The incidence of invasive fungal infections is rising due to the increase in susceptible populations. Current clinically available drugs have therapeutic limitations due to toxicity, a narrow spectrum of activity, and, more importantly, the consistent rise of fungal species that are intrinsically resistant or that develop resistance due to prolonged therapy. Thus, there is an urgent need for new broad-spectrum antifungal agents with low toxicity and a novel mechanism of action. We previously reported a new class of potent antifungal compounds, acylhydrazones, that target the fungal sphingolipid pathway.
KEYWORDS: Aspergillus, Candida, Cryptococcus, acylhydrazone, antifungal agents, invasive fungal infections
ABSTRACT
The incidence of invasive fungal infections is rising due to the increase in susceptible populations. Current clinically available drugs have therapeutic limitations due to toxicity, a narrow spectrum of activity, and, more importantly, the consistent rise of fungal species that are intrinsically resistant or that develop resistance due to prolonged therapy. Thus, there is an urgent need for new broad-spectrum antifungal agents with low toxicity and a novel mechanism of action. We previously reported a new class of potent antifungal compounds, acylhydrazones, that target the fungal sphingolipid pathway. Based upon our initial lead molecules, (E)-N′-(5-bromo-2-hydroxybenzylidene)-2-methylbenzohydrazide and D13, we performed a structure-activity relationship study, synthesizing ca. 300 new compounds. Of these, 5 compounds were identified to be the most promising for further studies, based on their broad-spectrum activity and low toxicity in mammalian cells lines. Among these top 5 lead compounds, we report here the impressive in vivo activity of 2,4-dibromo-N′-(5-bromo-2-hydroxybenzylidene)benzohydrazide (SB-AF-1002) in several models of systemic fungal infection. Our data show that SB-AF-1002 is efficacious and outperforms current standard-of-care drugs in models of invasive fungal infections, such as cryptococcosis, candidiasis, and aspergillosis. Specifically, animals treated with SB-AF-1002 not only survived the infection but also showed a clearing of fungal cells from key organs. Moreover, SB-AF-1002 was very effective in an aspergillosis model as a prophylactic therapy. SB-AF-1002 also displayed acceptable pharmacokinetic properties in mice, similar to those of the parent compound, D13. These results clearly indicate that our novel acylhydrazones constitute a new class of highly potent and efficacious antifungal agents which warrant further development for the treatment of invasive fungal infections.
INTRODUCTION
Invasive fungal infections (IFIs) are emerging as a serious threat to human health. In recent reports, IFIs were estimated to cause death at numbers similar to the numbers of deaths from tuberculosis or malaria (1). In the past few decades, the incidence of invasive mycoses has increased dramatically, principally due to the increase in the population susceptible to these infections, the emergence of new fungal species, and substantial progress in the diagnosis of these infections (2, 3). It was recently estimated that more than 300 million people are diagnosed with serious fungal infections (3) and that Cryptococcus, Candida, and Aspergillus infections account for 1.5 million to 2 million deaths annually (4). Individuals at high risk include immunocompromised subjects, such as HIV-positive/AIDS patients, organ transplant recipients, pediatric and geriatric cancer patients, and any patients undergoing immunosuppressive therapy for numerous conditions (5–8). Invasive fungal infections are associated with a prolonged or extended hospital stay, resulting in an increase in the overall health care cost (9). Current major classes of antifungal drugs, including azoles (e.g., fluconazole), polyenes (e.g., amphotericin B), and echinocandins (e.g., caspofungin) (10), come with therapeutic challenges, such as nephrotoxicity, drug-drug interactions, a narrow spectrum of activity, and more importantly, emerging resistance (11–14). For these reasons there is an urgent need for a new class of antifungal drugs targeting fungal pathways different from those engaged by standard-of-care (SoC) therapeutics.
In our previous studies, we demonstrated that a new target for antifungal drug development is the fungal sphingolipid pathway (15). In particular, we identified and characterized (E)-N′-(5-bromo-2-hydroxybenzylidene)-2-methylbenzohydrazide (BHBM), which targets the synthesis of the fungal, but not mammalian, sphingolipid glucosylceramide (GlcCer) and demonstrated its efficacy in vitro and in vivo against a series of pathogenic fungi. We also screened a series of BHBM analogs and identified D13, which has shown improved efficacy in killing different fungi and lower toxicity in mammalian cell lines and which resulted in increased survival in several animal models of fungal infections compared with the characteristics of the SoC therapeutics. These results have further validated the sphingolipid glucosylceramide (GlcCer) pathway to be a highly promising target for antifungal therapy (16). Following up on the encouraging results, we designed and synthesized a library of aromatic acylhydrazones for structure-activity relationship (SAR) studies (17). We identified 5 compounds that are highly potent and selective against several key strains of fungi in vitro. These new lead compounds are highly synergistic when used in combination with SoC therapeutics against both resistant and nonresistant fungal strains.
Among the 5 lead compounds, based on its improved broad-spectrum activity, selectivity, and low in vitro toxicity, we report here the findings of our preclinical studies on 2,4-dibromo-N′-(5-bromo-2-hydroxybenzylidene)benzohydrazide (SB-AF-1002) (Fig. 1) in animal models of invasive fungal infections, assessing its efficacy and pharmacokinetic properties in vivo.
FIG 1.
Structure of SB-AF-1002.
RESULTS
Synthesis of SB-AF-1002.
Commercially available 2,4-dibromobenzoic acid was converted to its methyl ester, followed by reaction with hydrazine monohydrate to give 2,4-dibromobenzohydrazide. This hydrazide was then condensed with 5-bromosalicylaldehyde to afford 2,4-dibromo-N′-(5-bromo-2-hydroxybenzylidene)benzohydrazide (SB-AF-1002) as an off-white solid in a high overall yield with high purity. See Materials and Methods for details.
In vivo efficacy against cryptococcosis.
SB-AF-1002 was tested in various mouse models of cryptococcosis to assess its efficacy in vivo against Cryptococcus neoformans, the pathogen causing this disease. In the first experiment, mice were treated orally (p.o.) with 20 mg/kg of body weight/day of SB-AF-1002 immediately after intranasal infection with 5 × 105 C. neoformans cells. Fluconazole at the same dose was used as a positive/comparator control, and a group of vehicle-treated mice was used as a negative control. At the end of the experiment, 100% of the mice treated with SB-AF-1002 survived, whereas only 30% of the mice treated with fluconazole survived and 100% of the vehicle-treated mice died, as expected, by day 30 (for SB-AF-1002 versus no drug, P = 0.0001; for SB-AF-1002 versus fluconazole, P = 0.0007) (Fig. 2a). At the end of the experiment (day 40), we determined the endpoint organ fungal burden by measuring the numbers of CFU from the lung and brain. Thus, brains and lungs were collected and processed for determination of the numbers of CFU. Seven mice out of 10 in the SB-AF-1002 group showed no CFU in the brain, 2 had numbers of CFU under the detection limit, and only 1 showed appreciable numbers of CFU. Five out of 10 mice showed no CFU in the lung, 2 had numbers of CFU under the detection limit, and 3 showed CFU in the lung, but the numbers were statistically significantly lower than those in the 3 mice in the fluconazole-treated group that survived (Fig. 2b and c).
