Criteria defining bedaquiline resistance for tuberculosis have been proposed addressing an emerging concern. We evaluated bedaquiline phenotypic drug susceptibility testing (pDST) criteria using drug-resistant tuberculosis clinical isolates tested at five reference laboratories. Isolates were tested at the proposed bedaquiline MGIT960 and 7H11 agar proportion (AP) critical concentrations and also at higher dilutions. The epidemiological cutoff value for the broth microdilution (BMD) plates (frozen and dry) was investigated.
KEYWORDS: Mycobacterium tuberculosis, bedaquiline, drug resistance, drug susceptibility testing, tuberculosis
ABSTRACT
Criteria defining bedaquiline resistance for tuberculosis have been proposed addressing an emerging concern. We evaluated bedaquiline phenotypic drug susceptibility testing (pDST) criteria using drug-resistant tuberculosis clinical isolates tested at five reference laboratories. Isolates were tested at the proposed bedaquiline MGIT960 and 7H11 agar proportion (AP) critical concentrations and also at higher dilutions. The epidemiological cutoff value for the broth microdilution (BMD) plates (frozen and dry) was investigated. Sanger sequencing was performed (atpE and Rv0678 genes) for any isolate testing resistant. The composite reference standard (CRS) defined susceptibility or resistance as is if all pDST methods agreed. If the pDST result was discordant, sequencing results were used for final classification. Geographically diverse and bedaquiline-unexposed isolates were tested (n = 495). The epidemiological cutoff value for BMD was confirmed to be 0.12 μg/ml. The majority of isolates were determined to be susceptible by all methods (467/495; 94.3%), and 28 were determined to be resistant by at least one method; 4 of these were determined to be resistant by all methods. Of the 28 resistant isolates, 12 harbored Rv0678 mutations exclusively. Isolates with insertions/deletions were more likely to be determined to be resistant by more than one method (5/7) compared to isolates with a single nucleotide polymorphism (1/5). Applying the CRS to 24 discordant pDST, BMD dry correctly detected most (15/24; 63%), followed by MGIT960 and BMD frozen (13/24; 61%) and lastly AP (12/24; 50%). Applying the CRS, the prevalence of bedaquiline resistance was 2.2% and ranged from 1.4 to 3.4%, depending on the method used. All methods performed well for bedaquiline susceptibility determination; however, resistance detected should be investigated by a second, alternative method.
INTRODUCTION
Globally, multidrug-resistant and extensively drug-resistant tuberculosis (MDR-TB and XDR-TB) have poor clinical outcomes, with only 55% of MDR-TB and 34% of XDR-TB cases achieving treatment success (1). There are, however, positive signs that outcomes are improving with the use of new and repurposed drugs. Bedaquiline (BDQ) is a novel antimycobacterial agent that has been shown to have significant in vitro and in vivo activity against Mycobacterium tuberculosis, and it is associated with improved clinical outcomes (2–4). Although it has delayed early clinical activity, it achieves potent clearance of M. tuberculosis, leading to a significant reduction in time to culture conversion (4–7). The World Health Organization (WHO) classified the drug as group A, to be used for the first-line treatment of drug-resistant TB (8).
The use of BDQ globally has increased significantly, with more than 60 countries reporting use of the drug by the end of 2017 (1), and it will continue to expand exponentially with the updated guidance from the WHO. Resistance to BDQ has emerged during treatment (9–11) and is expected to increase over time. Importantly, cross-resistance between BDQ and clofazimine, a WHO group B drug (8), has been shown in both laboratory-engineered and clinical isolates (12–14).
Increased MICs to BDQ are associated with mutations in the ATP synthase target atpE (2) and the efflux pump regulator Rv0678 (12, 15, 16), which have been observed in clinical isolates (12, 17). The mutations in the clinically relevant atpE and Rv0678 mutants include single nucleotide insertions, deletions and substitutions, large deletions, and random insertions of sequence elements (12, 15, 16). It is not possible to detect these mutations with the rapid molecular drug susceptibility testing systems available today, including the GeneXpert and Line Probe Assay platforms, but it is possible using targeted next-generation sequencing technology. Moreover, the association between specific mutations and BDQ MIC with clinical outcome has not been established yet.
As a result, there is a lack of algorithms to predict poor clinical outcomes due to these mutations, and whole-genome sequencing alone might not provide sufficient clinical guidance at this stage. Phenotypic drug susceptibility testing (pDST) for BDQ will be used for these purposes in the near future, and the combination of pDST with molecular methods, such as whole-genome sequencing or targeted next-generation sequencing for drug resistance surveillance purposes, will be valuable for improving their predictive capability.
