Select methicillin-susceptible Staphylococcus aureus (MSSA) strains may produce β-lactamases with affinity for first-generation cephalosporins (1GCs). In the setting of a high inoculum, these β-lactamases may promote the cleavage of 1GCs, a phenomenon known as the cefazolin inoculum effect (CzIE). We evaluated the prevalence and impact of CzIE on clinical outcomes among MSSA acute hematogenous osteomyelitis (AHO) cases. MSSA AHO isolates obtained from two children’s hospitals between January 2011 and December 2018 were procured through ongoing surveillance studies.
KEYWORDS: osteomyelitis, cefazolin inoculum effect, MSSA, pediatric, cephalosporin
ABSTRACT
Select methicillin-susceptible Staphylococcus aureus (MSSA) strains may produce β-lactamases with affinity for first-generation cephalosporins (1GCs). In the setting of a high inoculum, these β-lactamases may promote the cleavage of 1GCs, a phenomenon known as the cefazolin inoculum effect (CzIE). We evaluated the prevalence and impact of CzIE on clinical outcomes among MSSA acute hematogenous osteomyelitis (AHO) cases. MSSA AHO isolates obtained from two children’s hospitals between January 2011 and December 2018 were procured through ongoing surveillance studies. Isolates were tested for CzIE via a broth macrodilution assay using an inoculum of 107 CFU/ml; CzIE was defined as a cefazolin MIC of ≥16 μg/ml. Isolates were characterized by accessory gene regulator group (agr). The progression from acute to chronic osteomyelitis was considered an important outcome. A total of 250 cases with viable isolates were included. Notably, 14.4% of isolates exhibited CzIE with no observed temporal trend; and 4% and 76% of patients received a 1GC as an empirical and definitive therapy, respectively. CzIE isolates were more often resistant to clindamycin, belonged to agrIII, and associated with the development of chronic osteomyelitis. In multivariable analyses, agrIII, multiple surgical debridements, delayed source control, and CzIE were independently associated with progression to chronic osteomyelitis. A higher rate of chronic osteomyelitis was observed with CzIE isolates regardless of definitive antibiotic choice. CzIE is exhibited by 14.4% of MSSA AHO isolates in children. CzIE is independently associated with progression to chronic osteomyelitis in cases of AHO irrespective of final antibiotic choice. These data suggest that negative outcomes reported with CzIE may more accurately reflect strain-dependent virulence factors rather than true antibiotic failure.
INTRODUCTION
The agents of choice in the treatment of methicillin-susceptible Staphylococcus aureus (MSSA) infections are β-lactam antimicrobials (1, 2). A number of recent studies have illustrated that the use of first-generation cephalosporins (1GCs; e.g., cefazolin) is associated with similar or, in some reports, improved outcomes compared with antistaphylococcal penicillins (e.g., nafcillin and oxacillin) in the treatment of serious MSSA infections (3–5). Moreover, both cefazolin and nafcillin/oxacillin are regarded as appropriate agents in national guidelines for the treatment of serious MSSA infections, such as bacteremia and endocarditis (2, 6, 7). Notably, 1GCs are associated with fewer drug-related adverse events than nafcillin/oxacillin, including less instances of infusion reactions, rash, neutropenia, acute kidney injury, and transaminase elevation (3, 8).
The vast majority of S. aureus strains possess a β-lactamase encoded by blaZ (9–11). S. aureus β-lactamases were initially characterized serologically into types A to D. The various β-lactamase types have subtle differences in kinetics and substrate affinity (12, 13). Some S. aureus β-lactamase types have a degree of affinity for 1GCs, with the highest affinity among types A and C (13, 14). Despite this, drug inactivation in vivo is not believed to typically occur to a clinically meaningful degree.
As early as the 1970s, a subset of MSSA isolates was noted to exhibit striking elevations in 1GC MICs when a greater than standard inoculum was used in susceptibility testing (15, 16). This phenomenon, which affects 1GCs (including cefazolin and cephalexin) but not nafcillin/oxacillin, is referred to as the cephalosporin (or cefazolin) inoculum effect (CzIE) (17, 18). In a recent study of Argentinian adults with MSSA bacteremia treated with 1GC, it was noted that CzIE was an independent predictor of 30-day all-cause mortality (9). Such observations continue to raise questions regarding the efficacy of 1GCs in severe MSSA infections.
