burden of organisms needing to be sterilized and removing necrotic and purulent tissue where antibiotics may have reduced penetration. Thus, it would be interesting in further studies to explore whether adjunctive surgery can reduce the risk of treatment failure.
Importantly, there was no increased risk of treatment failure with age, the WHO category of lesions, the location, type or baseline size of lesions, diabetes, eGFR, the presence of immune reconstitution inflammatory syndrome (IRIS), or the use of corticosteroids during treatment. Interestingly, in patients excluded from this study, there were no reported treatment failures related to antibiotic durations of between 28 and 55 days. This may have been influenced by the selection in the 28- to 55-day group of a higher proportion of patients with small lesions (64% versus 20% with lesions ≤400 mm2; P < 0.001) and those where antibiotics were ceased early due to toxicity (26% versus 11% of patients; P < 0.001), as both of these factors potentially influence the risk of treatment failure (38).
There were some limitations to our study. First, we did not measure therapeutic drug levels and, therefore, were unable to assess whether there were low serum antibiotic levels in patients that may have contributed to their treatment failure. Additionally, as there were very few treatment failures, statistical power to show a difference in associations was limited. Furthermore, as in many categories of variables where there were no treatment failures, we were unable to perform a multivariate analysis of associated variables to take into account potential confounders. There were also a significant number of patients whose weight was not available, and this may have biased the results. Finally, neither genotypic nor phenotypic susceptibility testing has been performed on the mycobacterial isolates from relapsed cases, and, therefore, we are unable to determine whether antibiotic drug resistance may have been present and played a role in the treatment failure.
In conclusion, there is a low rate of treatment failure in Australian patients with BU treated with rifampin-based oral combination antibiotic therapy. Our study raises the possibility that treatment failure risk may be increased in males, those with a body weight of >90 kg, those with immune suppression, and in those taking rifampin-clarithromycin antibiotic regimens, but future pharmacokinetic and pharmacodynamics studies are required to determine the validity of these hypotheses.
MATERIALS AND METHODS
We examined treatment failure in BU patients who had been treated for at least 56 days with effective antibiotics from a prospective observational cohort at Barwon Health, Victoria, between 1 January 1998 and 31 December 2018. We used 56 days to exclude the possibility that treatment failure was related to an antibiotic treatment duration less than that currently recommended by the WHO. The definition of treatment failure was culture-positive recurrence of a lesion within 12 months of commencing antibiotics under the following conditions: (i) following failure to heal the initial lesion or (ii) a new lesion developing either at the original or at a new site.
A BU case was defined as the presence of a lesion clinically suggestive of BU plus any of the following: (i) a culture of M. ulcerans from the lesion, (ii) a positive PCR from a swab or biopsy of the lesion, or (iii) histopathology of an excised lesion showing a necrotic ulcer with the presence of acid-fast bacilli (AFB) consistent with acute BU infection. The initial size of the lesion was determined by measuring the induration diameter of lesions and calculating the surface area in square millimeters. WHO category was assigned according to published definitions (3).
WHO and Australian recommended standard dosages for antibiotics were used and included rifampin at 10 mg/kg/day (up to a maximum of 600 mg daily), clarithromycin at 7.5 mg/kg twice daily (up to a maximum of 500 mg twice daily), ciprofloxacin at 500 mg twice daily, and moxifloxacin at 400 mg daily (3, 4). Dosages of ciprofloxacin and clarithromycin were reduced in severe renal dysfunction (eGFR ≤ 30 ml/min) according to Australian guidelines (39).
Immune suppression was defined as current treatment with immunosuppressive medication (e.g., prednisolone), severe chronic renal failure (eGFR < 30), liver cirrhosis, or active malignancy. Body weight was measured in kilograms; however, body mass index (BMI) could not be calculated as heights were not recorded. Paradoxical reactions (IRIS) were determined by the treating clinician and defined by the presence of one or both of the following features: (i) clinical—an initial improvement on antibiotic treatment in the clinical appearance of a BU lesion followed by a clinically significant deterioration of the lesion or its surrounding tissues or the appearance of a new lesion(s), and/or (ii) histopathological—examination of excised tissue from the clinical lesion showing evidence of an intense inflammatory reaction consistent with a paradoxical reaction (40).
This study was approved by the Barwon Health Human Research and Ethics Committee. All previously gathered human medical data were analyzed in a deidentified fashion.
Data analysis.
Data were collected prospectively using Epi Info 6 (CDC, Atlanta, GA) and analyzed using Stata 14 (StataCorp, TX, USA). Comparison of parametric variables was performed using the chi-square test, and median values for nonparametric variables were compared using the Wilcoxon rank sum test.
