Dental caries is the most common disease in the human mouth. Streptococcus mutans is the primary cariogenic bacterium. Propolis is a nontoxic natural product with a strong inhibitory effect on oral cariogenic bacteria. The polyphenol-rich extract from propolis inhibits S. mutans growth and biofilm formation, as well as the genes involved in virulence and adherence, through the inhibition of glucosyltransferases (GTF). However, because the chemical composition of propolis is highly variable and complex, the mechanism of its antimicrobial action and the active compound are controversial and not completely understood.
KEYWORDS: propolis, caffeic acid phenethyl ester, Streptococcus mutans, cariogenicity
ABSTRACT
Dental caries is the most common disease in the human mouth. Streptococcus mutans is the primary cariogenic bacterium. Propolis is a nontoxic natural product with a strong inhibitory effect on oral cariogenic bacteria. The polyphenol-rich extract from propolis inhibits S. mutans growth and biofilm formation, as well as the genes involved in virulence and adherence, through the inhibition of glucosyltransferases (GTF). However, because the chemical composition of propolis is highly variable and complex, the mechanism of its antimicrobial action and the active compound are controversial and not completely understood. Caffeic acid phenethyl ester (CAPE) is abundant in the polyphenolic compounds from propolis, and it has many pharmacological effects. In this study, we investigated the antibacterial effects of CAPE on common oral cariogenic bacteria (Streptococcus mutans, Streptococcus sobrinus, Actinomyces viscosus, and Lactobacillus acidophilus) and its effects on the biofilm-forming and cariogenic abilities of S. mutans. CAPE shows remarkable antimicrobial activity against cariogenic bacteria. Moreover, CAPE also inhibits the formation of S. mutans biofilms and their metabolic activity in mature biofilms. Furthermore, CAPE can inhibit the key virulence factors of S. mutans associated with cariogenicity, including acid production, acid tolerance, and the bacterium’s ability to produce extracellular polysaccharides (EPS), without affecting bacterial viability at subinhibitory levels. In conclusion, CAPE appears to be a new agent with anticariogenic potential, not only via inhibition of the growth of cariogenic bacteria.
INTRODUCTION
Dental caries is the most common disease in the human mouth. The WHO has classified dental caries as one of the most important diseases in humans. Dental caries is a disease involving chronic, progressive destruction of hard tissue due to the influence of many factors, especially bacteria. Cariogenic bacteria form biofilms on the dental surface and metabolize carbohydrates to produce acid, which then causes demineralization of the hard tissues, leading to cavity formation. Streptococcus mutans is the most common cariogenic bacterium. The glucosyltransferase (GTF) produced by S. mutans catalyzes sucrose to produce glucan, which promotes bacterial adhesion and plaque formation; this process is considered to be an important cariogenic virulence factor in the pathogenesis of dental caries (1).
Propolis is a nontoxic natural product consisting of a variety of complex chemical components, and it has many pharmacological effects. Propolis has a wide range of biological activities, including antibacterial, anti-inflammatory, anesthetic, and cariostatic properties (2–4). Recently, studies have shown that propolis has an effective inhibitory effect on the growth of oral cariogenic bacteria, especially that of Gram-positive bacteria (5). It also significantly inhibits the growth, metabolism, acid production, glucosyltransferase activity, bacterial adhesion, and plaque biofilm formation in S. mutans, the primary cariogenic bacterium (6–8). In animal experiments, topical application of propolis twice daily (9) or its inclusion in drinking water available ad libitum (10) reduced the incidence of dental caries in rats. In clinical studies, propolis has been shown to significantly reduce the number of S. mutans cells in the mouth, and it has no side effects on the oral soft and hard tissues (11).
Some of the pharmacological properties of propolis extracts, such as its antidiabetic, antimicrobial, antiatherosclerotic, and antifungal properties, are mainly related to the polyphenol content of the propolis samples (9, 12, 13). The other pharmacological effects of the polyphenols in propolis, including their anticaries ability, have been confirmed. Propolis can inhibit S. mutans growth and biofilm formation; the molecular mechanism of this effect is that the polyphenols inhibit the S. mutans the virulence- and adhesion-associated genes by inhibiting glucosyltransferase, and the inhibitory action has a dose-dependent effect, thereby supporting the compound’s ability to prevent caries (13, 14).
Depending on the source of propolis, its components can vary (15). Leticia Barrientos analyzed the polyphenol content of 20 propolis samples from different sources by high-performance liquid chromatography (HPLC), and most of the propolis samples contained caffeic acid phenethyl ester (CAPE). CAPE was the second most abundant polyphenolic compound detected in the propolis samples (12). The pharmacological effects of CAPE, such as its anti-inflammatory (16), antioxidant (17, 18), antitumor (19), antiviral (20), antiallergic (21), and immunomodulatory (22) properties, have been confirmed by studies, and its potential application in oral diseases has also been studied. In experimental periodontitis, CAPE had more robust anti-inflammatory, antioxidant, and antiapoptotic effects than low doses of doxycycline (23). Its strong oxidation potential makes it a powerful oxidant as an alternative treatment for oral cancer. CAPE treatment can effectively suppress the proliferation, survival, and metastasis of oral cancer cells (24). It has been reported that CAPE renders its antitumor effect without causing cytotoxicity to normal cells (25). The literature shows that CAPE has antimicrobial activity against Enterococcus faecalis, Listeria monocytogenes, Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, and Haemophilus influenzae (26–29). The latest research showed that CAPE can reduce the thickness of S. mutans biofilm (30). Does CAPE exhibit pharmacological activity against oral cariogenic bacteria and affect the growth and metabolism of S. mutans? Furthermore, does its anticaries ability make CAPE, a drug with multiple pharmacological activities, an effective treatment for various oral diseases?
Hence, the purpose of this study was to investigate the antibacterial effects of CAPE on common oral cariogenic bacteria (Streptococcus mutans, Streptococcus sobrinus, Actinomyces viscosus, and Lactobacillus acidophilus) and its effects on the biofilm and cariogenic abilities of S. mutans. This study is the first to investigate the effects of CAPE on oral cariogenic bacteria and caries prevention. Compared with propolis, CAPE is a single component that is easier to obtain, making it more conducive to popularization and application in experimental research and clinical treatment.
RESULTS
Bacterial susceptibility.