There are no data available on the prevalence or clinical significance of CzIE in a pediatric population. Acute hematogenous osteomyelitis (AHO) is the most common invasive staphylococcal infection in children. S. aureus AHO is frequently associated with large deep-seated purulent collections which require surgical debridement (19, 20) and as such may be regarded as a high inoculum infection. As the proportion of invasive S. aureus disease attributable to MSSA among children in the United States continues to rise (19, 21, 22), understanding the impact of CzIE on clinical outcomes will be of increasing importance.
The primary goals of this study were to (i) describe the frequency and characteristics of CzIE among MSSA AHO isolates at two children’s hospitals and (ii) assess the impact of CzIE on AHO outcomes. The progression from acute to chronic osteomyelitis was considered a clinically significant outcome which is regarded as a surrogate for treatment failure.
RESULTS
A total of 250 viable MSSA AHO isolates from January 2011 to December 2018 met all inclusion criteria. Of those, 208 isolates were obtained from Texas Children’s Hospital (TCH) and 42 were from St. Louis Children’s Hospital (SLCH). The median patient age was 9.3 years (interquartile range [IQR], 5.4 to 12.2 years) (Table 1). Overall, 64.4% of cases were associated with isolated osteomyelitis and 35.6% with osteomyelitis and concomitant septic arthritis. A total of 57.6% of cases had bacteremia. Surgical drainage/debridement was performed in 63.2% of cases and 18% underwent ≥2 temporally distinct surgical procedures during the index admission.
TABLE 1.
Characteristics of study groupa
| Characteristic | Clinical valuesb |
|---|---|
| Age (yrs) | 9.3 (5.4–12.2) |
| Female gender | 100 (40) |
| Race | |
| White | 183 (73.2) |
| Black | 48 (19.2) |
| Asian | 6 (2.4) |
| Otherc | 5 (2) |
| Unknown/not disclosed | 8 (3.2) |
| Hispanic ethnicity | 71 (28.4) |
| Chronic medical comorbidities | 15 (6) |
| Duration of symptoms on presentation (days) | 5 (3–7) |
| Isolated osteomyelitis | 162 (64.4) |
| Osteomyelitis with concomitant septic arthritis | 88 (35.2) |
| Subperiosteal/intraosseous abscess | 108 (43.2) |
| Surgery performed | 158 (63.2) |
| Positive blood culture | 144 (57.6) |
| ICUd admission | 13 (5.2) |
| Length of stay (days) | 6 (5–9) |
| Total duration of therapy (days) | 42 (31–56) |
| Total duration of follow-up (days) | 92.5 (37–324) |
| Progression to chronic osteomyelitis | 13 (5.2) |
n = 250.
All continuous variables are presented as medians with interquartile ranges; categorical variables are presented as n (%).
Other with regard to race includes Native American/Alaskan Native, Native Hawaiian/Pacific Islander, and self-identified multiple races given the relatively small number of patients.
ICU, intensive care unit.
Significant differences in patient demographics, disease presentation, and management were noted between contributing institutions (see Table S1 in the supplemental material). MSSA isolates from both institutions were similar in terms of clindamycin resistance, accessory gene regulator (agr) group, and Panton-Valentine leucocidin (PVL) carriage.
Medical therapy.
Overall, the most common empirical antibiotic choices were clindamycin monotherapy (87, 34.5%) and vancomycin monotherapy (75, 30%); 38 patients (15.2%) received empirical vancomycin and nafcillin combination therapy. Only 10 subjects (4%) received a 1GC for empirical therapy with or without other agents. A full list of empirical therapy regimens is provided in Table S2 in the supplemental material.