FIG 2.
(a) Survival of mice infected intranasally with 5 × 105 C. neoformans cells and treated with 20 mg/kg/day of the compound through gavage. #, P = 0.0001 for SB-AF-1002 versus no drug; *, P = 0.0007 for SB-AF-1002 versus fluconazole (Fluco). (b and c) Endpoint numbers of CFU in the brain and lungs of mice that survived in the experiment whose results are presented in panel a. (d) Survival of mice infected intranasally with 5 × 105 C. neoformans cells with treatment starting at 5 days after infection with 20 mg/kg/day of compounds through gavage. #, P = 0.0001 for SB-AF-1002 versus no drug; *, P = 0.0016 for SB-AF-1002 versus fluconazole. (e and f) Endpoint numbers of CFU in the lungs and brains of mice that survived in the experiment whose results are presented in panel d.
The second study was performed to assess the efficacy of SB-AF-1002 in a more aggressive model of cryptococcosis. The mice were infected in the same manner as the previous experiment, although the treatment started 5 days after the infection to allow the development of meningitis. At the end of the experiment, 90% of the mice treated with SB-AF-1002 survived, whereas only 20% of the fluconazole-treated group survived (for SB-AF-1002 versus no drug, P = 0.0001; for SB-AF-1002 versus fluconazole, P = 0.0016) (Fig. 2d). The SB-AF-1002-treated mice that survived showed no CFU in the brain, and only 2 mice showed CFU in the lung, whereas the 2 mice in the fluconazole group had significant numbers of CFU in the brain and lungs (Fig. 2e and f).
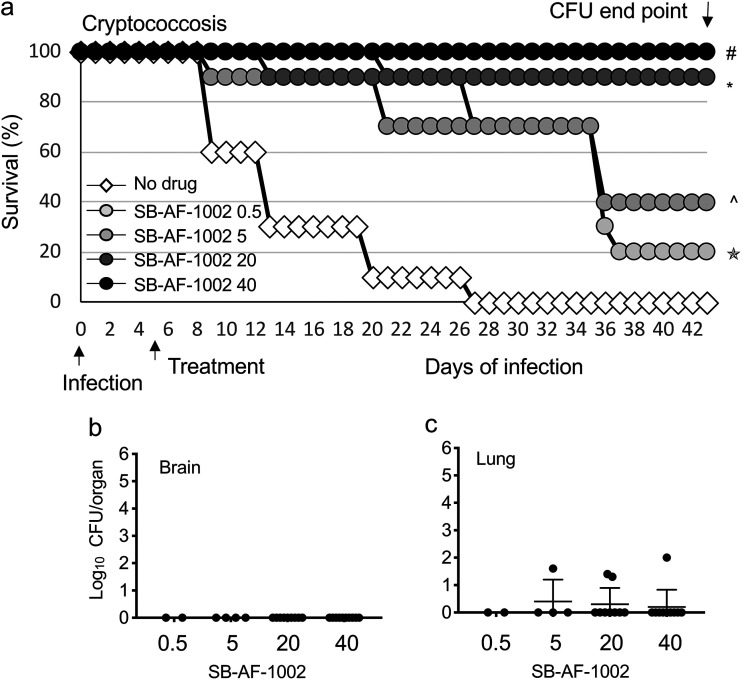
The third experiment was done to examine if SB-AF-1002 had a dose-related effect. Mice were infected as described above, and the treatment started 5 days after infection with 0.5, 5, 20, and 40 mg/kg/day of SB-AF-1002. All mice (100%) treated with 40 mg/kg/day survived, and 90% of the mice treated with 20 mg/kg/day survived, while 40% and 20% of the mice treated with 5 mg/kg/day and 0.5 mg/kg/day, respectively, survived, showing a dose-related response to SB-AF-1002 treatment (for SB-AF-1002 at 40 mg/kg versus no drug, P < 0.0001; for SB-AF-1002 at 20 mg/kg versus no drug, P = 0.0005; for SB-AF-1002 at 5 mg/kg versus no drug, P = 0.0016; for SB-AF-1002 at 0.5 mg/kg versus no drug, P = 0.136) (Fig. 3a). Among the mice that survived upon treatment with 40 mg/kg/day of SB-AF-1002, all except one showed a complete absence of cryptococcal cells in their brains and lungs (Fig. 3b and c). These results indicate that SB-AF-1002 is highly efficacious against cryptococcosis and outperformed fluconazole in these models.
FIG 3.
(a) Survival of mice infected intranasally with 5 × 105 C. neoformans cells and treated daily with increasing concentrations of SB-AF-1002 (05, 5, 20, and 40 mg/kg) through gavage, starting at day 5 postinfection. #, P < 0.0001 for SB-AF-1002 at 40 mg/kg versus no drug; *, P = 0.0005 for SB-AF-1002 at 20 mg/kg versus no drug; ^, P = 0.0016 for SB-AF-1002 at 5 mg/kg versus no drug; ✬, P = 0.136 for SB-AF-1002 at 0.5 mg/kg versus no drug. (b and c) Endpoint numbers of CFU in the lungs and brains of the mice that survived in the experiment whose results are presented in panel a.
In vivo efficacy against candidiasis.
SB-AF-1002 was examined in a model of invasive candidiasis. Mice were infected intravenously (i.v.) with 105 Candida albicans cells, and they were treated orally with 20, 40, and 80 mg/kg/day of SB-AF-1002 starting on the same day. Fluconazole at 20 mg/kg/day was used as a drug control, and a group of mice treated with vehicle only was used as the control for no treatment. The survival of mice treated with SB-AF-1002 was 20%, independent of the dose received, whereas all mice in the untreated and fluconazole-treated groups experienced 100% mortality (Fig. 4a). The kidneys of the surviving mice treated with SB-AF-1002 showed the presence of C. albicans cells (Fig. 4b).
FIG 4.
(a) Survival of mice infected intravenously with 105 C. albicans cells and starting a daily treatment on the day of infection with increasing concentration of SB-AF-1002 through gavage. There were no statistically significant differences between the groups. (b) Endpoint numbers of CFU in the left (L) and right (R) kidneys of the surviving mice treated with SB-AF-1002 at 20, 40, or 80 mg/kg. No CFU were observed in the left kidneys. (c) Survival of mice infected intravenously with 105 C. albicans cells and treated with 3 mg/kg/day of SB-AF-1002 intravenously. *, P = 0.0003 for SB-AF-1002 versus no drug. (d) Endpoint numbers of CFU in the left and right kidneys of the surviving mice in the experiment whose results are presented in panel c.
In the second survival study that was conducted, SB-AF-1002 was administered intravenously at a dose of 3 mg/kg/day. Interestingly, 100% of the mice treated with SB-AF-1002 survived, whereas 20% of the control group survived (for SB-AF-1002 versus no drug, P = 0.0003) (Fig. 4c), even though the surviving mice exhibited C. albicans cells in their kidneys (Fig. 4d). These results suggest that SB-AF-1002 may be efficacious against candidiasis when administered intravenously.