The MIC and minimum bactericidal concentration (MBC) of CAPE were determined for four cariogenic bacterial strains (Table 1). CAPE can inhibit and kill all of the bacterial strains at MICs ranging from 0.08 to 0.16 mg/ml, and MBCs are all 0.32 mg/ml. CAPE showed a stronger bacteriostatic effect against S. mutans than against the other strains. Dimethyl sulfoxide (DMSO) at 5% (the solvent control) had no effect on bacterial growth. The MIC values ranged from 2 × 103 to 8 × 103 mg/ml, and the MBC values ranged from 4 × 103 to 16 × 103 mg/ml for the chlorhexidine (CHX) control.
TABLE 1.
MIC and MBC values of CAPE and CHX against oral bacteria
| Bacterial strain | CAPE (mg/ml) |
CHXb (103 mg/ml) |
||
|---|---|---|---|---|
| MIC | MBCa | MIC | MBC | |
| Streptococcus mutans | 0.08 | 0.32 | 2 | 4 |
| Streptococcus sobrinus | 0.16 | 0.32 | 8 | 16 |
| Actinomyces viscosus | 0.16 | 0.32 | 2 | 4 |
| Lactobacillus acidophilus | 0.16 | 0.32 | 4 | 8 |
MBC, minimum bactericidal concentration.
CHX, chlorhexidine.
Since S. mutans is recognized as the primary cariogenic bacterium, it was selected for further evaluation in time-kill assays, biofilm assays, and metabolism assays.
Time-kill assays.
CAPE exhibited a strong short-term bactericidal activity against S. mutans (Fig. 1). At the MBC, CAPE caused an approximately 3-log reduction in the number of viable cells within 1 min, and all of the S. mutans cells were dead within 5 min. At a CAPE concentration of two times the MBC, all of the S. mutans cells were eliminated within 1 min, and there was no significant difference compared to the bactericidal effect of 0.12% CHX.
FIG 1.
Time-kill curves for CAPE against S. mutans. Solvent control, 5% DMSO; negative control, sterile water; positive control, 0.12% CHX. Results are expressed as lg(CFU/ml). Data are presented as mean ± standard deviation from at least three independent experiments.
Biofilm inhibition.
As shown in Fig. 2, CAPE showed a good inhibitory effect on S. mutans biofilm formation. CAPE (0.04 mg/ml) inhibited biofilm formation by at least 50%, and at 0.08 mg/ml CAPE inhibited biofilm formation by more than 90%. DMSO at 5% (the solvent control) had no influence on the biomass. At a low concentration of 0.02 mg/ml, CAPE did not significantly inhibit biofilm formation. CHX (the positive control) at a concentration of 1 × 103 mg/ml inhibited biofilm formation by at least 50%, and 2 × 103 mg/ml CHX inhibited biofilm formation by at least 90%.
FIG 2.

The effects of CAPE on inhibition of biofilm formation are expressed as the absorbance at 595 nm (A595). Solvent control, 5% DMSO; negative control, sterile water. Data are presented as mean ± standard deviation from at least three independent experiments (P < 0.05).
Scanning electron microscopy (SEM) photographs of the biofilms formed on glass slides are shown in Fig. 3. For the negative control, the S. mutans biofilm was uniform and thick. At the CAPE 50% minimum biofilm inhibition concentration (MBIC50), biofilm formation was greatly reduced, although a small number of planktonic bacteria were visible between the S. mutans cell clumps. The inhibitory effect of CHX at a concentration of 1 × 103 mg/ml is slightly weaker than that of the MBIC50 of CAPE. DMSO at 5% (the solvent control) had no effect on the biomass; however, organic solvents may cause some collapse of the biofilm.
FIG 3.
Scanning electron microscopy observation of S. mutans biofilm. (A and B) Reduction of biofilm formation with the presence of CAPE at the concentration of MBIC50 (0.04 mg/ml) compared with that in the presence of MBIC50 CHX (1 × 103mg/ml) (C and D), solvent control (E and F), and negative control (G and H).
Viability of S. mutans biofilms based on confocal microscopy.
CAPE caused a clear reduction in the 1-day-old S. mutans biofilms as assessed via live/dead staining assays imaged using confocal laser scanning microscopy (Fig. 4). The live bacteria were stained green, and the dead bacteria were stained red. In the solvent and negative-control groups, the S. mutans biofilms were thick and mostly green. This results also proves that 5% DMSO (the solvent control) had no influence on the biomass; however, organic solvents may cause some collapse of the biofilm, probably via dissolution of the extracellular matrix. In the CAPE group, as the CAPE concentration increased, the red color gradually increased, and the green color and the thickness of the biofilm gradually decreased.
FIG 4.
Representative images of live/dead staining of 1-day-old S. mutans biofilm after treatment with CAPE. The 3-dimensional reconstruction of S. mutans biofilms (live bacteria, stained green; dead bacteria, stained red).
As shown by the graph of the biomass (Fig. 5), the reduction effects and bactericidal activity of CAPE on 1-day-old S. mutans biofilm are very significant; as the CAPE concentration increased, the proportion and quantity of dead bacteria increased, the proportion and quantity of living bacteria decreased, and the total biomass of biofilm also decreased. So, the reduction effects of CAPE on 1-day-old S. mutans biofilm and its bactericidal effects were concentration dependent. The biofilm treated with 0.12% CHX in the positive-control group had a significant reduction in biomass, but the proportion of live bacteria was higher than that in the 0.08 and 0.16 mg/ml CAPE groups.
FIG 5.

Biomass of live and dead bacteria, calculated according to 5 random fields of view of S. mutans biofilms by COMSTAT. Data are presented as mean ± standard deviation.
CAPE inhibits acid production and acid tolerance of S. mutans in vitro.
We assessed the effect of CAPE on acid production by S. mutans by monitoring the lactic acid production in S. mutans cultures. The lactic acid production of the S. mutans cells was significantly inhibited by CAPE at sub-MIC levels (Fig. 6A). Dose-dependent inhibition was observed. DMSO at 5% (the solvent control) had no effect on the acid production of S. mutans.
FIG 6.
(A) Effects of CAPE on lactic acid production of S. mutans at sub-MIC levels. (B) Effect of CAPE on aciduricity of S. mutans at sub-MIC levels. Acid tolerance was determined by measuring the survival of S. mutans cells after 2 h of treatment at pH 5.0. Negative control (NC), sterile water; solvent control (SC), 5% DMSO. Data are presented as mean ± standard deviation.
The acidurity of S. mutans was also strongly inhibited by CAPE at sub-MIC levels (Fig. 6B). The survival of the cells at a pH of 5.0 was significantly reduced in the presence of CAPE compared to that of the control cells. In particular, CAPE at one-half MIC showed bactericidal activity against S. mutans at a pH of 5.0, resulting in no survival of the cells in this group.