A total of 189 patients (76%) received a 1GC for definitive therapy, 114 patients (45.6%) were prescribed cephalexin, 74 patients (29.6%) received cefazolin, and 1 patient received cefadroxil. Patients received a median of 4 days of non-1GC therapy (IQR, 3 to 6 days) prior to transitioning to 1GC. Specific dosing regimens of cephalosporins are provided in Table S3 in the supplemental material; patients were most commonly prescribed 100 mg/kg of body weight per day of cefazolin or cephalexin divided every 8 h. Other commonly used agents for definitive therapy included nafcillin (37, 14.8%) and clindamycin (14, 5.6%).
Overall, 127 patients (50.8%) were discharged on oral antibiotics. These patients received a median of 6 days of intravenous antibiotics prior to transition to oral antibiotics (IQR, 4 to 8 days). The rate of oral antibiotic use increased during the study period from 19% in 2011 to 75% in 2018 (P = 0.003).
Cefazolin inoculum effect.
Using a standard inoculum (105 CFU/ml), all isolates had a cefazolin MIC of ≤2 μg/ml (Fig. 1). When tested with a high inoculum (107 CFU/ml), 90 isolates (36%) exhibited a ≥4-fold increase in cefazolin MIC, although the majority had a high inoculum MIC of ≤4 μg/ml (53/90, 58.9%). Overall, 36 MSSA isolates (14.4%) exhibited significant CzIE in vitro, defined as a high inoculum MIC of ≥16 μg/ml (range, 16 to 128 μg/ml) (9, 23). CzIE isolates were more likely to be clindamycin resistant (25% versus 9.3%, P = 0.02) and to belong to agrIII (52.7% versus 9.8%, P < 0.001) than isolates not exhibiting CzIE (Table 2). There was no temporal trend with regard to the proportion of isolates exhibiting CzIE in the study period. A similar proportion of isolates from each contributing hospital exhibited CzIE. Additionally, among the subset tested, CzIE isolates exhibited higher cephalexin MICs when using a 107-CFU/ml inoculum than non-CzIE isolates (Fig. 2).
FIG 1.
Cefazolin MICs. Broth macrodilution MICs for cefazolin using standard (105 CFU/ml) (A) and high inocula (107 CFU/ml) (B). CzIE, cefazolin inoculum effect.
TABLE 2.
Comparison of infections by isolates with and without CzIE
| Characteristic | Clinical and microbiologic valuesa related to isolates: |
P value | |
|---|---|---|---|
| With CzIE (n = 36) | Without CzIE (n = 214) | ||
| Age (yrs) | 10.4 (6.9–11.9) | 9.1 (5.1–12.2) | 0.38 |
| Female gender | 13 (36.1) | 87 (40.6) | 0.71 |
| Race | 0.82 | ||
| White | 30 (83.3) | 153 (71.4) | |
| Black | 5 (13.8) | 43 (20.1) | |
| Asian | 0 | 6 (2.8) | |
| Otherb | 0 | 5 (2.3) | |
| Unknown/not disclosed | 1 (2.7) | 7 (3.3) | |
| Hispanic ethnicity | 10 (27.8) | 61 (28.5) | 1 |
| Duration of symptoms on presentation (days) | 4.5 (2–7) | 5 (3–7) | 0.52 |
| Admission CRPc (mg/dl) | 4.6 (3.8–7.7) | 6.6 (3.7–15.5) | 0.21 |
| Multifocal infection | 3 (8.3) | 7 (3.3) | 0.16 |
| Subperiosteal/intraosseous abscess | 17 (47.2) | 85 (39.7) | 0.46 |
| Maximum abscess diameterd (cm) | 2.9 (1.1–4) | 3 (1.5–5.8) | 0.3 |
| Surgical procedure performed | 20 (55.5) | 136 (63.5) | 0.36 |
| ≥2 surgical procedures performede | 2 (5.6) | 43 (20.1) | 0.04 |
| Delayed first source controlf | 5 (13.8) | 23 (10.7) | 0.57 |
| ICU admission | 1 (2.7) | 12 (5.6) | 0.7 |
| Positive blood culture | 26 (72.2) | 118 (55.1) | 0.07 |
| Duration of bacteremia (days) | 1 (1–2) | 1 (1–2) | 0.39 |