Quality control parameters for the BDQ pDST using 7H10 and 7H11 agar dilution, as well as 7H9 broth microdilution (BMD) MIC methods, have been established in a multicountry, multilaboratory study (18). BDQ DST using the MGIT960 system has also been reported. Keller et al. (19) proposed a BDQ MGIT960 epidemiological cutoff value (ECOFF) of 1.6 μg/ml, while Torrea et al. (20) proposed 1 μg/ml. In the latter study, the BDQ 7H11 agar MIC ECOFF was proposed at 0.25 μg/ml.
A recent study by Ismail et al. included 391 clinical M. tuberculosis isolates where whole-genome sequencing was performed, together with BDQ MGIT960 and 7H9 BMD MIC (17). Susceptible and intermediate categories were determined to have BDQ MICs of ≤0.12 and 0.25 μg/ml using BMD and of ≤1 and 2 μg/ml using MGIT960. The study by Ismail et al. was a single-country study and excluded agar dilution MIC.
In 2018, the WHO systematically reviewed the available data on BDQ pDST (agar dilution MIC and MGIT960) from both the literature and the sponsor. This review did not include BMD MIC method. Based upon the totality of the evidence, BDQ interim critical concentrations (CCs) of 0.25 and 1 μg/ml were established for the 7H11 agar proportion (AP) method and the MGIT960 system, respectively (21). The WHO-proposed CC was based on multiple studies that lacked standardization and did not include BMD. An external quality assessment (EQA) study by Kaniga et al. confirmed the appropriateness of the susceptible interpretive criterion by Ismail et al. and WHO interim CCs using a set of well-characterized isolates (22).
In this study, we evaluate the performance of the breakpoints (BPs) for BDQ pDST comparing different methods across multiple regions, assess the molecular basis of phenotypic resistance, and determine the prevalence of BDQ phenotypic resistance by each method among BDQ treatment-naive DR-TB patients.
RESULTS
A total of 495 geographically diverse isolates, representing 28 countries across four continents, were successfully tested using the different methodologies in five WHO Supranational Reference laboratories. The resistance profile of the isolates included: MDR-TB (276; 56%), pre-XDR-TB (135; 27%), XDR-TB (39; 8%), and the remainder a mixed pattern (45; 9%). For the BDQ BMD ECOFF determination, 494 isolates provided valid results for both frozen and dry microtiter plates. The histograms are shown in Fig. 1 and 2, respectively.
FIG 1.
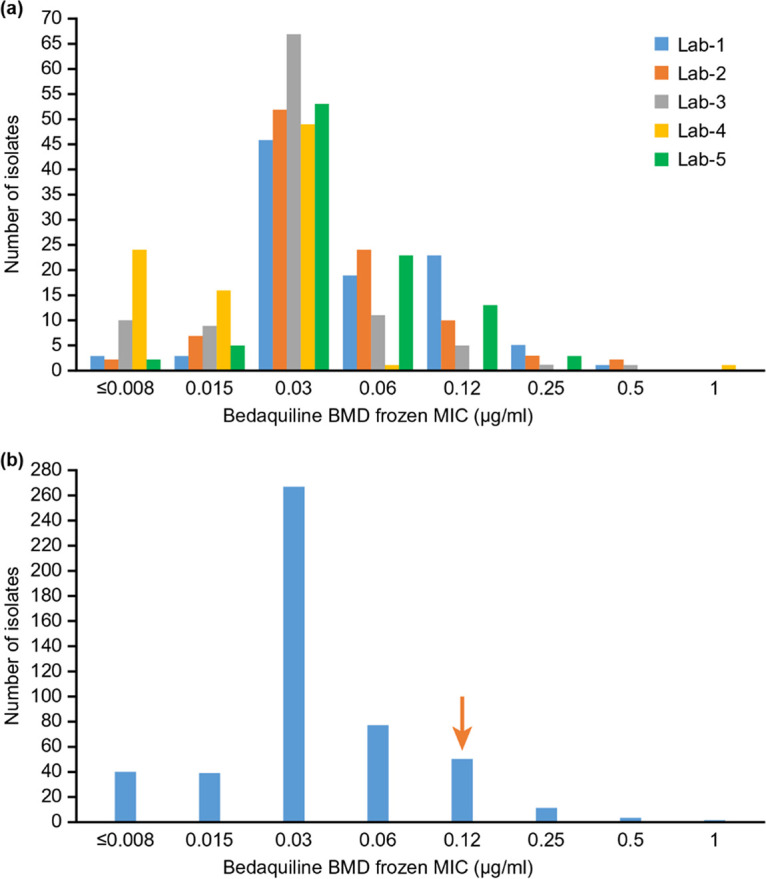
BDQ MIC distribution for BMD frozen plates by particular laboratory (a) and by all laboratories (n = 494) (b). The total number of isolates was 494 (Lab 1, 104; Lab 2, 100; Lab 3, 100; Lab 4, 91; and Lab 5, 99). The arrow indicates the ECOFF determined by visual inspection.