Growth curve assay of sub-MIC concentration at pH 5.0.
This experiment demonstrated the growth curve of S. mutans at a pH of 5.0 in the presence of CAPE at one-half of its MIC (Fig. 7). Because the acidurity of S. mutans was inhibited by CAPE, the survival of the cells in the experimental group gradually decreased over 2 h. CAPE caused an approximately 4-log reduction in the number of viable cells within 1 h, and all of the S. mutans cells were killed within 2 h.
FIG 7.
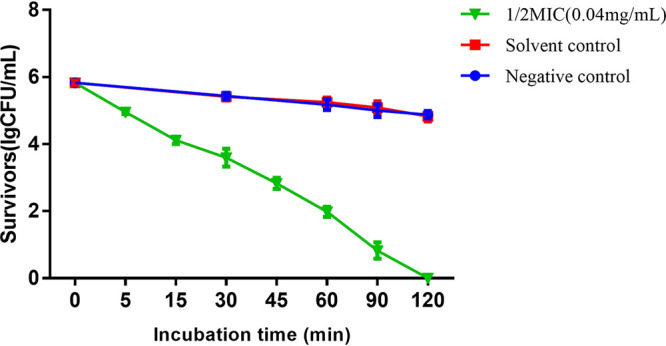
Growth curve of CAPE against S. mutans at pH 5.0 in 2 h; a kinetic killing effect was observed. Solvent control, 5% DMSO; negative control, sterile water. Results are expressed as lg(CFU/ml). Data are presented as mean ± standard deviation from at least three independent experiments.
CAPE inhibits the synthesis of water-insoluble EPS by S. mutans in vitro.
CAPE at sub-MIC levels significantly inhibited the ability of S. mutans cells to synthesize water-insoluble extracellular polysaccharide (EPS). As shown in Fig. 8, one-half of the MIC of CAPE reduced the amount of water-insoluble EPS to about half of that produced by the control cells. The production of water-insoluble EPS was significantly reduced at all of the tested CAPE concentrations compared with the control groups. CAPE showed a dose-dependent inhibitory effect on the ability of S. mutans cells to synthesize water-insoluble EPS.
FIG 8.

Effects of CAPE on polysaccharides synthesis of S. mutans at sub-MIC levels. Quantitative data of the water-insoluble EPS amount of S. mutans biofilms measured by the anthrone method. Solvent control (SC), 5% DMSO; negative control (NC), sterile water. Data are presented as mean ± standard deviation.
DISCUSSION
The use of natural products has been one of the most successful strategies for the discovery of new medicines (31). That propolis can inhibit and kill oral bacteria has been confirmed by a large number of studies. The mechanism of the antimicrobial action of propolis, including its effectiveness against cariogenic microorganisms, is controversial and not completely understood. The biological activity of propolis (ethanolic extracts of propolis [EEP]) may vary according to its composition and seems to be multidirectional (32), involving several mechanisms, such as disorganization of the cytoplasmatic membrane and the cell wall, partial bacteriolysis, formation of pseudomulticellular colonies, and inhibition of protein synthesis (33).
Due to the different sources of propolis, its chemical composition is highly variable and complex (15). The main bioactive components of propolis are flavonoids, terpenes, and phenolic compounds (34–36). Some research groups have started to study the anticaries effects of specific compounds in propolis. Kaempferol and quercetin are flavonols that are also commonly present in propolis (37, 38). Like CAPE, they also show inhibitory effects against oral bacteria, such as S. mutans, S. sobrinus, and A. viscosus (39). However, these strains were more susceptible to CAPE. Flavanones and some dihydroflavonols, as well as the sesquiterpene tt-farnesol, inhibited the growth of S. mutans and S. sobrinus (1).
CAPE is widely found in propolis from different sources and is abundant in the polyphenolic compounds found in propolis (12). Velázquez et al. showed that Mexican propolis has antibacterial activity, and they attributed this activity to the presence of CAPE (29). Here, we studied the anticariogenic activity of CAPE found among the polyphenol compounds in propolis.
Despite the complexity of the oral environment, oral streptococci, including S. mutans and S. sobrinus, are generally considered to be the primary etiologic agents of dental caries (40, 41). A. viscosus is one of the early colonies in plaque development (42), and S. mutans and L. acidophilus are capable of acidogenicity and aciduricity, and they can colonize the tooth surface, leading to enamel demineralization (43). However, S. mutans, which has a strong ability to form biofilms, is still considered to be the primary etiologic agent of dental caries (44). The various species of oral streptococci tested in our study showed differential sensitivities to CAPE under the same experimental conditions. The antimicrobial assays demonstrated that CAPE has a good bactericidal effect against cariogenic bacteria and good inhibitory activity on biofilm formation. The MIC values determined in our study show that S. mutans is the most susceptible to CAPE, and the MBC values for all of the tested bacterial strains were comparable.
Time-kill assays, which measure how fast an antimicrobial agent kills bacteria, showed that CAPE worked quickly against S. mutans and that the killing times were as short as only 5 or 1 min with CAPE concentrations of 1-fold or 2-fold the MBC, respectively. The mechanisms of CAPE activity against microorganisms are not clear, and the antibacterial effects of CAPE might be complex. Various studies show that RNA, DNA, and cellular proteins are all possible targets of CAPE (25–29). Furthermore, Takaisi-Kikuni and Schilcher suggest that the antimicrobial action of CAPE is probably based on inhibition of bacterial RNA polymerase (32). Moreover, Lee et al. reported that the antimicrobial effect of CAPE is related to outer membrane damage and reactive oxygen species synthesis in bacteria (45).