REFERENCES
- 1.O’Brien DP, Jeanne I, Blasdell K, Avumegah M, Athan E. 2018. The changing epidemiology worldwide of Mycobacterium ulcerans. Epidemiol Infect 147:1–8. doi: 10.1017/S0950268818002662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sizaire V, Nackers F, Comte E, Portaels F. 2006. Mycobacterium ulcerans infection: control, diagnosis, and treatment. Lancet Infect Dis 6:288–296. doi: 10.1016/S1473-3099(06)70464-9. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2012. Treatment of Mycobacterium ulcerans disease (Buruli ulcer): guidance for health workers. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.O'Brien DP, Jenkin G, Buntine J, Steffen CM, McDonald A, Horne S, Friedman ND, Athan E, Hughes A, Callan PP, Johnson PDR. 2014. Treatment and prevention of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: guideline update. Med J Aust 200:267–270. doi: 10.5694/mja13.11331. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien DP, Callan P, Friedman ND, Athan E, Hughes A, McDonald A. 2019. Mycobacterium ulcerans disease management in Australian patients: the re-emergence of surgery as an important treatment modality. ANZ J Surg 89:653–658. doi: 10.1111/ans.14829. [DOI] [PubMed] [Google Scholar]
- 6.Thangaraj HS, Adjei O, Allen BW, Portaels F, Evans MR, Banerjee DK, Wansbrough-Jones MH. 2000. In vitro activity of ciprofloxacin, sparfloxacin, ofloxacin, amikacin and rifampicin against Ghanaian isolates of Mycobacterium ulcerans. J Antimicrob Chemother 45:231–233. doi: 10.1093/jac/45.2.231. [DOI] [PubMed] [Google Scholar]
- 7.Ji B, Lefrancois S, Robert J, Chauffour A, Truffot C, Jarlier V. 2006. In vitro and in vivo activities of rifampin, streptomycin, amikacin, moxifloxacin, R207910, linezolid, and PA-824 against Mycobacterium ulcerans. Antimicrob Agents Chemother 50:1921–1926. doi: 10.1128/AAC.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 208:1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsollier L, Honore N, Legras P, Manceau AL, Kouakou H, Carbonnelle B, Cole ST. 2003. Isolation of three Mycobacterium ulcerans strains resistant to rifampin after experimental chemotherapy of mice. Antimicrob Agents Chemother 47:1228–1232. doi: 10.1128/aac.47.4.1228-1232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beissner M, Bretzel G, Nienhuis WA, Fleischmann E, Loscher T, Siegmund V, Thompson W, van der Werf TS, Herbinger K-H, Awua-Boateng N-Y, Fleischer B, Nitschke J, Adjei O, Agbenorku P, Klutse E. 2010. A genotypic approach for detection, identification, and characterization of drug resistance in Mycobacterium ulcerans in clinical samples and isolates from Ghana. Am J Trop Med Hyg 83:1059–1065. doi: 10.4269/ajtmh.2010.10-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owusu E, Newman MJ, Kotey NK, Akumwena A, Bannerman E. 2016. Susceptibility profiles of Mycobacterium ulcerans isolates to streptomycin and rifampicin in two districts of the eastern region of Ghana. Int J Microbiology 2016:8304524. doi: 10.1155/2016/8304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauty A, Ardant MF, Marsollier L, Pluschke G, Landier J, Adeye A, Goundote A, Cottin J, Ladikpo T, Ruf T, Ji B. 2011. Oral treatment for Mycobacterium ulcerans infection: results from a pilot study in Benin. Clin Infect Dis 52:94–96. doi: 10.1093/cid/ciq072. [DOI] [PubMed] [Google Scholar]
- 13.Nienhuis WA, Stienstra Y, Thompson WA, Awuah PC, Abass KM, Tuah W, Awua-Boateng NY, Ampadu EO, Siegmund V, Schouten JP, Adjei O, Bretzel G, van der Werf TS. 2010. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375:664–672. doi: 10.1016/S0140-6736(09)61962-0. [DOI] [PubMed] [Google Scholar]