FIG 2.
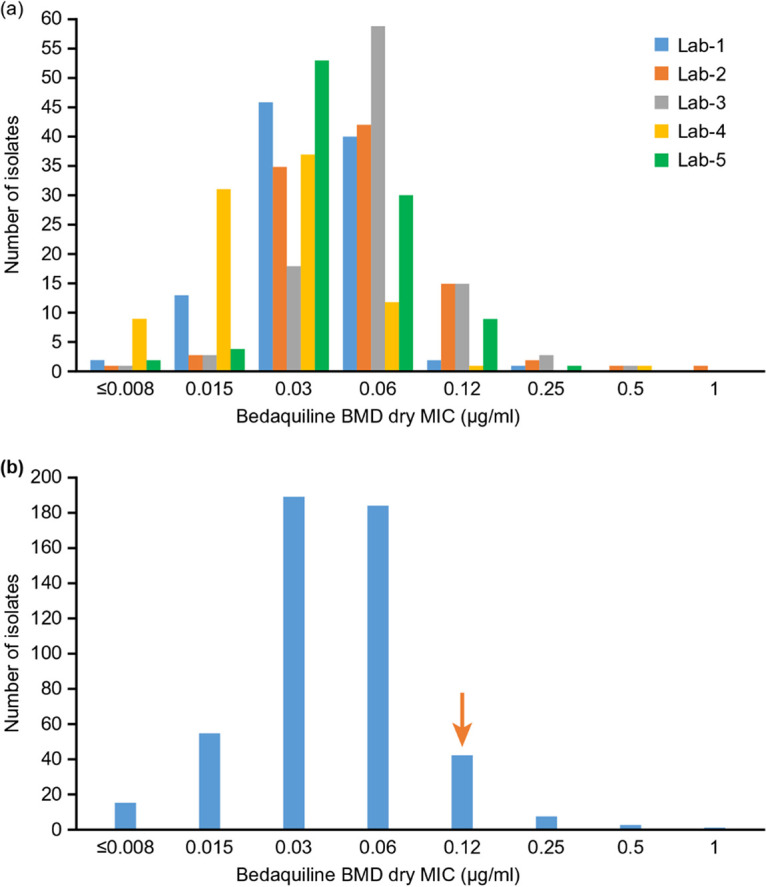
BDQ MIC distribution for BMD dry plates by particular laboratory (a) and by all laboratories (n = 494) (b). The total number of isolates was 494 (Lab 1, 104; Lab 2, 100; Lab 3, 100; Lab 4, 91; and Lab 5, 99). The arrow indicates the ECOFF determined by visual inspection.
The overall BMD MIC distributions were unimodal, with a mode of 0.03 μg/ml for both frozen and dry plates. For the dry plate, two laboratories had a mode of 0.06 μg/ml, which is probably normal assay variation. As expected, there were some differences in susceptibility of isolates to BDQ across laboratories. The ECOFF determined by visual observation of the histograms is 0.12 μg/ml (shown as the downward vertical arrows in figure). The raw data were entered into the ECOFFinder tool (23, 24). The wild-type (WT) ECOFF at 97.5% was 0.125 μg/ml, which was consistent with histogram-based ECOFF. The WT ECOFF 99% was 0.25 μg/ml (Fig. 3).
FIG 3.
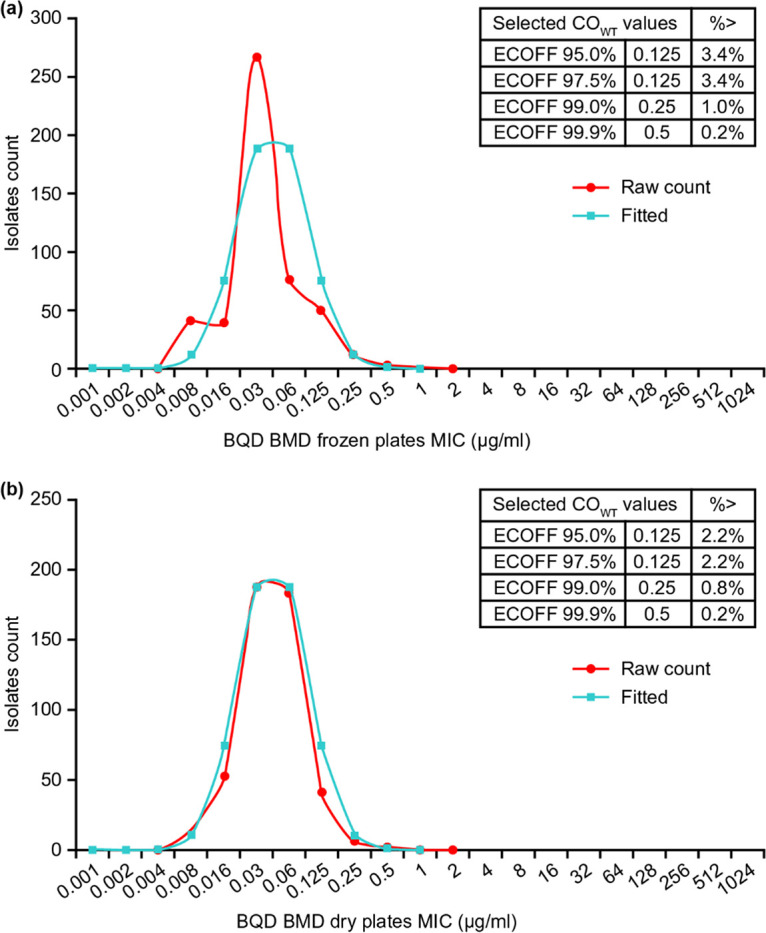
ECOFF plots and values for BMD frozen (a) and dry (b) plates.