Cariogenic bacteria usually grow in biofilms deposited on the surfaces of the teeth; therefore, an anticaries agent should effectively inhibit the formation of new biofilms and reduce the viability of existing biofilms, rather than only inhibiting the growth of planktonic bacteria (46). CAPE showed a good inhibitory effect on S. mutans biofilm formation. In the present study, CAPE inhibited the formation of S. mutans biofilms at low concentrations; its MBIC90 value was the same as its MIC, and its MBIC50 value was half of its MIC. Therefore, we choose a CAPE concentration at the MBIC50 (0.04 mg/ml) to observe the formation of S. mutans biofilms under a scanning electron microscope. The inhibitory effect of CAPE on biofilm formation may be mainly via inhibition of bacterial growth, as a reduced number of planktonic cells limits biofilm formation. Furthermore, we observed the effect of CAPE on an established, mature S. mutans biofilm under a confocal laser scanning microscope after staining. The mature biofilm was encapsulated by extracellular matrix and difficult to remove. Even if it was treated with the clinical concentration of 0.12% CHX for 24 h, bacterial cells could not be completely eliminated or killed in the biofilm. The structure of the CAPE-treated biofilm was observed to be loose and disordered, and the number of dead bacteria that emitted red fluorescence in the CAPE-treated S. mutans biofilm was significantly increased. As the concentration of CAPE increased, the number of dead bacteria increased and the biofilm thickness decreased. For a possible mechanism of mature S. mutans biofilms removal by CAPE, based on our results, we supposed that CAPE might be able to enter small channels in the biofilm, penetrate the thick extracellular matrix, and kill the cells, ultimately destroying and lysing the structure of S. mutans biofilms.
S. mutans is considered to be the most prominent pathogenic factor in dental caries (47). S. mutans cells release various organic acids, especially lactic acid, during the process of carbohydrate metabolism, resulting in demineralization of tooth enamel (48, 49). Therefore, it is very important to study interventions with the virulence factors associated with cariogenicity, such as acidogenicity and acid tolerance (50).
Because the pathology of dental caries is almost entirely related to bacterial metabolism of carbohydrates, the metabolites of bacteria, especially the acids produced after carbohydrate consumption, in turn exert physiological pressure on the oral flora. Due to the acidogenic and aciduric properties of S. mutans, under less favorable environmental conditions it is more competitive than less-acid-tolerant species, and it becomes more abundant in the cariogenic plaque (51). Therefore, stress tolerance, especially acidurity, is closely related to S. mutans virulence. However, there has not been a drug that can be used as a gold standard for inhibiting the acidurity of S. mutans in the current literature studies. In this study, we observed that acid production by S. mutans in vitro was inhibited by sub-MIC levels of CAPE, and dose-dependent inhibition was observed. The survival of S. mutans was affected by pH, while the number of living cells decreased slightly. In this study, CAPE at one-half of its MIC exhibited a strong ability to reduce the acidurity of S. mutans at pH 5, resulting in death of all of the cells. In previous studies of propolis, some researchers found similar results, and it was claimed that the pH and the propolis concentration might be altered due to solvents and that acidic propolis solutions were more effective against bacteria (52).
Previous reports have clarified that biofilms of cariogenic bacteria are responsible for the formation of extracellular polysaccharides due to glucosyltransferase activity (7). The EPS, especially water-insoluble glucans, mediate the adherence of S. mutans and other oral bacterial species to the tooth surfaces, contributing to the formation of plaque biofilms (53). In our experiments, we found that CAPE at sub-MIC levels significantly inhibited the synthesis of water-insoluble EPS and that this inhibitory effect was dose dependent. The accumulation of these dextrans in the dental biofilm is mediated by the expression of extracellular glucosyltransferases enzymes, which are important for the sucrose-dependent adhesion to the tooth surfaces (54). A polyphenol-rich extract from propolis inhibited expression of glycosyltransferases (GtfB, GtfC, and GtfD) and their related regulatory genes, e.g., VicK, VicR, and CcpA (14). The inhibition of EPS production by CAPE may also occur via inhibition of GTF gene expression, but this possibility must be confirmed through further research.
Conclusion.
In conclusion, our results suggest that CAPE shows a remarkable antimicrobial activity against cariogenic bacteria. The inhibitory actions of CAPE against S. mutans may be partly due to its effects on some key virulence factors associated with cariogenicity, including acid production, acid tolerance and the ability to produce extracellular polysaccharides. Further studies involving animal models and safe application forms are worth performing to evaluate its effects in vivo, including the treatment methods and duration of CAPE in the oral cavity, and the application of a carrier in combination with other anticaries drugs. CAPE appears to be a promising source of new agents that may prevent dental caries and other oral diseases.
MATERIALS AND METHODS
Materials.
Materials consisted of caffeic acid phenethyl ester (CAPE) (>98% purity; Solarbio Life Science, Beijing, China), dimethyl sulfoxide (DMSO; Sigma, Taufkirchen, Germany), chlorhexidine (CHX; Sigma-Aldrich, Steinheim, Germany), brain heart infusion, (BHI; Oxoid, Basingstoke, Hampshire, UK), phosphate-buffered saline, (PBS; pH 7.0), crystal violet staining solution, (CV; Sigma), buffered peptone water (BPW; Nissui, Tokyo, Japan), and tryptone-yeast extract medium containing 20 mM glucose (TYEG).
A stock solution of CAPE was prepared in 50% DMSO at 12.8 mg/ml.
Bacterial strains and growth conditions.
Streptococcus mutans UA159, Streptococcus sobrinus 6715, Actinomyces viscosus ATCC 15987, and Lactobacillus acidophilus ATCC 14931 were obtained from the State Key Laboratory of Oral Diseases at Sichuan University (Chengdu, China). The bacteria were cultured anaerobically in BHI (85% N2, 10% H2, and 5% CO2) at 37°C (55).
Bacterial susceptibility assay.
The MIC and minimal bactericidal concentration (MBC) of CAPE were tested in 96-well U-bottomed microtiter plates using a modified broth microdilution method. Two-fold serial dilutions of CAPE were prepared in 50% DMSO at concentrations between 12.8 and 0.4 mg/ml in a volume of 20.0 μl in each well. Next, 80.0 μl of BHI broth and 100.0 μl of bacterial culture containing 2.0 × 106 CFU/ml were added to each well. Each microwell contained final CAPE concentrations ranging from 0.04 to 1.28 mg/ml in 5% DMSO and a final bacterial concentration of 1.0 × 106 CFU/ml. Microwells containing CHX (rather than CAPE) at concentrations ranging from 512.0 to 0.5 × 103 mg/ml were used as positive controls. Solvent and blank controls were incubated with 5% DMSO or BHI broth only, respectively. The cultivation process was performed according to the 2012 Clinical and Laboratory Standards Institute (CLSI) standards. The microtiter plates were anaerobically incubated at 37°C for 46 to 48 h. A microplate spectrophotometer (Multiskan GO; Thermo Scientific, USA) was used to measure the optical density at 600 nm (OD600) of each microwell. The MIC was defined as the lowest CAPE concentration in well for which there was no difference between the of OD600 values of the CAPE-treated well and the blank BHI broth well (56).