| Duration of fever after admission (days) | 3 (2–4) | 3 (2–4) | 0.37 |
| Definitive treatment with 1GC | 30 (83.3) | 159 (74.3) | 0.29 |
| Discharge on oral antibiotics | 20 (55.6) | 105 (49.1) | 0.58 |
| Length of stay (days) | 6 (5–10) | 6 (5–9) | 0.9 |
| Duration of follow-up (days) | 68 (39–494) | 77 (35–247) | 0.32 |
| Progression to chronic osteomyelitis | 5 (13.8) | 8 (3.7) | 0.03 |
| Clindamycin resistance | 9 (25) | 20 (9.3) | 0.01 |
| PVLg positive | 5 (13.8) | 47 (21.9) | 0.38 |
| Vancomycin MIC of >1 μg/ml | 12 (33.3) | 100 (46.7) | 0.15 |
| agrIh | 13 (36.1) | 140 (65.4) | 0.001 |
| agrII | 2 (5.6) | 31 (14.5) | 0.19 |
| agrIII | 19 (52.7) | 21 (9.8) | <0.001 |
| agrIV | 1 (2.7) | 8 (3.7) | 1 |
| agr nontypeable | 1 (2.7) | 14 (6.5) | 0.7 |
All continuous variables are presented as medians with interquartile ranges; categorical variables are presented as n (%).
Other with regard to race includes Native American/Alaskan Native, Native Hawaiian/Pacific Islander, and self-identified multiple races.
CRP, C-reactive protein.
Only 61 cases had abscess size documented.
The number of surgical procedures performed refers only to those performed during the index admission.
Delayed source control was defined as >3 calendar days from the time of admission until first surgical source control.
PVL, Panton-Valentine leucocidin.
agr refers to accessory gene regulator group.
FIG 2.
Cephalexin MICs. Broth macrodilution MICs for cefazolin using standard (105 CFU/ml) (A) and high inocula (107 CFU/ml) (B).
Infections caused by isolates with and without CzIE were similar in terms of patient age, demographics, the presence of bone abscesses, and the need for surgery and medical management (Table 2). Patients with CzIE isolate infections less often underwent multiple surgical debridement procedures (5.6% versus 20.1%, P = 0.04). A similar proportion of patients in both groups received definitive treatment with a 1GC. A greater proportion of infections caused by isolates with CzIE evolved into chronic osteomyelitis by the time of last follow-up (13.8% versus 3.7%, P = 0.03).
Chronic osteomyelitis.
Overall, 13 (5.2%) MSSA osteoarticular infections (OAIs) progressed to chronic osteomyelitis. With regard to characteristics of the infecting organisms themselves, the development of chronic osteomyelitis was associated with isolates exhibiting CzIE and those belonging to agrIII in univariable analyses (Table 3); there was no association with the carriage of PVL and development of chronic osteomyelitis.
TABLE 3.
Univariable associations of chronic osteomyelitis and microbiologic and molecular characteristics of MSSA
| Characteristic of MSSA | No. (%) of patients with: |
P value | |
|---|---|---|---|
| Progression to chronic osteomyelitis (n = 13) | No progression to chronic osteomyelitis (n = 237) | ||
| Clindamycin resistant | 3 (23.1) | 26 (10.9) | 0.18 |
| CzIEa | 5 (38.5) | 31 (13.1) | 0.02 |
| ≥ 4-fold increase in cefazolin MIC with high inoculum | 6 (46.2) | 84 (35.4) | 0.55 |
| Vancomycin Etest MIC of >1 μg/mlb | 3 (23.1) | 109 (45.9) | 0.15 |
| PVL positive | 5 (38.5) | 47 (19.8) | 0.15 |
| agrI | 6 (46.1) | 147 (62) | 0.26 |
| agrII | 2 (15.4) | 31 (13.1) | 0.67 |
| agrIII | 4 (30.7) | 36 (15.2) | 0.1 |
| agrIV | 0 | 9 (3.8) | 1 |
| agr nontypeable | 1 (7.6) | 14 (5.9) | 0.56 |
CzIE was defined as a high-inoculum cefazolin MIC of ≥16 μg/ml.