In vivo efficacy against invasive aspergillosis.
SB-AF-1002 was used in a mouse model of aspergillosis. Mice were immunosuppressed the day before infection and then infected and subsequently treated orally with 20, 40, or 80 mg/kg/day of SB-AF-1002, starting on the day of infection. A drug control group treated with voriconazole at a dose of 20 mg/kg/day, as well as a vehicle control group, was included. In the SB-AF-1002-treated group, 80% of the mice survived, independent of the doses used, and 50% of the mice survived in the voriconazole-treated group, whereas 100% of the untreated mice died within 8 days, as expected (for SB-AF-1002 versus no drug, P = 0.0012) (Fig. 5a). Lungs from SB-AF-1002-treated mice appeared to show a dose-dependent decrease in the number of PFU, albeit the difference between the 20- and 40-mg/kg/day doses was not statistically significant (Fig. 5b).
FIG 5.
(a) Survival of mice infected intranasally with 5 × 105 A. fumigatus conidia. Animals were immunosuppressed (IS) at 1 day prior to infection and treated with increasing concentrations of SB-AF-1002 (20, 40, and 80 mg/kg/day) or with voriconazole (Vori) at 20 mg/kg/day through gavage starting on the same day of infection. *, P = 0.0012 for SB-AF-1002 versus no drug. (b) Endpoint numbers of PFU in the lungs of mice that survived in the experiment whose results are presented in panel a. (c) Survival of mice infected intranasally with 5 × 105 A. fumigatus conidia and treated with SB-AF-1002 at 40 mg/kg/day or voriconazole at 20 mg/kg/day starting at 5 days before infection. Animals were immunocompromised at 1 day prior to infection. *, P < 0.0001 for SB-AF-1002 versus no drug; #, P = 0.013 for SB-AF-1002 versus voriconazole; ^, P = 0.0269 for voriconazole versus no drug. (d) Endpoint numbers of PFU in the lungs of mice that survived the experiment whose results are presented in panel c.
The second experiment was conducted to assess the efficacy of SB-AF-1002 as a preventive therapy against aspergillosis. Thus, at 5 days before immunosuppression, a group of 10 mice started treatment orally with a 20-mg/kg dose of SB-AF-1002 daily. The second group was treated with a 20-mg/kg dose of voriconazole daily, and the third group of mice was untreated. After 5 days, the mice were immunosuppressed and they were infected with Aspergillus fumigatus on the following day. We found a 100% survival of mice pretreated with SB-AF-1002, in contrast to the only 30% survival of mice receiving voriconazole (Fig. 5c). As expected, 100% of untreated mice in the control group died within 8 days (for SB-AF-1002 versus voriconazole, P = 0.013; for SB-AF-1002 versus no drug, P < 0.001) (Fig. 5c). When the lungs were examined, there was no trace of PFU in the SB-AF-1002-treated group, whereas lungs recovered from the voriconazole-treated group showed significant numbers of PFU (Fig. 5d). The voriconazole-treated mice also exhibited lethargy and significant weight loss, which required euthanasia. In addition, the livers of the voriconazole-treated mice were substantially larger than normal, and a protuberance in the upper right abdomen was observed during the course of the experiment (data not shown).
Pharmacokinetic studies.
In order to ascertain the distribution of SB-AF-1002 in the bloodstream, preliminary pharmacokinetic studies were carried out. The plasma concentration of SB-AF-1002 was monitored following intravenous (i.v.) or oral (p.o.) administration. Following i.v. administration, the concentration of SB-AF-1002 decreased from 10,000 ng/ml to nearly 100 ng/ml over a period of 4 h and remained at 100 ng/ml until 8 h (Fig. 6a). When administered orally, SB-AF-1002 persisted in the circulation at ∼1,000 ng/ml until 8 h and gradually decreased to a negligible 10 ng/ml at the end of the 24-h period (Fig. 6b).
FIG 6.
Pharmacokinetic studies of SB-AF-1002 administered intravenously (A) or orally (B) using a single dose of 1 mg/kg or 20 mg/kg, respectively.
Affinity toward hERG potassium channel.
In order to assess the affinity for the cardiac potassium channel, SB-AF-1002 was tested for its ability to block dofetilide binding to the human ether-a-go-go-related gene (hERG) potassium channel. The SB-AF-1002 50% inhibitory concentration (IC50) of 24.495 μM indicates that there is a low probability of cardiac effects mediated through this K channel. For comparison, the dofetilide IC50 was 0.009 μM. The FDA criterion for defining the probability that a drug is positive for hERG binding is an IC50 of <1 μM (low probability, IC50 > 10 μM; moderate probability, 1 μM < IC50 < 10 μM; high probability, IC50 < 1 μM).
Caco-2 cell permeability.
The permeation of SB-AF-1002 in the Caco-2 cell line was assessed. The efflux ratio of the compound was 2.54. It possessed a higher apparent permeability value in the secretory direction from basolateral to apical (Papp B to A) than the permeability value in the absorptive direction (Papp A to B). The recovery for SB-AF-1002 from the apical (AP) to the basolateral (BL) direction was lower than 10%, whereas the recovery from the BL to AP direction was 40% (Table 1).
TABLE 1.
Permeability results for test compounds in Caco-2 cell line
| Compound |
Papp (10−6 cm/s) |
Efflux ratio | Recovery (%) |
||
|---|---|---|---|---|---|
| A to B direction | B to A direction | AP to BL direction | BL to AP direction | ||
| Propranolol | 34.38 | 19.50 | 0.57 | 90.69 | 95.53 |
| Digoxin | 0.61 | 20.25 | 33.17 | 82.73 | 95.58 |
| SB-AF-1002 | 0.85 | 2.17 | 2.54 | 9.48 | 40.10 |
Metabolic stability studies.
The metabolic stability of SB-AF-1002 was assessed using mouse and human liver microsomes. SB-AF-1002 possessed a longer half-life (t1/2) in human liver microsomes than in mouse microsomes and also a slower intrinsic clearance (Table 2). SB-AF-1002 was also found to be less stable in the presence of the reducing enzyme NADPH. After 60 min, nearly 75% of the compound remained in the human microsomes, whereas only 11% remained in the mouse liver microsomes. In the absence of NADPH, however, more than 80% of the compound remained in both human and mouse microsomes (Table 3).
TABLE 2.
Metabolic stability of test compounds in human and mouse liver microsomes
| Compound | Species | t1/2 (min) | CLinta (μl/min/mg protein) | Scaled-up CLint (ml/min/kg) |
|---|---|---|---|---|
| Verapamil | Human | 12.19 | 113.72 | 142.62 |
| Mouse | 9.15 | 151.48 | 662.71 | |
| SB-AF-1002 | Human | 120.41 | 11.51 | 14.44 |
| Mouse | 19.43 | 71.32 | 312.01 |
CLint, intrinsic clearance.
TABLE 3.