For the assays to determine the MBC, aliquots (100.0 ml) were taken from the wells in the MIC assays described above that showed no visual bacterial growth and spread on BHI agar. These cultures were incubated anaerobically at 37°C for 48 h. The lowest CAPE concentration that completely prevented bacterial growth on the BHI agar was defined as the MBC (57). The MIC and MBC assays were performed 3 times for all strains.
Time-kill assay.
The time-kill assay was used to demonstrate the short-term bactericidal effect of CAPE on S. mutans (58). Briefly, CAPE solution (200 μl) and BHI (800 μl) were added to 1 ml of bacterial culture containing 2.0 × 106 CFU/ml; the final CAPE concentrations were equal to the MBC and twice the MBC in a final volume of 2.0 ml. CHX (at a concentration of 0.12%) was used in place of CAPE as a positive control, and 5% DMSO was used as the solvent control. After 0 s, 10 s, 20 s, 30 s, 40 s, 50 s, 1 min, 2 min, 3 min, 4 min, 5 min, 15 min, 30 min, 45 min, and 1 h of anaerobic incubation at 37°C, aliquots (100.0 μl) were withdrawn and 10-fold serially diluted. Aliquots (100.0 ml) of the dilutions were spread on BHI agar and incubated anaerobically at 37°C for 48 h. The time-kill kinetic curves were constructed by plotting the log10 (CFU/ml) versus the incubation time. The assays were performed in triplicate on three different occasions.
Biofilm susceptibility assay.
The effects of CAPE on S. mutans biofilm formation cultivated on saliva-coated plates were assessed in 24-well flat-bottomed microtiter plates using the modified microdilution method (59). First, saliva samples were collected from healthy volunteers. The volunteers were required to not have any food or drink for 2 h before donating saliva, and they spit directly into the saliva collection tubes. The saliva samples were centrifuged at 2,600 × g for 10 min, and the supernatant was aspirated out for use. Next, 200 μl of saliva supernatant was added to each well in a sterile 24-well plate, and the plate was incubated at 37°C. The lid was left open for 1 h to dry the saliva coating. Next, the plate was disinfected under UV light for 1 h to prepare it for biofilm formation.
S. mutans cells were grown in BHI broth supplemented with 1.00% (wt/vol) sucrose (BHIS) to a final concentration of 1.0 × 106 CFU/ml, and 2-fold serial dilutions of CAPE were added to final concentrations ranging from 0.02 to 0.16 mg/ml. Untreated bacterial culture was used as a negative control, CHX treatment was used as a positive control (at concentrations 1 × 103 mg/ml and 2 × 103 mg/ml), 5% DMSO was used as the solvent control. After anaerobic incubation at 37°C for 24 h, the culture supernatants were decanted, and all of the wells were washed 3 times with PBS. The biofilms were fixed with methanol for 15 min, and then the wells were stained with 0.10% (wt/vol) crystal violet (Sigma) for 5 min. The microwells were gently rinsed with water to remove the excess dye. Subsequently, 200.0 μl of 95.00% ethanol was added to each well, and the microtiter plate was shaken at room temperature for 40 min. Finally, the absorbance of each well at 595 nm (A595) was measured using a microplate spectrophotometer (Multiskan GO) to determine the biofilm biomass. The inhibition rate is calculated as [1 − (A595 of the test/A595 of the negative control)] × 100%. The minimum biofilm inhibition concentration (MBIC) was defined as the lowest CAPE concentration that showed 50% (or 90%) or higher inhibition of biofilm formation (MBIC50 or MBIC90) (60).
Scanning electron microscopy.
Electron microscopy was used to observe the formation of S. mutans biofilms in the presence of CAPE. Sterile glass slides were transferred into 24-well flat-bottomed microplates, and the saliva coating was formed on the glass slides in advance. S. mutans cells were grown in BHIS to a final concentration of 1.0 × 106 CFU/ml, and CAPE was added to a final concentration equal to the MBIC50. Untreated bacterial culture was used as the negative control, and 5% DMSO was used as the solvent control. CHX treatment was used as a positive control at a concentration of MBIC50 (1 × 103 mg/ml). Following anaerobic incubation at 37°C for 24 h, the culture supernatants were decanted, and the glass slides were washed 3 times with PBS and air dried at 37°C. The slides were fixed overnight in 2.50% glutaraldehyde solution, washed twice with PBS, and then dehydrated in solutions with increasing concentrations of ethanol (35.00, 50.00, and 75.00% for 30 min each and two cycles of 90.00 and 100.00% for 30 min each) (61). The samples were dried, sputter coated with gold, and observed under a scanning electron microscope (Inspect F; FEI, Eindhoven, The Netherlands) at 20.0 kV.
Confocal laser scanning microscopy.
The bacterial viability after CAPE treatment was assessed using the Live/Dead BacLight bacterial viability kit (L-7012; Molecular Probes, Invitrogen, Carlsbad, CA) (62). S. mutans biofilms were formed on saliva-coated glass slides by culturing for 24 h in a 24-well flat-bottomed microplate. The culture supernatant was decanted and the planktonic cells were removed by washing with PBS. BHIS and CAPE were added to the microplates at final concentrations ranging from 0.04 to 0.16 mg/ml followed by anaerobic incubation at 37°C for 24 h. Sterile saline (0.85% NaCl) was used as the negative control, 5% DMSO was used as the solvent control, and 0.12% CHX alone was used as the positive control. After treatment, the biofilms were stained using SYTO 9 and propidium iodide following the manufacturer’s instructions (Invitrogen) and then observed under a confocal laser scanning microscope (CLSM) (DMIRE2; Leica, Wetzlar, Germany) equipped with a 60× oil immersion lens objective (63). Five fields of view were photographed for each sample; biomembrane three-dimensional (3D) reconstruction was performed with Imaris 7.0.0 (Bitplane, Zürich, Switzerland), and COMSTAT was used for biomass quantification (64).
Lactic acid measurement.
The effect of CAPE on the acid production of S. mutans was detected using subinhibitory concentrations without affecting bacterial viability. For lactic acid measurement, mid-log-phase S. mutans cells were harvested and washed twice with PBS. The S. mutans cells were resuspended in buffered peptone water (BPW) supplemented with 0.2% sucrose in a 24-well plate at a final concentration of 1.0 × 107 CFU/ml, and CAPE was added at final concentrations of one-half MIC, one-quarter MIC, and one-eighth MIC. Untreated bacterial suspension was used as the negative control, and 5% DMSO was used as the solvent control. Following anaerobic incubation at 37°C for 2 h, the S. mutans cells were removed by centrifugation (8,000 × g, 5 min, 4°C), and a lactic acid assay kit (MAK064) was used to measure the lactate concentrations in the supernatants. The absorbance at 570 nm (A570) was measured using a microplate spectrophotometer, and standard curves were used to calculate the lactate concentrations (65). The assays were independently performed 3 times.