No isolate had a vancomycin Etest MIC of >2 μg/ml.
Patients who developed chronic osteomyelitis more often had subperiosteal/intraosseous abscesses (69.2% versus 41.8%, P = 0.08, Table 4), bacteremia (84.6% versus 56.1%, P = 0.048), multiple surgical debridements during the period of acute infection (53.8% versus 15.8%, P = 0.003), and experienced a delay in first source control (>3 days until source control [24]; 30.7% versus 9.2%, P = 0.03). There were no significant associations observed between antibiotic choices, route or duration of therapy, and progression to chronic osteomyelitis. With regard to 1GCs, there was no observed association with specific agents or dosing regimens and chronic osteomyelitis (see Table S4 in the supplemental material).
TABLE 4.
Univariable associations of chronic osteomyelitis, patient characteristics, and management
| Characteristic | Valuesa for patients with: |
P value | |
|---|---|---|---|
| Progression to chronic osteomyelitis (n = 13) | No progression to chronic osteomyelitis (n = 237) | ||
| Age (yrs) | 8.9 (4.2–13.9) | 8.3 (3.1–11.6) | 0.26 |
| Female gender | 4 (30.7) | 96 (40.5) | 0.57 |
| Race | 0.9 | ||
| White | 10 (76.9) | 173 (72.9) | |
| Black | 3 (23.1) | 45 (18.9) | |
| Asian | 0 | 6 (2.5) | |
| Otherb | 0 | 5 (2.1) | |
| Unknown/not disclosed | 0 | 8 (3.4) | |
| Hispanic ethnicity | 2 (15.4) | 69 (29.1) | 0.35 |
| Duration of symptoms on presentation (days) | 5 (4–20) | 5 (3–7) | 1 |
| Multifocal infection | 0 | 11 (4.6) | 1 |
| Subperiosteal/intraosseous abscess | 9 (69.2) | 99 (41.8) | 0.08 |
| Maximum abscess diameterc (cm) | 4.8 (2.9–6.1) | 3 (1.4–4.4) | 0.21 |
| ICU admission | 2 (15.4) | 11 (4.6) | 0.14 |
| Positive blood culture | 11 (84.6) | 133 (56.8) | 0.048 |
| Duration of bacteremia (days) | 1 (1–1) | 1 (1–2) | 0.9 |
| Duration of fever after admission (days) | 1 (0–2) | 2 (0–3) | 0.11 |
| Medical management | |||
| Empiric therapy | 0.18 | ||
| Clindamycin monotherapy | 3 (23.1) | 84 (35.4) | |
| Vancomycin monotherapy | 6 (46.2) | 69 (29.1) | |
| 1GC monotherapy | 1 (7.7) | 9 (3.8) | |
| Vancomycin + nafcillin | 3 (23.1) | 35 (14.8) | |
| Other | 0 | 40 (16.8) | |
| Definitive therapy | 0.26 | ||
| 1GC | 8 (61.5) | 181 (76.4) | |
| Nafcillin | 5 (38.4) | 32 (13.5) | |
| Clindamycin | 0 | 14 (5.9) | |
| Other | 0 | 10 (4.2) | |
| Oral antibiotic at discharge | 5 (38.5) | 120 (50.6) | 0.57 |
| Duration of intravenous antibiotics (days) | 22.5 (10–55) | 14.5 (6–34) | 0.17 |
| Total duration of antibiotics (days) | 46 (32–61) | 42 (31–53) | 0.71 |
| Surgical management | |||
| Surgical procedure performed | 11 (84.6) | 145 (61.2) | 0.14 |
| ≥2 surgical procedures | 7 (53.8) | 38 (15.8) | 0.003 |
| Delayed first source control | 4 (30.7) | 22 (9.2) | 0.03 |
| Duration of follow-up (days) | 61 (32–82) | 79 (36–285) | 0.15 |
All continuous variables are presented as medians with interquartile ranges; categorical variables are presented as n (%).