Metabolic stability of test compounds in human and mouse liver microsomes
| Compound | Species | Assay format | % remaining at: |
||||
|---|---|---|---|---|---|---|---|
| 0 min | 15 min | 30 min | 45 min | 60 min | |||
| Verapamil | Human | With NADPH | 100.00 | 28.59 | 11.61 | 5.43 | 3.23 |
| Without NADPH | 100.00 | 99.39 | 100.00 | 95.15 | 93.33 | ||
| Mouse | With NADPH | 100.00 | 14.64 | 4.72 | 1.79 | 1.04 | |
| Without NADPH | 100.00 | 79.63 | 91.36 | 90.12 | 96.91 | ||
| SB-AF-1002 | Human | With NADPH | 100.00 | 94.84 | 78.13 | 74.92 | 73.06 |
| Without NADPH | 100.00 | 100.00 | 93.85 | 86.15 | 82.31 | ||
| Mouse | With NADPH | 100.00 | 58.09 | 30.37 | 19.43 | 11.96 | |
| Without NADPH | 100.00 | 100.82 | 81.07 | 84.43 | 86.89 | ||
Permeation through the BBB.
To assess if SB-AF-1002 could pass the blood-brain barrier (BBB), we used a commercially available kit (BBB kit MBT-24; Pharmaco-Cell Company Ltd.). Similar to the parent compound, D13 (16), SB-AF-1002 was able to penetrate the BBB in the monkey brain model in the first 5 min, although its ability to pass through decreased after 15 min. The Papp value was lower than that for the positive control (caffeine), but it was still able to penetrate during the first 5 min and later decreased to the level of the negative control (cyclosporine). We will further investigate this time-dependent phenomenon (Table 4).
TABLE 4.
Papp value of SB-AF-1002 compared to those of cyclosporine and caffeine in monkey BBB kit
| Drug | Time (min) | Papp (10−6 cm/s) |
|---|---|---|
| Cyclosporine | 5 | 1 |
| Cyclosporine | 15 | 1 |
| Cyclosporine | 30 | 1 |
| Caffeine | 5 | 20 |
| Caffeine | 15 | 25 |
| Caffeine | 30 | 25 |
| SB-AF-1002 | 5 | 6 |
| SB-AF-1002 | 15 | 2 |
| SB-AF-1002 | 30 | 1.1 |
DISCUSSION
In this study, we assessed the in vivo efficacy of the acylhydrazone SB-AF-1002 in different animal models of fungal infections. SB-AF-1002, which was among our top 5 lead compounds previously published, was chosen for an advanced preclinical study for its characteristics of being highly active in vitro against a variety of fungal clinical isolates and for its low toxicity in mammalian cells (17). In this study, we showed that SB-AF-1002 is highly efficacious in animal models of cryptococcosis, candidiasis, and aspergillosis.
In the cryptococcosis model, we found 100% survival when we started the treatment immediately after infection, whereas we found 30% survival in the group that was treated with the current standard of care. Moreover, we found a 90% survival when we delayed the treatment at the same concentration and 100% survival when we doubled the dose, even when we started the therapy after 5 days of infection in order to allow the development of meningitis. Not only did SB-AF-1002 outperform fluconazole, but most importantly, the mice showed an almost complete clearance of cryptococcal cells from the lungs and brains. These results show that this effect is dose dependent, further confirming our previous dose-dependent results obtained using an in vitro killing assay (17). All these results clearly indicate the effectiveness of SB-AF-1002 against cryptococcosis.
In the candidiasis model, we found that when SB-AF-1002 was used orally, the survival rate was only 20%, independent of the dose used, while none of the mice in the control group and untreated group survived. But when it was administered intravenously, 100% of mice survived, although SB-AF-1002 was unable to completely clear the kidneys of the Candida cells, and 20% of the mice in the control group survived. Even if SB-AF-1002 did not show any in vitro activity on C. albicans, with an MIC >16 μg/ml (17), we decided to use it in an in vivo model, based on the fact that the parent compound, D13, behaved similarly in vitro but displayed some activity in vivo against Candida infection (16). Oral administration of SB-AF-1002 did not significantly improve survival, but intravenous administration resulted in 100% survival (Fig. 4). Our acylhydrazones affect the production of fungal sphingolipids, particularly GlcCer, and a Candida mutant lacking GlcCer proved to be less virulent in the animal model (18). The difference between oral and intravenous administration of SB-AF-1002 could be due to pharmacokinetic characteristics and the organ distribution of the drug, which are under investigation.
In the aspergillosis model, not only did SB-AF-1002 outperform voriconazole, the standard of care, with a survival rate of 80% in a therapeutic model of infection, but, interestingly, it was also able to effectively prevent the disease. In fact, when SB-AF-1002 was administered 6 days before infection, the survival rate was 100%, whereas it was 30% for the voriconazole group, and Aspergillus cells were unable to colonize the lungs. This effect is remarkable, and it is most likely due to the effect of the drug on hyphal formation through the inhibition of fungal sphingolipids (16, 19).
Moreover, SB-AF-1002 was effective without the addition of grapefruit juice. In the aspergillosis mouse model, when mice are treated with voriconazole, they require the inclusion of grapefruit juice in their diet, to block the rapid metabolism of the drug by the liver (20). When used in the preventive model, the voriconazole-treated group was lethargic and lost weight sooner than the mice in the voriconazole-treated group in the aspergillosis model, and we noticed an engorged liver during necropsy, an effect most likely due to the use of the juice. The hepatomegaly was not observed in the SB-AF-1002-treated group at any time during the administration of the drug. This suggests that SB-AF-1002 is not metabolized as fast as voriconazole is in mice.
Pharmacokinetic studies help to determine the plasma concentration of an administered drug over a period of time (21). The concentration of SB-AF-1002 started decreasing immediately following intravenous administration, but it could be detected in the plasma up to 8 h, thus displaying a typical pharmacokinetic profile for a drug administered intravenously. In contrast, SB-AF-1002 displayed better bioavailability when administered orally, as the concentration at the end of 8 h was 10 times higher than that which was seen after intravenous administration.
Metabolic stability studies provide information, such as intrinsic clearance and metabolic liabilities, indicating bioavailability, which would help us to determine the dose and frequency of administration for a drug (22). SB-AF-1002 showed a longer in vitro t1/2 and a slower intrinsic clearance in human liver microsomes than that in mouse liver microsomes, which suggests that it could reach a higher exposure in humans than in mice if liver metabolism is the primary route of clearance.
hERG encodes a potassium channel that is essential for cardiac repolarization (23). A wide range of drugs block the hERG channel, resulting in cardiac arrhythmia (24). Thus, it is essential to screen compounds for this cardiac safety indicator in early stages of drug discovery (25). SB-AF-1002 was tested for its affinity to a hERG channel in a patch clamp assay. SB-AF-1002 had an IC50 of 24.495 μM, showing, rather, a low affinity to the hERG channel. This result suggests that SB-AF-1002 would not be cardiotoxic at the concentrations used to treat fungal infections.
Caco-2 cells are a cancer cell line from the human colon epithelium and are used as a model to assess the intestinal absorption of drugs (26). The efflux ratio of SB-AF-1002 was 2.54, while that of a known P-glycoprotein (P-gp) substrate, digoxin, was 33.17. The results suggest that SB-AF-1002 is a poor substrate for efflux pumps, such as P-gp, and, thus, that it would be transported across the membrane by passive diffusion without disturbance by efflux pumps. However, we noted that the percentage of recovery of SB-AF-1002 was less than 10% from the AP to the BL direction and nearly 40% from the BL to AP direction. This could imply that some amount of the drug either accumulates in the apical side and is not transported to the basolateral side or is metabolized by Caco-2 cells.