Acid tolerance assay.
The effect of CAPE on the acid tolerance of S. mutans was determined by measuring the viability of the bacteria after 2 h of exposure to a pH of 5.0 (66). The S. mutans cells were grown in TYEG broth until the mid-log phase, harvested, and then resuspended at a final concentration of 1.0 × 107 CFU/ml in TYEG broth buffered with 40 mM phosphate/citrate buffer (pH 5.0). CAPE was added at final concentrations of one-half MIC, one-quarter MIC, and one-eighth MIC. Untreated bacterial suspension was used as the negative control, and 5% DMSO was used as the solvent control. After anaerobic incubation at 37°C for 2 h, samples were removed before and after incubation at pH 5.0 for viable counts. The assays were independently performed 3 times.
Growth curve assay of sub-MIC concentration at pH 5.0.
A growth curve was used to show the number of S. mutans cells in the presence of CAPE at one-half of the MIC at pH 5.0. Mid-log-phase S. mutans cells were harvested and resuspended at a final concentration of 1.0 × 107 CFU/ml in TYEG broth buffered with 40 mM phosphate/citrate buffer (pH 5.0). CAPE was added to a final concentration of one-half MIC. Untreated bacterial suspension was used as the negative control, and 5% DMSO was used as the solvent control. After 0, 5, 15, 30, 45, 60, 90, and 120 min of anaerobic incubation at 37°C, samples were removed for viable counts. The growth curve was constructed by plotting the log10 (CFU/ml) versus the incubation time. The assays were performed in triplicate on three different occasions.
Water-insoluble EPS measurement.
The water-insoluble EPS of the biofilms was measured by the anthrone method (67). Saliva coatings were formed in advance on the bottoms of 24-well plates. S. mutans were grown in BHIS to a final concentration of 1.0 × 106 CFU/ml to form biofilms in the plates, and CAPE was added at a sub-MIC final concentration. Untreated bacterial suspension was used as the negative control, and 5% DMSO was used as the solvent control. After anaerobic incubation at 37°C for 24 h, the biofilms were collected in PBS buffer and then centrifuged to obtain the precipitate. The precipitate was washed twice with sterile water and resuspended in 4 ml of 0.4 M NaOH. After centrifugation, the supernatant was mixed with triplex anthrone reagent at 95°C for 6 min. The absorbance at 625 nm (A625) was measured, and the concentrations of water-insoluble EPS were calculated using standard curves. The experiments were independently performed 3 times.
Statistical analysis.
All of the experiments were performed 3 times on different occasions. The differences between the experimental group and the control group were analyzed using SPSS 20.0 (IBM, Chicago, IL) at a significance level of 0.05. To compare the means among groups, one-way analysis of variance (ANOVA) was performed, followed by Tukey’s honestly significant difference (HSD) test.
REFERENCES
- 1.Koo H, Rosalen PL, Cury JA, Yong KP, Bowen WH. 2002. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother 46:1302–1309. doi: 10.1128/aac.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumazawa S, Hamasaka T, Nakayama T. 2004. Antioxidant activity of propolis of various geographic origins. Food Chemistry 84:329–339. doi: 10.1016/S0308-8146(03)00216-4. [DOI] [Google Scholar]
- 3.Burdock GA. 1998. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol 36:347–363. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 4.Ghisalberti EL. 1979. Propolis: a review. Bee World 60:59–84. doi: 10.1080/0005772X.1979.11097738. [DOI] [Google Scholar]
- 5.Kalogeropoulos N, Konteles SJ, Troullidou E, Mourtzinos I, Karathanos VT. 2009. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chemistry 116:452–461. doi: 10.1016/j.foodchem.2009.02.060. [DOI] [Google Scholar]
- 6.Ophori EA, Eriagbonye BN, Ugbodaga P. 2010. Antimicrobial activity of propolis against Streptococcus mutans. Afr J Biotechnol 9:4966–4969. [Google Scholar]
- 7.Duarte S, Koo H, Bowen WH, Hayacibara MF, Cury JA, Ikegaki M, Rosalen PL. 2003. Effect of a novel type of propolis and its chemical fractions on glucosyltransferases and on growth and adherence of mutans streptococci. Biol Pharm Bull 26:527–531. doi: 10.1248/bpb.26.527. [DOI] [PubMed] [Google Scholar]
- 8.Alencar SM, Oldoni TLC, Castro ML, Cabral ISR, Costa-Neto CM, Cury JA, Rosalen PL, Ikegaki M. 2007. Chemical composition and biological activity of a new type of Brazilian propolis: red propolis. J Ethnopharmacol 113:278–283. doi: 10.1016/j.jep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Koo H, Rosalen PL, Cury JA, Park YK, Ikegaki M, Sattler A. 1999. Effect of Apis mellifera propolis from two Brazilian regions on caries development in desalivated rats. Caries Res 33:393–400. doi: 10.1159/000016539. [DOI] [PubMed] [Google Scholar]
- 10.Ikeno K, Ikeno T, Miyazawa C. 1991. Effects of propolis on dental caries in rats. Caries Res 25:347–351. doi: 10.1159/000261390. [DOI] [PubMed] [Google Scholar]
- 11.Hegde KS, Bhat SS, Rao A, Sain S. 2013. Effect of propolis on Streptococcus mutans counts: an in vivo study. Int J Clin Pediatr Dent 6:22–25. doi: 10.5005/jp-journals-10005-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrientos L, Herrera CL, Montenegro G, Ortega X, Veloz J, Alvear M, Cuevas A, Saavedra N, Salazar LA. 2013. Chemical and botanical characterization of Chilean propolis and biological activity on cariogenic bacteria Streptococcus mutans and Streptococcus sobrinus. Braz J Microbiol 44:577–585. doi: 10.1590/S1517-83822013000200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veloz JJ, Saavedra N, Lillo A, Alvear M, Barrientos L, Salazar LA. 2015. Antibiofilm activity of Chilean propolis on Streptococcus mutans is influenced by the year of collection. Biomed Res Int 2015:291351. doi: 10.1155/2015/291351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veloz JJ, Saavedra N, Alvear M, Zambrano T, Barrientos L, Salazar LA. 2016. Polyphenol-rich extract from propolis reduces the expression and activity of Streptococcus mutans glucosyltransferases at subinhibitory concentrations. Biomed Res Int 2016:4302706. doi: 10.1155/2016/4302706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bankova V. 2005. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol 99:114–117. doi: 10.1016/j.jep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, Mestre JR, Grunberger D, Sacks PG, Tanabe T. 1999. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res 59:2347–2352. [PubMed] [Google Scholar]