Of the total set of isolates, 467 (94.3%) were determined to be susceptible by all methods, and 28 were determined to be resistant by at least one method with four of these resistant by all. Of the 28 resistant isolates, 16 were classified as wild type for the BDQ genes of interest (Table 1). The remaining 12 isolates had mutations in the Rv0678 gene. Isolates that had a genetic insertion, denoted by “ins” in Table 1, were more likely to have more than one method classifying resistance (5/7) compared to isolates with a single nucleotide polymorphism (SNP; 1/5). None of the isolates had an atpE mutation.
TABLE 1.
Rv0678 variants and number of DST methods classifying resistance (n = 28)a
|
Rv0678 (NP_215192.1) mutation |
No. of methods classifying resistance |
Total | ||||
|---|---|---|---|---|---|---|
| Mutation type | Nucleotide change | 1 | 2 | 3 | 4 | |
| Single nucleotide polymorphism | c. 220 C>G | 1 | 1 | |||
| c. 350 T>G | 1 | 1 | ||||
| c. 320 G>T | 1 | 1 | ||||
| c. 74 G>A | 1 | 1 | ||||
| c. 136 T>C | 1 | 1 | ||||
| Insertion | c. 466 insC | 1 | 1 | |||
| c. 126 insG/WT | 1 | 1 | ||||
| c. 135 insG/WT | 1 | 1 | ||||
| c. 139 insG | 1 | 1 | ||||
| c. 144 insC | 1 | 1 | ||||
| c. 198 insG/c. 417 G>A/WT | 1 | 1 | ||||
| c. ins46 TGTGATC | 1 | 1 | ||||
| None | WT | 11 | 1 | 2 | 2 | 16 |
| Total | 17 | 3 | 4 | 4 | 28 | |
RAV, resistance-associated variant; WT, wild-type (includes the –11C>A mutation in one isolate).
Of the 28 strains classified as phenotypically resistant, 17 (61%) were classified as resistant by only one method and 11 (39%) were classified as resistant by more than one method. Among the 17, 11 (65%) isolates did not harbor any mutation; 9 of these 11 isolates showed no growth (i.e., were susceptible) when tested at a double-dilution higher concentration. The remaining six isolates had a mutation, and five of these showed no growth when tested at a double-dilution higher concentration. Thus, these isolates, although classified as resistant, had an MIC very close to the cutoff applied.
Among the 11 isolates classified as resistant by more than one method, six (55%) had a mutation. These six isolates were tested with all four methods (i.e., 24 individual tests), 18 tests confirmed resistance at the BP. These were then further tested at a double-dilution higher concentration; 10 (10/18; 56%) showed no growth. Of the five isolates that did not harbor a mutation and tested by all four methods (i.e., 20 individual tests), 16 tests confirmed resistance at the individual BP. Nine of the 16 individual tests (56%) showed no growth at a double-dilution higher concentration.
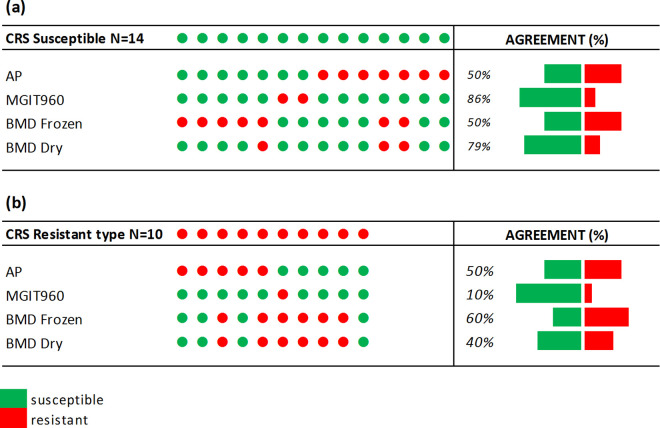
Applying the composite reference standard (CRS) to the 24 discordant isolates, 14 were reclassified as susceptible, and 10 remained resistant. Of the 14 CRS susceptible isolates, MGIT960 correctly classified 12/14 (86%), followed by BMD dry (11/14; 79%), while the other two methods classified 7/14 each (50%) (Fig. 4a). The majority (12/14) were classified by more than one method as susceptible. Among the 10 CRS resistant isolates, the pattern was reversed with BMD frozen detecting the most (6/10; 60%), followed by AP (5/10; 50%), BMD dry (4/10; 40%), and MGIT (1/10; 10%) (Fig. 4b). Similarly, the majority (8/10) were classified by more than one method as resistant. Of the 24 discordant isolates using the CRS as the final classification (susceptible or resistant), BMD dry classified the most correctly (15/24; 63%), followed by MGIT960 and BMD frozen (13/24; 54%) and lastly by AP (12/24; 50%) (Fig. 4).