The BBB can prevent the passage of many compounds from blood to the brain (27). Because most invasive fungal infections eventually affect the brain tissue, having a compound that can traverse the BBB is essential (28). To assess this in vitro, we used a commercially available monkey brain cell monolayer model (BBB kit MBT-24; Pharmaco-Cell Company Ltd.). SB-AF-1002 was able to pass through the brain cell monolayer rapidly with a time dependency similar to that of the related compound D13 (16), suggesting that SB-AF-1002 and D13 may be useful in treating fungal infections of the central nervous system (CNS).
In conclusion, our studies confirmed that acylhydrazones are a powerful class of antifungal compounds with a mechanism of action distinct from that of the current standard of care that may be used to treat invasive fungal infections, such as cryptococcosis, candidiasis, and aspergillosis. These results warrant further research and development of this new class of compounds with compelling systemic antifungal efficacy.
MATERIALS AND METHODS
Strains, media, and reagents.
A series of clinical isolates and reference strains were used in this study, including Cryptococcus neoformans (strain H99), Candida albicans (strain A39), and Aspergillus fumigatus (strain Af239). The strains were obtained from the existing collection at Maurizio Del Poeta’s laboratory (Stony Brook University). Yeast extract-peptone-dextrose (YPD), yeast nitrogen base (YNB), RPMI 1640 medium, and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Invitrogen Life Technologies (Grand Island, NY). Fluconazole was purchased from Sigma-Aldrich (St. Louis, MO). Voriconazole was purchase from Pfizer (Rey Brook, NY).
Chemical synthesis.
2,4-Dibromobenzoic acid (5.00 g, 18.0 mmol) was converted to its methyl ester, followed by reaction with hydrazine monohydrate (18.0 g, 360.0 mmol), to give 2,4-dibromobenzohydrazide (3.40 g, 65% yield), following the procedure previously published by us (17). To a solution of 2,4-dibromobenzohydrazide (3.00 g, 10.3 mmol) and 5-bromosalicylaldehyde (2.1 g, 10.5 mmol) in methanol (20 ml), 5 drops of glacial acetic acid were added. The reaction mixture was stirred at room temperature overnight, which resulted in precipitation of the product. Addition of water (50 ml) to the reaction mixture, followed by filtration and washing of the precipitate with dichloromethane (100 ml) and acetone (30 ml), resulted in pure SB-AF-1002 as an off-white solid (4.20 g, 88% yield): melting point > 220°C; 1H nuclear magnetic resonance (NMR) (500 MHz, dimethyl sulfoxide [DMSO]-d6) δ 6.80 (d, 1H, 35%, J = 8.7 Hz), 6.90 (d, 1H, 65%, 8.7 Hz), 7.35 (m, 1H, 65%), 7.43 (m, 1H), 7.54 (d, 1H, 65%, J = 8.2 Hz), 7.71 (dd, 1H, 35%, J = 8.2 Hz, J = 1.8 Hz), 7.73 (dd, 1H, 65%, J = 8.2 Hz, J = 1.8 Hz), 7.81 (d, 1H, 65%, J = 2.5 Hz), 8.26 (s, 1H, 35%), 8.46 (s, 1H, 65%), 10.22 (br s, 1H, 35%), 11.00 (br s, 1H, 65%), 12.18 (s, 1H); 13C NMR (125 MHz, DMSO-d6) δ 110.51, 110.52, 118.5, 118.7, 119.8, 120.6, 121.3, 121.6, 122.7, 123.8, 129.0, 130.0, 130.2, 130.7, 130.8, 130.9, 133.5, 133.9, 134.1, 134.8, 136.1, 137.2, 141.5, 145.6, 155.7, 156.4, 162.5, 168.3; high-resolution mass spectrometry (electrospray ionization/time of flight) calculated for C14H9Br3N2O2H+: 474.8287; found: 474.8292 (Δ = −1.1 ppm). High-performance liquid chromatography: Kinetex PFP 2.6 μM, 100- by 2.1-mm, 100-Å column; acetonitrile and water; flow rate of 0.2 ml/min; at times of 0 to 30 min, a gradient of 40 to 95% acetonitrile; at 7.8 min, purity was >99%.
Animal study for cryptococcosis.
For survival studies, 4-week-old CBA/J (Envigo) female mice were used. They were divided into 10 mice for each treatment or control group. For the first survival study, mice were infected intranasally with 20 μl of a suspension containing 5 × 105 Cryptococcus neoformans cells. Mice were treated starting on the day of infection with 20 mg/kg/day of SB-AF-1002 and fluconazole in a final volume of 100 μl of 30% polyethylene glycol (PEG) in a saline buffer. The untreated control group mice received 100 μl of 30% PEG in a saline buffer. Gavage was used as the route of administration. The second survival experiment was performed as described above, but the treatment started 5 days after the infection to allow the development of meningitis. Mice were fed ad libitum and monitored every day for discomfort and meningitis signs. Mice showing weight loss, lethargy, tremor, or an inability to reach food or water were sacrificed, and survival was counted as the time until that day. The third survival study was performed using the same design described above for the treatment starting after 5 days, but different concentrations of SB-AF-1002, i.e., 0.5, 5, 20, and 40 mg/kg/day, were used. At the end of each experiment, all the surviving animals were sacrificed, and their brain and lungs were collected and homogenized through a Stomacher80 Biomaster blender (Seward). After dilution in phosphate-buffered saline (PBS), 100 μl was plated on YPD plates, the plates were incubated for 48 h at 30°C, and the colonies were counted.
Animal study for candidiasis.
For survival studies, 8-week-old CBA/J (Envigo) female mice were used. They were divided into 10 mice for each treatment or control group. Mice were infected intravenously with 100 μl of a suspension containing 105 cells of the Candida albicans A39 strain and treated through gavage with 20, 40, and 80 mg/kg/day of SB-AF-1002 in a final volume of 100 μl of 30% PEG in a saline buffer. Fluconazole was used as a drug control. The untreated control group mice received 100 μl of 30% PEG in a saline buffer. The second experiment was performed following the same route of infection, but the treatment was done with 3-mg/kg/day SB-AF-1002 through intravenous injection. Mice were fed ad libitum, monitored every day for discomfort, and subsequently sacrificed. At the end of each experiment, all the surviving animals were sacrificed and the kidneys were collected and homogenized through a Stomacher80 Biomaster blender (Seward). After dilution in PBS, 100 μl was plated on YPD plates, the plates were incubated for 48 h at 30°C, and the colonies were counted.
Animal study for aspergillosis.