- 14.Phillips RO, Robert J, Abass KM, Thompson W, Sarfo FS, Wilson T, Sarpong G, Gateau T, Chauty A, Omollo R, Ochieng Otieno M, Egondi TW, Ampadu EO, Agossadou D, Marion E, Ganlonon L, Wansbrough-Jones M, Grosset J, Macdonald JM, Treadwell T, Saunderson P, Paintsil A, Lehman L, Frimpong M, Sarpong NF, Saizonou R, Tiendrebeogo A, Ohene SA, Stienstra Y, Asiedu KB, van der Werf TS. 2020. Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited Buruli ulcer lesions: a randomised, open-label, non-inferiority phase 3 trial. Lancet 395:1259–1267. doi: 10.1016/S0140-6736(20)30047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Brien DP, McDonald A, Callan P, Robson M, Friedman ND, Hughes A, Holten I, Walton A, Athan E. 2012. Successful outcomes with oral fluoroquinolones combined with rifampicin in the treatment of Mycobacterium ulcerans: an observational cohort study. PLoS Negl Trop Dis 6:e1473. doi: 10.1371/journal.pntd.0001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman ND, Athan E, Hughes AJ, Khajehnoori M, McDonald A, Callan P, Rahdon R, O'Brien DP. 2013. Mycobacterium ulcerans disease: experience with primary oral medical therapy in an Australian cohort. PLoS Negl Trop Dis 7:e2315. doi: 10.1371/journal.pntd.0002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarfo FS, Phillips R, Asiedu K, Ampadu E, Bobi N, Adentwe E, Lartey A, Tetteh I, Wansbrough-Jones M. 2010. Clinical efficacy of combination of rifampin and streptomycin for treatment of Mycobacterium ulcerans disease. Antimicrob Agents Chemother 54:3678–3685. doi: 10.1128/AAC.00299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips RO, Sarfo FS, Abass MK, Abotsi J, Wilson T, Forson M, Amoako YA, Thompson W, Asiedu K, Wansbrough-Jones M. 2014. Clinical and bacteriological efficacy of rifampin-streptomycin combination for two weeks followed by rifampin and clarithromycin for six weeks for treatment of Mycobacterium ulcerans disease. Antimicrob Agents Chemother 58:1161–1166. doi: 10.1128/AAC.02165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien DP, Friedman ND, McDonald A, Callan P, Hughes A, Walton A, Athan E. 2018. Wound healing: natural history and risk factors for delay in Australian patients treated with antibiotics for Mycobacterium ulcerans disease. PLoS Negl Trop Dis 12:e0006357. doi: 10.1371/journal.pntd.0006357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Ingen J, Aarnoutse RE, Donald PR, Diacon AH, Dawson R, Plemper van Balen G, Gillespie SH, Boeree MJ. 2011. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis 52:e194–e199. doi: 10.1093/cid/cir184. [DOI] [PubMed] [Google Scholar]
- 21.Seijger C, Hoefsloot W, Bergsma-de Guchteneire I, Te Brake L, van Ingen J, Kuipers S, van Crevel R, Aarnoutse R, Boeree M, Magis-Escurra C. 2019. High-dose rifampicin in tuberculosis: experiences from a Dutch tuberculosis centre. PLoS One 14:e0213718. doi: 10.1371/journal.pone.0213718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boeree MJ, Diacon AH, Dawson R, Narunsky K, Du Bois J, Venter A, Phillips PP, Gillespie SH, McHugh TD, Hoelscher M, Heinrich N, Rehal S, van Soolingen D, van Ingen J, Magis-Escurra C, Burger D, Plemper van Balen G, Aarnoutse RE, PanACEA Consortium . 2015. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med 191:1058–1065. doi: 10.1164/rccm.201407-1264OC. [DOI] [PubMed] [Google Scholar]
- 23.Aarnoutse RE, Kibiki GS, Reither K, Semvua HH, Haraka F, Mtabho CM, Mpagama SG, van den Boogaard J, Sumari-de Boer IM, Magis-Escurra C, Wattenberg M, Logger JGM, Te Brake LHM, Hoelscher M, Gillespie SH, Colbers A, Phillips PPJ, Plemper van Balen G, Boeree MJ, Pan AC. 2017. Pharmacokinetics, tolerability, and bacteriological response of rifampin administered at 600, 900, and 1,200 milligrams daily in patients with pulmonary tuberculosis. Antimicrob Agents Chemother 61:e01054-17. doi: 10.1128/AAC.01054-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gumbo T, Louie A, Deziel MR, Liu W, Parsons LM, Salfinger M, Drusano GL. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother 51:3781–3788. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Converse PJ, Almeida DV, Tasneen R, Saini V, Tyagi S, Ammerman NC, Li S-Y, Anders NM, Rudek MA, Grosset JH, Nuermberger EL. 2018. Shorter-course treatment for Mycobacterium ulcerans disease with high-dose rifamycins and clofazimine in a mouse model of Buruli ulcer. PLoS Negl Trop Dis 12:e0006728. doi: 10.1371/journal.pntd.0006728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omansen TF, Almeida D, Converse PJ, Li SY, Lee J, Stienstra Y, van der Werf T, Grosset JH, Nuermberger EL. 2019. High-dose rifamycins enable shorter oral treatment in a murine model of Mycobacterium