FIG 4.
Diagrammatic representation of BDQ DST results for discordance isolates by method stratified by CRS susceptible (n = 14) (a) and resistant (n = 10) (b). AP, agar proportion; BMD, broth microdilution.
The prevalence of BDQ resistance by all methods, including the CRS is shown in Table 2. Applying the CRS, the prevalence was 2.2% (11/492) and varied by method: 1.4% (MGIT960), 2.2% (BMD dry), 3.2% (AP), and 3.4% (BMD frozen).
TABLE 2.
Prevalence of BDQ resistance by method and composite reference standarda
| DST method | CC or BP (μg/ml) | No. | % resistant (range) |
|---|---|---|---|
| AP | 0.25 | 495 | 3.2 (1.9–5.2) |
| BMD frozen | 0.12 | 494 | 3.4 (2.0–5.5) |
| BMD dry | 0.12 | 494 | 2.2 (1.1–3.9) |
| MGIT960 | 1 | 492 | 1.4 (0.6–2.9) |
| CRS | NA | 492 | 2.2 (1.1–3.9) |
CC, critical concentration; BP, MIC breakpoint; NA, not applicable; CRS, composite reference standard. The CC is indicated for the agar proportion (AP) and MGIT960 methods; the BP is indicated for BMD frozen and BMD dry methods.
DISCUSSION
Resistance to BDQ is an emerging concern impacting on the successful management of drug-resistant TB globally. This study provides important data on the robustness of different methods for susceptibility testing against BDQ. Isolates from 28 countries across 4 continents were processed in five supranational TB reference laboratories according to standardized methods. Our findings have confirmed the critical concentration criteria set out by the WHO applied to AP and MGIT960 at concentrations of 0.25 and 1 μg/ml, respectively. We have also confirmed the validity of the BP proposed for the BMD methods. EUCAST has established noncommercial BMD as the reference method for mycobacterial DST (25), and our findings, albeit using a commercially manufactured assay, confirm the appropriateness of this selection. The MGIT960 and AP methods are established and widely used across TB laboratories globally and, applying the WHO criteria, they were comparable to BMD.
The ECOFFs determined in this study for both the frozen and dry BMDs matched those in the Ismail et al. study based on ECOFFs of 95, 97.5, and 99% (17). Although the ECOFF 99% is preferred, the Kaniga et al. study showed that using a well-characterized EQA panel, the ECOFF of 0.12 μg/ml based upon 97.5% was the most robust concentration to clearly delineate wild-type from non-wild-type populations (22). In the present study, applying this cutoff yielded good overall performance for correctly classifying susceptibility.
All methods were highly concordant for excluding resistance in patients without prior BDQ exposure with 94.3% (467/495) classified as susceptible across all methods. The prevalence of BDQ resistance was determined to be 2.2% when applying the composite reference standard. The prevalence ranged between 1.4 and 3.4% depended on the method applied, and such variance is expected at this low prevalence. The relatively low prevalence observed is appropriate, since BDQ resistance is not expected to be high currently, and these isolates were not selected based on prior BDQ exposure. Our findings are within the range of what was reported in the Ismail et al. study from South Africa (17) and in the study by Villellas et al. when applying the revised BPs to their data (12). The latter study included baseline isolates from two phase 2b clinical trials.
The genetic basis of resistance to BDQ is not fully elucidated; however, mutations in Rv0678 and the atpE gene have been associated with resistance (12, 15, 16). Not all variants in these genes confer resistance and, for Rv0678, prior exposure and activation of the encoded efflux pump impacts the MIC and consequent resistance development (17). Isolates that harbored insertions rather than SNPs were also more likely to test resistant by more than one method, indicating a significant impact on the functional activity of the efflux pump and development of true resistance.