Other with regard to race includes Native American/Alaskan Native, Native Hawaiian/Pacific Islander, and self-identified multiple races.
Only 61 cases had abscess size documented.
The results of multivariable analyses revealed significant associations between progression to chronic osteomyelitis and multiple surgical debridements (P = 0.01), delayed source control (P = 0.03), agrIII (P = 0.04), and CzIE (P = 0.03) (Table 5). When CzIE alone was substituted with CzIE and 1GC treatment and forced into the logistic regression model, we observed no association with chronic osteomyelitis (P = 0.3).
TABLE 5.
Multivariable analyses of risk factors for MSSA chronic osteomyelitisa
| Factor | Multivariable P value | Adjusted OR | 95% CI |
|---|---|---|---|
| Bone abscess | 0.54 | 1.58 | 0.37–6.7 |
| Positive blood culture | 0.09 | 4.1 | 0.8–20.26 |
| ≥2 surgical procedures | 0.01 | 6.99 | 1.58–31.1 |
| Delayed first source control | 0.03 | 2.59 | 1.16–11.11 |
| agrIII | 0.04 | 1.71 | 1.03–7.81 |
| CzIE | 0.03 | 13.4 | 1.1–18.21 |
OR, odds ratio; CI, confidence interval.
Antibiotic choice, inoculum effect, and outcomes.
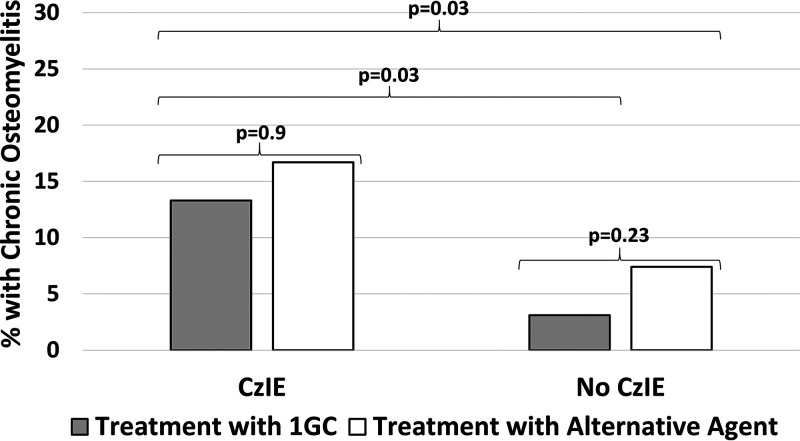
Subjects were stratified by inoculum effect status to assess the effect of definitive antibiotic choice on the development of chronic osteomyelitis. A 1GC was definitive therapy in 30/36 (83.3%) CzIE subjects and 159/214 (74.2%) of non-CzIE subjects (P = 0.29). Isolates with CzIE were associated with higher rates of progression to chronic osteomyelitis than those without CzIE regardless of whether treatment was with a 1GC or a non-1GC regimen (Fig. 3).
FIG 3.
Relationship between CzIE, definitive antibiotic choice, and development of chronic osteomyelitis. Higher rates of chronic osteomyelitis were observed among CzIE isolates regardless of whether patients were treated with a 1GC or an alternative agent. Comparisons performed with Fisher’s exact test.
Whole-genome sequencing.
Whole-genome sequencing was performed on all 36 isolates exhibiting CzIE to determine sequence types (STs) and β-lactamase types (Fig. 4). The majority of cases were ST30 (19/36, 52.7%), with the next most common being ST8 (5/36, 13.9%). Among ST30, 15/19 (78.9%) belonged to agrIII. Type A β-lactamase was most common, being noted in 27/36 cases (75%), followed by type C (8/36, 22.2%) and type B (1/36, 2.7%). Type A β-lactamase predominated in ST30 strains (18/19, 94.7%), while all ST8 strains possessed type C β-lactamase.
FIG 4.