ulcerans disease. Antimicrob Agents Chemother 63:e01478-18. doi: 10.1128/AAC.01478-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daskapan A, Idrus LR, Postma MJ, Wilffert B, Kosterink JGW, Stienstra Y, Touw DJ, Andersen AB, Bekker A, Denti P, Hemanth Kumar AK, Jeremiah K, Kwara A, McIlleron H, Meintjes G, van Oosterhout JJ, Ramachandran G, Rockwood N, Wilkinson RJ, van der Werf TS, Alffenaar JC. 2019. A systematic review on the effect of HIV infection on the pharmacokinetics of first-line tuberculosis drugs. Clin Pharmacokinet 58:747–766. doi: 10.1007/s40262-018-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace RJ Jr, Brown BA, Griffith DE, Girard W, Tanaka K. 1995. Reduced serum levels of clarithromycin in patients treated with multidrug regimens including rifampin or rifabutin for Mycobacterium avium-M. intracellulare infection. J Infect Dis 171:747–750. doi: 10.1093/infdis/171.3.747. [DOI] [PubMed] [Google Scholar]
- 29.Alffenaar JW, Nienhuis WA, de Velde F, Zuur AT, Wessels AM, Almeida D, Grosset J, Adjei O, Uges DR, van der Werf TS. 2010. Pharmacokinetics of rifampin and clarithromycin in patients treated for Mycobacterium ulcerans infection. Antimicrob Agents Chemother 54:3878–3883. doi: 10.1128/AAC.00099-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Ingen J, Egelund EF, Levin A, Totten SE, Boeree MJ, Mouton JW, Aarnoutse RE, Heifets LB, Peloquin CA, Daley CL. 2012. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med 186:559–565. doi: 10.1164/rccm.201204-0682OC. [DOI] [PubMed] [Google Scholar]
- 31.Lettieri JT, Rogge MC, Kaiser L, Echols RM, Heller AH. 1992. Pharmacokinetic profiles of ciprofloxacin after single intravenous and oral doses. Antimicrob Agents Chemother 36:993–996. doi: 10.1128/aac.36.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fong IW, Ledbetter WH, Vandenbroucke AC, Simbul M, Rahm V. 1986. Ciprofloxacin concentrations in bone and muscle after oral dosing. Antimicrob Agents Chemother 29:405–408. doi: 10.1128/aac.29.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaw MT, Emran NA, Lin Z. 2018. Mutations inside rifampicin-resistance determining region of rpoB gene associated with rifampicin-resistance in Mycobacterium tuberculosis. J Infect Public Health 11:605–610. doi: 10.1016/j.jiph.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Honore N, Cole ST. 1993. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob Agents Chemother 37:414–418. doi: 10.1128/aac.37.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Requena-Mendez A, Davies G, Ardrey A, Jave O, Lopez-Romero SL, Ward SA, Moore DA. 2012. Pharmacokinetics of rifampin in Peruvian tuberculosis patients with and without comorbid diabetes or HIV. Antimicrob Agents Chemother 56:2357–2363. doi: 10.1128/AAC.06059-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McIlleron H, Rustomjee R, Vahedi M, Mthiyane T, Denti P, Connolly C, Rida W, Pym A, Smith PJ, Onyebujoh PC. 2012. Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: implications for international dosing guidelines. Antimicrob Agents Chemother 56:3232–3238. doi: 10.1128/AAC.05526-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowan R, Athan E, Friedman ND, Hughes A, McDonald A, Callan P, Fyfe J, O’Brien DP. 2015. Mycobacterium ulcerans treatment – can antibiotic duration be reduced in selected patients? PLoS Negl Trop Dis 9:e0003503. doi: 10.1371/journal.pntd.0003503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brien DP, Friedman ND, Cowan R, Walton A, Athan E. 2020. Six versus eight weeks of antibiotics for small Mycobacterium ulcerans lesions in Australian patients. Clin Infect Dis 70:1993–1997. doi: 10.1093/cid/ciz532. [DOI] [PubMed] [Google Scholar]
- 39.Therapeutic Guidelines. 2016. Therapeutic guidelines: antibiotic. Therapeutic Guidelines, Melbourne, Australia: www.tg.org.au. Accessed 25 June 2020. [Google Scholar]
- 40.O'Brien DP, Robson ME, Callan PP, McDonald AH. 2009. “Paradoxical” immune-mediated reactions to Mycobacterium ulcerans during antibiotic treatment: a result of treatment success, not failure. Med J Aust 191:564–566. doi: 10.5694/j.1326-5377.2009.tb03313.x. [DOI] [PubMed] [Google Scholar]