- 17.Rao CV, Desai D, Kaul B, Amin S, Reddy BS. 1992. Effect of caffeic acid esters on carcinogen-induced mutagenicity and human colon adenocarcinoma cell growth. Chemico-Biological Interactions 84:277–290. doi: 10.1016/0009-2797(92)90129-9. [DOI] [PubMed] [Google Scholar]
- 18.Sud’ina GF, Mirzoeva OK, Pushkareva MA, Korshunova GA, Sumbatyan NV, Varfolomeev SD. 1993. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett 329:21–24. doi: 10.1016/0014-5793(93)80184-V. [DOI] [PubMed] [Google Scholar]
- 19.Chen YJ, Shiao MS, Wang SY. 2001. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs 12:143–149. doi: 10.1097/00001813-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Fesen MR, Pommier Y, Leteurtre F, Hiroguchi S, Yung J, Kohn KW. 1994. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem Pharmacol 48:595–608. doi: 10.1016/0006-2952(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 21.Park S-G, Lee D-Y, Seo S-K, Lee S-W, Kim S-K, Jung W-K, Kang M-S, Choi YH, Yea SS, Choi I, Choi I-W. 2008. Evaluation of anti-allergic properties of caffeic acid phenethyl ester in a murine model of systemic anaphylaxis. Toxicol Appl Pharmacol 226:22–29. doi: 10.1016/j.taap.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Park JH, Lee JK, Kim HS, Chung ST, Eom JH, Kim KA, Chung SJ, Paik SY, Oh HY. 2004. Immunomodulatory effect of caffeic acid phenethyl ester in BALB/c mice. Int Immunopharmacol 4:429–436. doi: 10.1016/j.intimp.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Yiğit U, Kırzıoğlu FY, Uğuz AC, Nazıroğlu M, Özmen Ö. 2017. Is caffeic acid phenethyl ester more protective than doxycycline in experimental periodontitis? Arch Oral Biol 81:61–68. doi: 10.1016/j.archoralbio.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Kuo Y-Y, Jim W-T, Su L-C, Chung C-J, Lin C-Y, Huo C, Tseng J-C, Huang S-H, Lai C-J, Chen B-C, Wang B-J, Chan T-M, Lin H-P, Chang W-SW, Chang C-R, Chuu C-P. 2015. Caffeic acid phenethyl ester is a potential therapeutic agent for oral cancer. Int J Mol Sci 16:10748–10766. doi: 10.3390/ijms160510748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murtaza G, Karim S, Akram MR, Khan SA, Azhar S, Mumtaz A, Asad MHHB. 2014. Caffeic acid phenethyl ester and therapeutic potentials. Biomed Res Int 2014:145342. doi: 10.1155/2014/145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishimoto N, Kakino Y, Iwai K, Mochida KYO, Fujita T. 2005. In vitro antibacterial, antimutagenic and anti-influenza virus activity of caffeic acid phenethyl esters. Biocontrol Sci 10:155–161. doi: 10.4265/bio.10.155. [DOI] [Google Scholar]
- 27.Kujumgiev A, Bankova V, Ignatova A, Popov S. 1993. Antibacterial activity of propolis, some of its components and their analogs. Pharmazie 48:785–786. [PubMed] [Google Scholar]
- 28.Serkedjieva J, Manolova N, Bankova V. 1992. Anti-influenza virus effect of some propolis constituents and their analogues (esters of substituted cinnamic acids). J Nat Prod 55:294–302. doi: 10.1021/np50081a003. [DOI] [PubMed] [Google Scholar]
- 29.Velazquez C, Navarro M, Acosta A, Angulo A, Dominguez Z, Robles R, Robles-Zepeda R, Lugo E, Goycoolea FM, Velazquez EF, Astiazaran H, Hernandez J. 2007. Antibacterial and free-radical scavenging activities of Sonoran propolis. J Appl Microbiol 103:1747–1756. doi: 10.1111/j.1365-2672.2007.03409.x. [DOI] [PubMed] [Google Scholar]
- 30.Veloz JJ, Alvear M, Salazar LA. 2019. Antimicrobial and antibiofilm activity against Streptococcus mutans of individual and mixtures of the main polyphenolic compounds found in Chilean propolis. Biomed Res Int 2019:7602343. doi: 10.1155/2019/7602343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey A. 2000. Strategies for discovering drugs from previously unexplored natural products. Drug Discov Today 5:294–300. doi: 10.1016/s1359-6446(00)01511-7. [DOI] [PubMed] [Google Scholar]
- 32.Takaisi-Kikuni NB, Schilcher H. 1994. Electron microscopic and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Med 60:222–227. doi: 10.1055/s-2006-959463. [DOI] [PubMed] [Google Scholar]
- 33.Mirzoeva OK, Grishanin RN, Calder PC. 1997. Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol Res 152:239–246. doi: 10.1016/S0944-5013(97)80034-1. [DOI] [PubMed] [Google Scholar]
- 34.Bankova V, Christov R, Kujumgiev A, Marcucci MC, Popov S. 1995. Chemical composition and antibacterial activity of Brazilian propolis. Z Naturforsch C J Biosci 50:167–172. doi: 10.1515/znc-1995-3-402. [DOI] [PubMed] [Google Scholar]
- 35.Bonvehí JS, Coll FV, Jordà RE. 1994. The composition, active components and bacteriostatic activity of propolis in dietetics. J Am Oil Chem Soc 71:529–532. doi: 10.1007/BF02540666. [DOI] [Google Scholar]
- 36.Salatino A, Teixeira ÉW, Negri G, Message D. 2005. Origin and chemical variation of Brazilian propolis. Evid Based Complement Alternat Med 2:33–38. doi: 10.1093/ecam/neh060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrera CAM, Barrientos L, Montenegro G, Salazar LA. 2009. Actividad fungicida de seis extractos de propóleos comerciales chilenos sobre Candida spp. Cien Inv Agr 37:75–84. [Google Scholar]
- 38.Saavedra NBL, Herrera C, Alvear M, Montenegro G, Salazar LA. 2011. Effect of Chilean propolis on cariogenic Lactobacillus fermentum bacteria. Cien Inv Agr 38:117–125. [Google Scholar]