Many of the isolates with discordant pDST results had an MIC that was a single dilution above the breakpoint. It should also be noted that Rv0678 mutations have variable MICs, making a clear assessment of resistance determination challenging (11, 26). Testing on an alternative phenotypic method, however, does seem to be a reasonable approach to confirm resistance at this stage when discordance is detected. If this is not possible, repeat testing with the same method at the BP, and a dilution higher may clarify a technical error or an MIC that is close to the BP. The use of sequencing would be useful, but interpretation may be challenging, particularly for isolates that have an SNP rather than an insertion or deletion.
A limitation of the study was that we did not sequence all isolates. There was, however, high agreement across all methods for BDQ susceptibility, and not sequencing the susceptible isolates is unlikely to have affected the results. The ECOFF for BMD did include the later-defined R strains and may have skewed the results to the right. This is unlikely to have been significant since they accounted for <2.5% of the total (12/494) and accommodates the ECOFF at 97.5%. Our application of the CRS using sequencing to resolve discordant pDST may not be completely accurate, since the genetic basis of BDQ resistance is not fully understood. Nonetheless, the use of genetic results provides an independent method unaffected by technical challenges with phenotypic testing and is a reasonable approach.
Overall, this study has provided further evidence of the robustness of the methods for use as a diagnostic tool for determination of BDQ phenotypic resistance and BMD as a reference method for BDQ DST. We confirmed the criteria previously set, and based on the multicountry study design, using the standardized protocols, these findings are generalizable. The BMD dry plate performed well classifying the largest number of strains correctly and highly comparable to the existing WHO-endorsed methods (MGIT960 and AP). The BMD dry method also offers practical advantages over the frozen plates and should be considered for review by the WHO and regulatory agencies for clinical use in the future.
MATERIALS AND METHODS
We conducted a multicountry study, which included five laboratories from the WHO Supranational TB Reference Laboratory Network: Belgium, Italy, Japan, Pakistan, and South Africa. Each site tested approximately 100 BDQ treatment-naive DR-TB clinical isolates collected from different geographic areas, using a standardized approach with multiple methods: AP, MGIT960, and BMD (frozen and dry).
For the 7H11 AP method, laboratories were provided with BDQ active pharmaceutical ingredient, lot number A17HB1824 (Janssen Pharmaceutica, Beerse, Belgium). For the 7H9 BMD MIC testing, the following materials were provided by Thermo Fisher Scientific (Oakwood Village, OH): frozen panels containing prediluted 2× drugs in 2× medium (7H9 broth/oleic acid albumin dextrose catalase [OADC]) and dry panels containing lyophilized prediluted 1× drugs. All dilutions were made in polystyrene microtiter plates. The BDQ concentration range was 0.008 to 4 μg/ml. For MGIT960 DST, lyophilized BDQ vials containing 170 μg/vial potency-adjusted BDQ (Becton Dickinson, Franklin Lakes, NJ) was reconstituted in 2 ml of dimethyl sulfoxide/vial. Clinical isolates were tested by MGIT960 and AP at the WHO-recommended CCs. In addition, MGIT960 and AP were performed at one and two doubling concentrations higher than the CCs, respectively.
The multicountry, multilaboratory-validated BDQ breakpoints (BPs) for the BMD and CCs for AP and MGIT960 were applied to categorize the clinical isolates into susceptible and resistant, and comparisons were made. Since resistance is not expected in this set of isolates, only when an isolate was determined to be resistant by any pDST method was sequencing performed. Sequencing targeted the Rv0678 and atpE genes which have been associated with resistance in clinical isolates (11, 12, 17). The pDST resistance classification was adjusted based upon the occurrence of mutations related to these genes. We also evaluated the classification of isolates using a composite reference standard (CRS). This was defined as follows: (i) where all test methods agreed as susceptible or resistant, these results were accepted; (ii) where pDST results were discordant, these isolates were considered resistant only if an atpE or Rv0678 mutation was also present; and (iii) in the absence of the above, isolates were reclassified as susceptible to BDQ. We considered mutations associated with both resistance and susceptible profile in literature when reclassifying and, if a mutation was not described, we assumed it to be a resistance determinant (2, 10–17, 20, 27). Discordance across different methods against the CRS where graphically tabulated. The prevalence of BDQ resistance among the total isolates tested was then determined for each method as well as the CRS.
Data availability. The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
ACKNOWLEDGMENTS
We thank the staff in the laboratories that provided the necessary support for the successful completion of the study. R.H. thanks Sabira Tahseen, Alamdar Hussain, Joveria Farooqi, Sarah Baber, and Zabin Wajidali for their contributions to the study. N.A.I. thanks the staff of the Centre for Tuberculosis for their contribution to the study, in particular Lavania Joseph, Farzana Ismail, and Dumisani Ngcamu. D.M.C. thanks Marco Rossi for his contribution to the study. We acknowledge the support of Briony Chisholm for assistance with editing and formatting of the manuscript and also Zoetic Science, an Ashfield company, Macclesfield, UK, for formatting of the manuscript and figures, which was funded by Janssen.