For survival studies, 8-week-old CBA/J (Envigo) female mice were used. They were divided into 10 mice for each treatment or control group. On the day prior to infection, the mice were immunosuppressed by using triamcinolone acetonide (1 mg/mouse) subcutaneously (29). On day 0, the mice were infected intranasally with 20 μl of a suspension containing 2 × 104 viable conidia of the Aspergillus fumigatus Af293 strain and were treated through gavage with 20-, 40-, and 80-mg/kg/day SB-AF-1002 in a final volume of 100 μl of 30% PEG in a saline buffer. Voriconazole was used as a drug control. The untreated control group mice received 30% PEG in a saline buffer. The mice were fed ad libitum, and the voriconazole group was given 50% grapefruit juice in place of normal drinking water to avoid liver metabolism of the drugs (18). The mice were monitored every day for discomfort and subsequently sacrificed. At the end of the experiment, all the surviving animals were sacrificed and the lungs were collected and homogenized through Stomacher80 Biomaster blender (Seward). After dilution in PBS, 100 μl was plated on YPD plates, the plates were incubated for 48 h at 30°C, and the colonies were counted.
Animal study approval.
Mice were fed ad libitum and monitored every day for discomforts and signs of disease. Mice showing weight loss, lethargy, tremor, or an inability to reach food or water were euthanized, and survival was counted as the time until that day. Euthanasia was performed with CO2 asphyxiation with a 100% fraction of inspired CO2 for 2 min, followed by cervical dislocation. Mouse experiments were performed in full compliance with a protocol approved by Stony Brook University (study number 341888; IACUC number 2012-1967) and in compliance with the United States Animal Welfare Act (Public Law 98-198). The experiments were carried out in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Pharmacokinetic studies.
(i) Formulation preparation. For an intravenous (i.v.) dose of 1 mg/kg, a stock solution of 0.5 mg/ml was prepared using 5% DMSO, 5% Solutol excipient in water. SB-AF-1002 (1.09 mg) was dissolved in 0.109 ml of DMSO with vortexing and sonification. This was followed by the addition of 0.109 ml of Solutol excipient with vortexing and sonification, and finally, 1.962 ml of water was added with vortexing and sonification until a clear solution was obtained. This 0.5-mg/ml solution (2 ml) was administered to achieve the desired 1-mg/kg concentration. In the case of oral (p.o.) administration, a stock solution of a 2-mg/ml solution of SB-AF-1002 was prepared using 5% DMSO, 5% Solutol excipient in water. To prepare this stock solution, 2.25 mg of SB-AF-1002 was dissolved in 0.056 ml of DMSO with vortexing and sonification, followed by addition of 0.056 ml of Solutol excipient with vortexing and sonification, and then, 1.013 ml of water was added with vortexing and sonification until a clear solution was achieved. This stock solution (10 ml) was administered orally to achieve the 20-mg/kg dose.
(ii) Analytical method. The desired serial concentrations of working solutions were achieved by diluting a stock solution of analyte with a 50% acetonitrile-in-water solution. Working solutions (3 μl each; 10, 20, 50, 100, 500, 1,000, 5,000, 10,000 ng/ml) were added to 30 μl of the blank CD1 mouse plasma to achieve calibration standards of 1 to 1,000 ng/ml (1, 2, 5, 10, 50, 100, 500, 1,000 ng/ml) in the total volume of 33 μl. Five quality control (QC) samples at 1 ng/ml, 2 ng/ml, 50 ng/ml, 400 ng/ml, and 800 ng/ml for plasma were prepared independently of those used for the calibration curves. These QC samples were prepared on the day of analysis in the same way as the calibration standards. Standards (33 μl), 33 μl of the QC samples, and 33 μl of unknown samples (30 μl of plasma with 3 μl of blank solution) were added to 200 μl of acetonitrile containing the internal standard mixture for precipitating the protein. Then, the samples were vortexed for 30 s. After centrifugation at 4°C and 4,000 rpm for 15 min, the supernatant was diluted 2 times with water. Diluted supernatant (20 μl) was injected into the liquid chromatography-tandem mass spectrometry (LC-MS/MS) system for quantitative analysis.
Metabolic stability studies.
To each well was added 40 μl of a 10 mM NADPH solution. The final concentration of NADPH was 1 mM. The mixture was prewarmed at 37°C for 5 min. The negative-control samples were prepared by replacing the NADPH solutions with 40 μl of ultrapure H2O. The negative control was used to exclude the misleading factor that resulted from instability of the chemical itself. Samples with NADPH were prepared in duplicate. Negative controls were prepared in singlet. The reaction was started with the addition of 4 μl of 200 μM control compound or test compound solutions. Verapamil was used as a positive control in this study. The final concentration of test compound or control compound was 2 μM. Aliquots of 50 μl were taken from the reaction solution at 0, 15, 30, 45, and 60 min. The reaction was stopped by the addition of 4 volumes of cold acetonitrile with the internal standard (100 nM alprazolam, 200 nM imipramine, 200 nM labetalol, 2 μM ketoprofen). Samples were centrifuged at 3,220 × g for 40 min. An aliquot of 90 μl of the supernatant was mixed with 90 μl of ultrapure H2O and then used for LC-MS/MS analysis.
Caco-2 cell permeability studies.
(i) Preparation of Caco-2 cells. To each well of the Transwell insert and reservoir were added 50 μl and 25 ml of cell culture medium. Then, the HTS Transwell plates were incubated at 37°C in 5% CO2 for 1 h before cell seeding. Caco-2 cells were diluted to 6.86 × 105 cells/ml with culture medium, and 50 μl of the cell suspension was dispensed into the filter well of the 96-well HTS Transwell plate. The cells were cultivated for 14 to 18 days in a cell culture incubator at 37°C in 5% CO2 with 95% relative humidity. The cell culture medium was replaced every other day, beginning no later than 24 h after initial plating.
(ii) Preparation of stock solutions. Test compounds were prepared in DMSO as 10 mM stock solutions. The stock solutions of the positive controls were prepared in DMSO at a concentration of 10 mM. Digoxin and propranolol were used as control compounds in this assay.
(iii) Assessment of cell monolayer integrity. Medium was removed from the reservoir and each Transwell insert and replaced with prewarmed fresh culture medium. The transepithelial electrical resistance (TEER) across the monolayer was measured using a Millicell epithelial volt-ohm measuring system (Millipore, USA). The plate was returned to the incubator once the measurement was done. The TEER value was calculated according to the following equation: TEER measurement (in ohms) × area of membrane (in square centimeters) = TEER value (in ohms · square centimeters). The TEER value should be greater than 230 Ω · cm2, which indicates that the Caco-2 cell monolayer is well qualified.