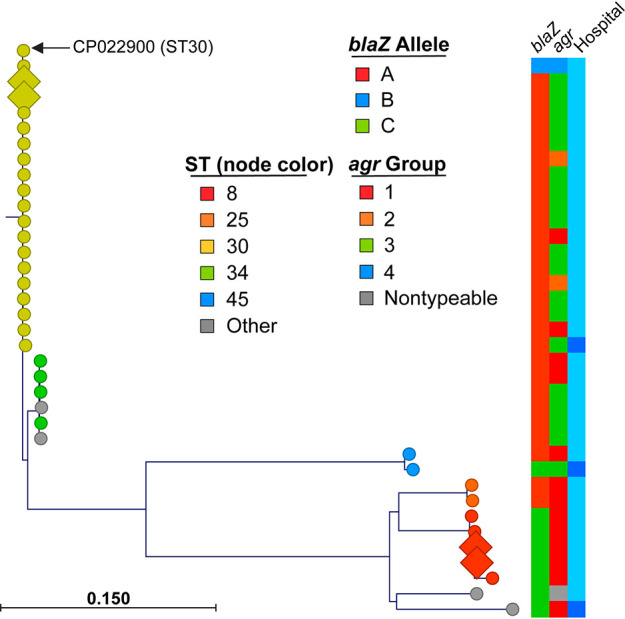
Phylogenetic tree of CzIE isolates. Whole-genome sequencing from a total of 36 CzIE strains was used to identify single nucleotide polymorphisms relative to the ST30 reference strain (GenBank accession number CP022900) core genome, as described in the Materials and Methods. Node shape (diamonds versus circles) corresponds to isolates with progression to chronic osteomyelitis, while color indicates sequence type (ST; legend). Metadata layers are indicated and defined in the legend; final metadata layer indicates geographic site of isolate, with light blue corresponding to TCH and dark blue representing SLCH.
DISCUSSION
In the treatment of serious MSSA infections outside the central nervous system, 1GCs are generally regarded as equally efficacious as antistaphylococcal penicillins (5). Controversy has surrounded the clinical impact of CzIE and how this phenomenon should influence therapeutic decisions (25). A number of studies in animal models as well as at least one clinical study in adults suggest that the presence of CzIE reduces the efficacy of 1GC therapy (9, 26). However, other observational clinical studies have not illustrated an increased risk of treatment failure or attributable mortality with CzIE infection (10, 27). Here, we present the first study of the epidemiology and impact of CzIE among pediatric osteoarticular infections, demonstrating an association between infections caused by CzIE strains and progression to chronic osteomyelitis, a surrogate for treatment failure.
Across published studies, the definition of CzIE varies. A cefazolin MIC of ≥16 μg/ml has previously been used by CLSI to define cefazolin resistance in S. aureus (28) and may represent a clinically meaningful measure; this definition was used in our study as by others (9). In our study population, 14.4% of MSSA isolates exhibited CzIE. In contrast, 55% of MSSA bloodstream isolates exhibited CzIE in a study from Argentina (9) using an identical definition. When applying the definition of a ≥4-fold increase in MIC comparing high and standard inoculums, 36% of our isolates exhibited CzIE. In a recent study of MSSA isolates from four hospitals in the Chicago area, Wang et al. reported that 16.7% of all isolates exhibited a 4-fold increase in cefazolin MIC comparing high and standard inoculums (29). Using a similar definition of CzIE, investigators at Emory University reported CzIE in 27% of MSSA bloodstream isolates obtained from adults, although only 4.3% had a cefazolin MIC of ≥16 μg/ml (11). Such findings suggest a degree of geographic variability in CzIE prevalence.
We also observed an association with CzIE and clindamycin resistance, occurring in 25% of isolates. Even though β-lactam antibiotics are clearly preferred for MSSA, this is notable, as clindamycin is commonly used empirically in suspected AHO. While the exact reasons for this association are unclear, such a finding was previously reported by investigators in Korea who found that 21% of CzIE isolates exhibited concomitant clindamycin resistance; this was particularly common among CzIE isolates with type A β-lactamase occurring in 44% of them (27). These findings may be associated with the underlying strain type. Wi et al. reported that among Korean CzIE isolates, 75% belonged to agrIII (30). Similar to these data, we found that CzIE was disproportionately occurring in agrIII isolates in our North American population.