This study was sponsored by Johnson & Johnson Global Public Health; a Division of Janssen Pharmaceutica, NV; and the Bill and Melinda Gates Foundation.
K.K. is a full-time employee of Janssen and potential stockholder of Johnson & Johnson. A.A. has received funding from Janssen Pharmaceutica, NV. OSR (D.M.C. and E.B.) have received funding covering the cost of staff and reagents for this study. The Institute of Tropical Medicine, Antwerp (C.D. and G.T.), has received funding for the study activities from Janssen. R.H. has received funding from Janssen Pharmaceutica for the BDQ DREAM and EQA studies. S.M. has received funding from Janssen Pharmacetica, NV. S.S. has received funding from Janssen Pharmaceutica for the BDQ DREAM and EQA studies. N.A.I. has received funding from Janssen Pharmaceutica for surveillance activities related to bedaquiline. S.V.O. has received funding to prepare and provide training for BDQ-related Janssen Pharmaceutica activities.
All authors substantially contributed to the study’s design and protocol and execution of the work described. All authors were involved in the development of the primary manuscript and interpretation of the data and have read and approved the final version and met the criteria for authorship as established by the ICMJE.
REFERENCES
- 1.World Health Organization. Global TB Report. 2018. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1. Accessed 7 December 2018. [Google Scholar]
- 2.Andries K, Verhasselt P, Guillemont J, Göhlmann WH, Neefs J-M, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 3.Schnippel K, Ndjeka N, Maartens G, Meintjes G, Master I, Ismail N, Hughes J, Ferreira H, Padanilam X, Romero R, Te Riele J, Conradie F. 2018. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med 6:699–706. doi: 10.1016/S2213-2600(18)30235-2. [DOI] [PubMed] [Google Scholar]
- 4.Mbuagbaw L, Guglielmetti L, Hewison C, Bakare N, Bastard M, Caumes E, Fréchet-Jachym M, Robert J, Veziris N, Khachatryan N, Kotrikadze T, Hayrapetyan A, Avaliani Z, Schünemann HJ, Lienhardt C. 2019. Outcomes of bedaquiline treatment in patients with multidrug-resistant tuberculosis. Emerg Infect Dis 25:936–943. doi: 10.3201/eid2505.181823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diacon AH, Donald PR, Pym A, Grobusch M, Patientia RF, Mahanyele R, Bantubani N, Narasimooloo R, De Marez T, van Heeswijk R, Lounis N, Meyvisch P, Andries K, McNeeley DF. 2012. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother 56:3271–3276. doi: 10.1128/AAC.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borisov SE, Dheda K, Enwerem M, Romero Leyet R, D’Ambrosio L, Centis R, Sotgiu G, Tiberi S, Alffenaar J-W, Maryandyshev A, Belilovski E, Ganatra S, Skrahina A, Akkerman O, Aleksa A, Amale R, Artsukevich J, Bruchfeld J, Caminero JA, Carpena Martinez I, Codecasa L, Dalcolmo M, Denholm J, Douglas P, Duarte R, Esmail A, Fadul M, Filippov A, Davies Forsman L, Gaga M, Garcia-Fuertes J-A, García-García J-M, Gualano G, Jonsson J, Kunst H, Lau JS, Lazaro Mastrapa B, et al. 2017. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J 49:1700387. doi: 10.1183/13993003.00387-2017. [DOI] [PubMed] [Google Scholar]
- 7.Diacon AH, Pym A, Grobusch MP, de los Rios JM, Gotuzzo E, Vasilyeva I, Leimane V, Andries K, Bakare N, De Marez T, Haxaire-Theeuwes M, Lounis N, Meyvisch P, De Paepe E, van Heeswijk RPG, Dannemann B, TMC207-C208 Study Group. 2014. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 371:723–732. doi: 10.1056/NEJMoa1313865. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. 2019. WHO consolidated guidelines on drug-resistant tuberculosis treatment. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 9.Nguyen TVA, Anthony RM, Banuls AL, Nguyen TVA, Vu DH, Alffenaar JC. 2018. Bedaquiline resistance: its emergence, mechanism, and prevention. Clin Infect Dis 66:1625–1630. doi: 10.1093/cid/cix992. [DOI] [PubMed] [Google Scholar]
- 10.Ghodousi A, Rizvi AH, Baloch AQ, Ghafoor A, Khanzada FM, Qadir M, Borroni E, Trovato A, Tahseen S, Cirillo DM. 2019. Acquisition of cross-resistance to bedaquiline and clofazimine following treatment for tuberculosis in Pakistan. Antimicrob Agents Chemother 63:e00915-19. doi: 10.1128/AAC.00915-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimenkov DV, Nosova EY, Kulagina EV, Antonova OV, Arslanbaeva LR, Isakova AI, Krylova LY, Peretokina IV, Makarova MV, Safonova SG, Borisov SE, Gryadunov DA. 2017. Examination of bedaquiline- and linezolid-resistant Mycobacterium tuberculosis isolates from the Moscow region. J Antimicrob Chemother 72:1901–1906. doi: 10.1093/jac/dkx094. [DOI] [PubMed] [Google Scholar]