(iv) Assay procedures. The Caco-2 cell plate was removed from the incubator, washed twice with prewarmed Hanks balanced salt solution (HBSS; 10 mM HEPES, pH 7.4), and then incubated at 37°C for 30 min. The stock solutions of the control compounds were diluted in DMSO to get 1 mM solutions and then diluted with HBSS (10 mM HEPES, pH 7.4) to get 5 μM working solutions. The stock solutions of the test compounds were diluted in DMSO to get 1 mM solutions and then diluted with HBSS (10 mM HEPES, pH 7.4) to get 5 μM working solutions. The final concentration of DMSO in the incubation system was 0.5%. To determine the rate of drug transport in the apical-to-basolateral direction, 75 μl of a 5 μM working solution of test compound was added to the Transwell insert (apical compartment) and the wells in the receiver plate (basolateral compartment) were filled with 235 μl of HBSS (10 mM HEPES, pH 7.4). To determine the rate of drug transport in the basolateral-to-apical direction, 235 μl of a 5 μM working solution of test compound was added to the receiver plate wells (basolateral compartment), and then the Transwell inserts (apical compartment) were filled with 75 μl of HBSS (10 mM HEPES, pH 7.4). Time zero samples were prepared by transferring 50 μl of a 5 μM working solution to the wells of a 96-well deep-well plate, followed by the addition of 200 μl cold acetonitrile or methanol containing appropriate internal standards. The plates were incubated at 37°C for 2 h. At the end of the incubation, 50-μl samples from the donor sides (the apical compartment for AP → BL flux and the basolateral compartment for BL → AP flux) and the receiver sides (the basolateral compartment for AP → BL flux and the apical compartment for BL → AP flux) were transferred to the wells of a new 96-well plate, followed by the addition of 4 volumes of cold acetonitrile or methanol containing appropriate internal standards. The samples were vortexed for 5 min and then centrifuged at 3,220 × g for 40 min. An aliquot of 100 μl of the supernatant was mixed with an appropriate volume of ultrapure water before LC-MS/MS analysis. To determine the Lucifer yellow leakage after a 2-h transport period, a stock solution of Lucifer yellow was prepared in ultrapure water and diluted with HBSS (10 mM HEPES, pH 7.4) to reach a final concentration of 100 μM. One hundred microliters of the Lucifer yellow solution was added to each Transwell insert (apical compartment), followed by filling of the wells in the receiver plate (basolateral compartment) with 300 μl of HBSS (10 mM HEPES, pH 7.4). The plates were incubated at 37°C for 30 min. Eighty-microliter samples were removed directly from the apical and basolateral wells (using the basolateral access holes) and transferred to the wells of new 96-well plates. The Lucifer yellow fluorescence signal was measured (to monitor the monolayer integrity) in a fluorescence plate reader at 485-nm excitation and 530-nm emission wavelengths.
hERG affinity.
The patch clamp assay was performed as follows. The coverslip was removed from the cell culture dish and placed on the microscope stage in a bath chamber. A desirable cell was located using the ×10 objective. The tip of the electrode was located under the microscope using the ×10 objective by focusing above the plane of the cells. Once the tip was in focus, the electrode was advanced downwards toward the cell using the coarse controls of the manipulator, while the objective was simultaneously moved to keep the tip in focus. When directly over the cell, the ×10 objective was switched to a ×40 objective and the fine controls of the manipulator were used to approach the surface of the cell in small steps. Gentle suction was applied through the side port of the electrode holder to form a gigaohm seal. The Cfast was used to remove the capacity current that was in coincidence with the voltage step. The whole-cell configuration was obtained by applying repetitive, brief, strong suction until the membrane patch has ruptured. The membrane potential was set to −60 mV at this point to ensure that the hERG channels were not open. The spikes of capacity current were then canceled using the Cslow on the amplifier. The holding potential was set to −90 mV for 900 ms; the current was recorded at 50 kHz and filtered at 10 kHz. Leaking current was tested at −80 mV for 500 ms. The hERG current was elicited by depolarizing at +30 mV for 4.8 s, and then the voltage was taken back to −50 mV for 5.2 s to remove the inactivation and observe the deactivating tail current. The maximum amount of tail current size was used to determine the hERG current amplitude. The current was recorded for 120 s to assess the current stability. Only stable cells with recording parameters above the threshold were applied for the drug administrations. First, the vehicle control was applied to the cells to establish the baseline. After allowing the current to stabilize for 3 min, compound was applied. The hERG current in the presence of test compound was recorded for approximately 5 min to reach steady state, and then 5 sweeps were captured. For dose-response testing, compound was applied to the cells accumulatively from low to high concentrations. The positive control (dofetilide) was used in this experiment to test the same batch of cells to ensure the good performance of the cells and operations.
Permeability of the blood-brain barrier.
A commercially available kit (BBB kit MBT-24, Pharmaco-Cell Company Ltd.) was used to determine the permeability of the blood-brain barrier. This kit permits a permeability assay to be performed using an in vitro reproduction of the blood-brain barrier using monkey brain. Briefly, in a12-well plate there is a triple culture with endothelial cells in the luminal part and pericytes and astrocytes in the luminal side connected by tight junctions. The luminal side of the well represents the blood side, whereas the abluminal side represents the brain side. The integrity of the blood-brain barrier is assessed by the measurement of transendothelial electrical resistance (TEER), which indicates the formation of tight junctions. To have an efficacious system, the TEER should be >150 Ω · cm2. The kit is kept frozen at −80°C until it is ready to use. Once thawed, it is activated by adding medium and changing the medium after 3 h and again after 24 h. After that, a wait of 3 days occurs to allow the cells to adjust, and then the cells may be used for the experiment on day 4. For drug testing, after assessing the TEER, the drugs are added in the chosen medium at the desired concentration and at different time points. At the end of each time point, the TEER was measured to assess if the BBB was still intact. After that, the medium was collected and sent to the mass spectrometry laboratory to measure the concentration of the samples on the brain side and calculate the apparent permeability (Papp) value. A Papp value greater than 20 indicates that the compound is very good in passing the barrier, a value of between 10 and 20 indicates that the compound is good in passing the barrier, a value of between 2 and 10 indicates that the compound can pass through the barrier but that only an extremely small amount may pass, and a value of lower than 2 indicates that the compound cannot pass the BBB. SB-AF-1002 was tested alongside caffeine as a positive control and cyclosporine as a negative control.
Statistics.
All data are expressed as the mean ± standard deviation. No samples or animals were excluded from the analysis. For animal studies, group sizes were chosen so that they were sufficient to reach a statistical power of at least 80% (http://www.statisticalsolutions.net/pss_calc.php). Mice were randomly assigned to treatment groups. For the survival studies, statistical analysis was performed using the Kruskal-Wallis test. Statistical analysis for tissue burden and for trophic form and ascus counts was performed using analysis of variance (ANOVA). Data met the assumption of a normal distribution, as determined by statistical software, and variance was similar between groups that were statistically compared. Statistical tests were carried out using GraphPad Prism (La Jolla, CA, USA) software for Mac (v. 400). The replicates used were biological replicates. Results were considered significant at a P value of ≤0.05.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI116420 and AI125770 to Maurizio Del Poeta. Maurizio Del Poeta is a Burroughs Welcome Investigator in Infectious Diseases.
We acknowledge Jean Rooney, senior veterinary technician, and Sandra Scherrer, veterinary technician, at Stony Brook University for help and suggestions during the animal experiments.
Maurizio Del Poeta is a cofounder and chief scientific officer (CSO) of MicroRid Technologies Inc. J. Brian McCarthy is a cofounder and chief executive officer (CEO) of MicroRid Technologies Inc. John Mallamo is a cofounder and chief research development officer (CRDO) of MicroRid Technologies Inc. All other authors have no conflict of interest.