- 39.Guan X, Zhou Y, Liang X, Xiao J, He L, Li J. 2012. Effects of compounds found in Nidus Vespae on the growth and cariogenic virulence factors of Streptococcus mutans. Microbiol Res 167:61–68. doi: 10.1016/j.micres.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Beighton D. 2005. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Commun Dent Oral Epidemiol 33:248–255. doi: 10.1111/j.1600-0528.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 41.Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380. doi: 10.1128/MMBR.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruby JD, Li Y, Luo Y, Caufield PW. 2002. Genetic characterization of the oral Actinomyces. Arch Oral Biol 47:457–463. doi: 10.1016/s0003-9969(02)00023-7. [DOI] [PubMed] [Google Scholar]
- 43.Marsh PD. 2006. Dental plaque as a biofilm and a microbial community—implications for health and disease. BMC Oral Health 6:S14. doi: 10.1186/1472-6831-6-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL. 2012. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One 7:e47722. doi: 10.1371/journal.pone.0047722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee HS, Lee SY, Park SH, Jin HL, Sang KA, Choi YM, Choi DJ, Chang JH. 2013. Antimicrobial medical sutures with caffeic acid phenethyl ester and their in vitro/in vivo biological assessment. Med Chem Commun (Camb) 4:777–782. doi: 10.1039/c2md20289a. [DOI] [Google Scholar]
- 46.Tu H, Fan Y, Lv X, Han S, Zhou X, Zhang L. 2016. Activity of synthetic antimicrobial peptide GH12 against oral streptococci. Caries Res 50:48–61. doi: 10.1159/000442898. [DOI] [PubMed] [Google Scholar]
- 47.Bowen WH. 1999. Wither or whither caries research? Caries Res 33:1–3. doi: 10.1159/000016489. [DOI] [PubMed] [Google Scholar]
- 48.Dashper SG, Reynolds EC. 1992. pH regulation by Streptococcus mutans. J Dent Res 71:1159–1165. doi: 10.1177/00220345920710050601. [DOI] [PubMed] [Google Scholar]
- 49.Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury JA. 2006. The role of sucrose in cariogenic dental biofilm formation—new insight. J Dent Res 85:878–887. doi: 10.1177/154405910608501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banas JA. 2004. Virulence properties of Streptococcus mutans. Front Biosci 9:1267–1277. doi: 10.2741/1305. [DOI] [PubMed] [Google Scholar]
- 51.Xu X, Zhou XD, Wu CD. 2011. Tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob Agents Chemother 55:1229–1236. doi: 10.1128/AAC.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mello BCBS, Hubinger MD. 2012. Antioxidant activity and polyphenol contents in Brazilian green propolis extracts prepared with the use of ethanol and water as solvents in different pH values. Int J Food Sci Technol 47:2510–2518. doi: 10.1111/j.1365-2621.2012.03129.x. [DOI] [Google Scholar]
- 53.Hamada S, Slade HD. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev 44:331–384. doi: 10.1128/MMBR.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krzyściak W, Jurczak A, Kościelniak D, Bystrowska B, Skalniak A. 2014. The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis 33:499–515. doi: 10.1007/s10096-013-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao R, Tong Z, Lin Y, Xue Y, Wang W, Kuang R, Wang P, Tian Y, Ni L. 2011. Antimicrobial and antibiofilm activity of pleurocidin against cariogenic microorganisms. Peptides 32:1748–1754. doi: 10.1016/j.peptides.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Song J, Choi B, Jin EJ, Yoon Y, Choi KH. 2012. Curcumin suppresses Streptococcus mutans adherence to human tooth surfaces and extracellular matrix proteins. Eur J Clin Microbiol Infect Dis 31:1347–1352. doi: 10.1007/s10096-011-1448-y. [DOI] [PubMed] [Google Scholar]
- 57.Isogai E, Isogai H, Takahashi K, Okumura K, Savage PB. 2009. Ceragenin CSA-13 exhibits antimicrobial activity against cariogenic and periodontopathic bacteria. Oral Microbiol Immunol 24:170–172. doi: 10.1111/j.1399-302X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 58.Li F, Weir MD, Fouad AF, Xu HHK. 2013. Time-kill behavior against eight bacterial species and cytotoxicity of antibacterial monomers. J Dent 41:881–891. doi: 10.1016/j.jdent.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179. doi: 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 60.Wei GX, Campagna AN, Bobek LA. 2006. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Chemother 57:1100–1109. doi: 10.1093/jac/dkl120. [DOI] [PubMed] [Google Scholar]
- 61.Weber K, Delben J, Bromage TG, Duarte S. 2014. Comparison of SEM and VPSEM imaging techniques with respect to Streptococcus mutans biofilm topography. FEMS Microbiol Lett 350:175–179. doi: 10.1111/1574-6968.12334. [DOI] [PubMed] [Google Scholar]
- 62.Zhou L, Ding Y, Chen W, Zhang P, Chen Y, Lv X. 2013. The in vitro study of ursolic acid and oleanolic acid inhibiting cariogenic microorganisms as well as biofilm. Oral Dis 19:494–500. doi: 10.1111/odi.12031. [DOI] [PubMed] [Google Scholar]
- 63.Zheng X, Zhang K, Zhou X, Liu C, Li M, Li Y, Wang R, Li Y, Li J, Shi W, Xu X. 2013. Involvement of gshAB in the interspecies competition within oral biofilm. J Dent Res 92:819–824. doi: 10.1177/0022034513498598. [DOI] [PubMed] [Google Scholar]
- 64.Zhang K, Wang S, Zhou X, Xu HHK, Weir MD, Ge Y, Li M, Wang S, Li Y, Xu X, Zheng L, Cheng L. 2015. Effect of antibacterial dental adhesive on multispecies biofilms formation. J Dent Res 94:622–629. doi: 10.1177/0022034515571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Wang X, Jiang W, Wang K, Luo J, Li W, Zhou X, Zhang L. 2018. Antimicrobial peptide GH12 suppresses cariogenic virulence factors of Streptococcus mutans. J Oral Microbiol 10:1442089. doi: 10.1080/20002297.2018.1442089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Svensäter G, Larsson UB, Greif ECG, Cvitkovitch DG, Hamilton IR. 1997. Acid tolerance response and survival by oral bacteria. Oral Microbiol Immunol 12:266–273. doi: 10.1111/j.1399-302x.1997.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 67.Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, Vacca-Smith AM, Bowen WH. 2003. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother 52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]