- 12.Villellas C, Coeck N, Meehan CJ, Lounis N, de Jong B, Rigouts L, Andries K. 2017. Unexpected high prevalence of resistance-associated Rv0678 variants in MDR-TB patients without documented prior use of clofazimine or bedaquiline. J Antimicrob Chemother 72:684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Wang B, Hu M, Huo F, Guo S, Jing W, Nuermberger E, Lu Y. 2017. Primary clofazimine and bedaquiline resistance among isolates from patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 61:e00239-17. doi: 10.1128/AAC.00239-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ismail N, Peters RPH, Ismail NA, Omar SV. 2019. Clofazimine exposure in vitro selects efflux pump mutants and bedaquiline resistance. Antimicrob Agents Chemother 63:e02141-18. doi: 10.1128/AAC.02141-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartkoorn RC, Uplekar S, Cole ST. 2014. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:2979–2981. doi: 10.1128/AAC.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ismail NA, Omar SV, Joseph L, Govender N, Blows L, Ismail F, Koornhof H, Dreyer AW, Kaniga K, Ndjeka N. 2018. Defining bedaquiline susceptibility, resistance, cross-resistance and associated genetic determinants: a retrospective cohort study. EBioMedicine 28:136–142. doi: 10.1016/j.ebiom.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaniga K, Cirillo DM, Hoffner S, Ismail NA, Kaur D, Lounis N, Metchock B, Pfyffer GE, Venter A. 2016. A multilaboratory, multicountry study to determine bedaquiline MIC quality control ranges for phenotypic drug susceptibility testing. J Clin Microbiol 54:2956–2962. doi: 10.1128/JCM.01123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller PM, Homke R, Ritter C, Valsesia G, Bloemberg GV, Bottger EC. 2015. Determination of MIC distribution and epidemiological cutoff values for bedaquiline and delamanid in Mycobacterium tuberculosis using the MGIT 960 system equipped with TB eXiST. Antimicrob Agents Chemother 59:4352–4355. doi: 10.1128/AAC.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torrea G, Coeck N, Desmaretz C, Van De Parre T, Van Poucke T, Lounis N, de Jong BC, Rigouts L. 2015. Bedaquiline susceptibility testing of Mycobacterium tuberculosis in an automated liquid culture system. J Antimicrob Chemother 70:2300–2305. doi: 10.1093/jac/dkv117. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. 2018. Technical report on critical concentrations for TB drug susceptibility testing of medicines used in the treatment of drug-resistant TB. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 22.Kaniga K, Aono A, Borroni E, Cirillo DM, Desmaretz C, Hasan R, Joseph L, Mitarai S, Shakoor S, Torrea G, Ismail NA, Omar SV. 2020. Validation of bedaquiline phenotypic drug susceptibility testing methods and breakpoints: a multi-laboratory, multi-country study. J Clin Microbiol 58:e01677-19. doi: 10.1128/JCM.01677-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterization of bacterial wild-type MIC value distributions and the determination of epidemiological cutoff values. Clin Microbiol Infect 12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2020. ECOFFinder tool. Clinical and Laboratory Standards Institute, Wayne, PA: https://clsi.org/meetings/microbiology/ecoffinder/#:~:text=Lab%20Week%202020-,ECOFFinder,type%20bacterial%20or%20fungal%20populations. Accessed 7 January 2020. [Google Scholar]
- 25.EUCAST. 2020. European Committee on Antimicrobial Susceptibility Testing. EUCAST, Växjö, Sweden: https://www.eucast.org/mycobacteria/methods_in_mycobacteria. Accessed 29 June 2020. [Google Scholar]
- 26.Peretokina IV, Krylova LY, Antonova OV, Kholina MS, Kulagina EV, Nosova EY, Safonova SG, Borisov SE, Zimenkov DV. 2020. Reduced susceptibility and resistance to bedaquiline in clinical Mycobacterium tuberculosis isolates. J Infect 80:527–535. doi: 10.1016/j.jinf.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Segala E, Sougakoff W, Nevejans-Chauffour A, Jarlier V, Petrella S. 2012. New mutations in the mycobacterial ATP synthase: new insights into the binding of the diarylquinoline TMC207 to the ATP synthase C-ring structure. Antimicrob Agents Chemother 56:2326–2334. doi: 10.1128/AAC.06154-11. [DOI] [PMC free article] [PubMed] [Google Scholar]