Cristina Lazzarini and Krupanandan Haranahalli conceptualized and performed the experiments, collected the data, performed statistical analyses, and wrote the manuscript. Iwao Ojima and Maurizio Del Poeta conceptualized the experiments, discussed the data, and edited the manuscript. J. Brian McCarthy and John Mallamo discussed the data and edited the manuscript.
REFERENCES
- 1.Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. 2010. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 3.Singh N. 2003. Treatment of opportunistic mycoses: how long is long enough? Lancet Infect Dis 3:703–708. doi: 10.1016/S1473-3099(03)00802-8. [DOI] [PubMed] [Google Scholar]
- 4.Tuite NL, Lacey K. 2013. Overview of invasive fungal infections. Methods Mol Biol 968:1–23. doi: 10.1007/978-1-62703-257-5_1. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong-James D, Meintjes G, Brown GD. 2014. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol 22:120–127. doi: 10.1016/j.tim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Mousset S, Buchheidt D, Heinz W, Ruhnke M, Cornely OA, Egerer G, Krüger W, Link H, Neumann S, Ostermann H, Panse J, Penack O, Rieger C, Schmidt-Hieber M, Silling G, Südhoff T, Ullmann AJ, Wolf HH, Maschmeyer G, Böhme A. 2014. Treatment of invasive fungal infections in cancer patients—updated recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 93:13–32. doi: 10.1007/s00277-013-1867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snydman DR, Singh N, Dromer F, Perfect JR, Lortholary O. 2008. Cryptococcosis in solid organ transplant recipients: current state of the science. Clin Infect Dis 47:1321–1327. doi: 10.1086/592690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. 2005. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 41:1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 9.Ashley ED, Drew R, Johnson M, Danna R, Dabrowski D, Walker V, Prasad M, Alexander B, Papadopoulos G, Perfect JR. 2012. Cost of invasive fungal infections in the era of new diagnostics and expanded treatment options. Pharmacotherapy 32:890–901. doi: 10.1002/j.1875-9114.2012.01124. [DOI] [PubMed] [Google Scholar]
- 10.Lepak AJ, Andes DR. 2014. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med 5:a019653. doi: 10.1101/cshperspect.a019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brüggemann RJM, Alffenaar JWC, Blijlevens NMA, Billaud EM, Kosterink JGW, Verweij PE, Burger DM, Saravolatz LD. 2009. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis 48:1441–1458. doi: 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- 12.Ghannoum MA, Rice LB. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 12:501–517. doi: 10.1128/CMR.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanafani ZA, Perfect JR. 2008. Resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis 46:120–128. doi: 10.1086/524071. [DOI] [PubMed] [Google Scholar]
- 14.Lyman CA, Walsh TJ. 1992. Systemically administered antifungal agents: a review of their clinical pharmacology and therapeutic applications. Drugs 44:9–35. doi: 10.2165/00003495-199244010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Mor V, Rella A, Farnoud AM, Singh A, Munshi M, Bryan A, Naseem S, Konopka JB, Ojima I, Bullesbach E, Ashbaugh A, Linke MJ, Cushion M, Collins M, Ananthula HK, Sallans L, Desai PB, Wiederhold NP, Fothergill AW, Kirkpatrick WR, Patterson T, Wong LH, Sinha S, Giaever G, Nislow C, Flaherty P, Pan X, Cesar GV, de Melo Tavares P, Frases S, Miranda K, Rodrigues ML, Luberto C, Nimrichter L, Del Poeta M. 2015. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. mBio 6:e00647-15. doi: 10.1128/mBio.00647-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazzarini C, Haranahalli K, Rieger R, Ananthulae HK, Desai PB, Ashbaugh A, Linke MJ, Cushion MT, Ruzsicska B, Haley J, Ojima I, Del Poeta M. 2018. Acylhydrazones as antifungal agents targeting the synthesis of fungal sphingolipids. Antifungal Agents Chemother 62:e00156-18. doi: 10.1128/AAC.00156-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haranahalli K, Lazzarini C, Sun Y, Zambito J, Pathiranage S, McCarthy JB, Mallamo J, Del Poeta M, Ojima I. 2019. SAR studies on aromatic acylhydrazone-based inhibitors of fungal sphingolipids synthesis as next generation antifungal agents. J Med Chem 62:8249–8273. doi: 10.1021/acs.jmedchem.9b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noble SM, French S, Kohn LA, Chen V, Johnson AD. 2010. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet 42:590–598. doi: 10.1038/ng.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levery SB, Momany M, Lindsey R, Toledo MS, Shayman JA, Fuller M, Brooks K, Doong RL, Straus AH, Takahashi HK. 2002. Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett 525:59–64. doi: 10.1016/s0014-5793(02)03067-3 (Erratum, 526: 151, .) [DOI] [PubMed] [Google Scholar]
- 20.Sugar AM, Liu XP. 2000. Effect of grapefruit juice on serum voriconazole concentrations in the mouse. Med Mycol 38:209–212. doi: 10.1080/mmy.38.3.209.212. [DOI] [PubMed] [Google Scholar]
- 21.Wright DFB, Winter HR, Duffull SB. 2011. Understanding the time course of pharmacological effect: a PKPD approach. Br J Clin Pharmacol 71:815–823. doi: 10.1111/j.1365-2125.2011.03925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masimirembwa CM, Bredberg U, Andersson TB. 2003. Metabolic stability for drug discovery and development. Clin Pharmacokinet 42:515–528. doi: 10.2165/00003088-200342060-00002. [DOI] [PubMed] [Google Scholar]
- 23.Lamothe SM, Guo J, Li W, Yang T, Zhang S. 2016. The human ether-a-go-go-related gene (hERG) potassium channel represents an unusual target for protease-mediated damage. J Biol Chem 291:20387–20401. doi: 10.1074/jbc.M116.743138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanguinetti MC, Tristani-Firouzi M. 2006. hERG potassium channels and cardiac arrhythmia. Nature 440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 25.Jing Y, Easter A, Peters D, Kim N, Enyedy IJ. 2015. In silico prediction of hERG inhibition. Future Med Chem 7:571–586. doi: 10.4155/fmc.15.18. [DOI] [PubMed] [Google Scholar]
- 26.Van Breemen RB, Li Y. 2005. Caco-2 cell permeability assays to measure drug absorption. Expert Opin Drug Metab Toxicol 1:175–185. doi: 10.1517/17425255.1.2.175. [DOI] [PubMed] [Google Scholar]
- 27.Pardridge WM. 2012. Drug transport across the blood–brain barrier. J Cereb Blood Flow Metab 32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flores VG, Cervantes Tovar RM, Zaldivar PG, Martinez EA. 2012. Meningitis due to Cryptococcus neoformans: treatment with posaconazole. Curr HIV Res 10:620–623. doi: 10.2174/157016212803305970. [DOI] [PubMed] [Google Scholar]
- 29.Clemons KV, Schwartz JA, Stevens DA. 2011. Therapeutic and toxicologic studies in a murine model of invasive pulmonary aspergillosis. Med Mycol 49:834–847. doi: 10.3109/13693786.2011.577822. [DOI] [PubMed] [Google Scholar]