In multivariable analyses, CzIE was an independent predictor for the progression of acute osteomyelitis to chronic infection, a proxy for treatment failure. This finding is consistent with that of a recent study in adults with MSSA bacteremia which suggested an association with CzIE and mortality (9). Importantly, we found that children with AHO caused by CzIE MSSA have similar rates of progression to chronic osteomyelitis when treated with either 1GC or non-1GC regimens. These results suggest that poor outcomes previously observed with CzIE strains may more accurately reflect some intrinsic virulence factor rather than antibiotic failure per se, as negative outcomes occurred irrespective of antibiotic choice. Such findings may explain why 1GCs and antistaphylococcal penicillins have been found to have a similar overall efficacy in the treatment of serious MSSA infections (3–5) despite the existence of CzIE in 10% to 20% of North American MSSA isolates (29). Based on the results of the present study, clinicians may be able to successfully use 1GCs to treat CzIE osteoarticular MSSA infections, although careful follow-up is urged. Arguably, 1GCs may be the preferred agent even in the setting of CzIE, given their favorable side effect profile relative to antistaphylococcal penicillins; the rate of adverse drug reactions was almost 3-fold higher among adults receiving nafcillin than those receiving cefazolin in one study (8). Our findings must be interpreted with caution given the relatively small number of patients in the present study who received definitive treatment with a non-1GC regimen. Furthermore, these results are in direct contrast to work in adults by Lee et al. (31) which showed that CzIE MSSA infections treated with cefazolin were associated with higher rates of treatment failure than those treated with nafcillin. Interestingly, these investigators found that CzIE and non-CzIE MSSA infections have overall similar outcomes. In addition, studies in rat endocarditis models demonstrate that the use of cefazolin in a CzIE MSSA infection is associated with a more severe disease course which may be ameliorated by the addition of a β-lactamase inhibitor with the 1GC (26, 32). Such discrepancies could in part be related to differences in populations studied (adults with comorbidities versus otherwise healthy children), as well as measures of treatment success/failure. Definitively addressing this question would require a clinical trial comparing the outcomes of CzIE MSSA infections treated with antistaphylococcal penicillins versus those with 1GC; however, significant challenges would exist for the execution of such a study. Of note, the majority of patients in our study receiving a 1GC were treated with cephalexin at an every-8-hour dosing interval with a median dose of 100 mg/kg/day. Studies from the 1970s found that cephalexin peak serum concentrations in children following a 25 mg/kg dose were 20 to 25 μg/ml (33). Given the cephalexin MIC90 of 8 μg/ml, concerns may exist about the potential failure of this particular therapy; however, the observed MIC range for cephalexin was similar to that found by other investigators (18). Furthermore, a recent observational study at Rady Children’s found that patients with MSSA AHO treated with cephalexin at a median of 91 mg/kg/day had similar cure rates when doses were administered with either every 8 h or every 6 h (34). For our study, all patients received some duration of intravenous therapy and many underwent surgical drainage procedures prior to starting cephalexin; these interventions may have reduced the bacterial load enough to obviate CzIE.
The agrIII strains were also associated with chronic osteomyelitis. agr is a complex quorum sensing regulatory system controlling the expression of numerous staphylococcal adhesins and virulence factors (35). Isolates exhibiting agr dysfunction and dysregulation of downstream gene products have previously been associated with progression to chronic infection in animal models of osteomyelitis (36). While we did not specifically assess for agr dysfunction, differences in agr-associated gene regulation attributable to specific agr polymorphisms may partially explain our findings. Interestingly, agrIII is rarely associated with community-acquired methicillin-resistant S. aureus (MRSA) in the United States, with most USA300 strains (either MRSA or MSSA) being agrI (21, 24). Approximately 25% of invasive non-USA300 MSSA strains belong to agrIII (21) and thus may represent a small but virulent subset of MSSA. USA300 strains have previously been associated with large purulent collections (19) that often require multiple debridement procedures; this may explain in part why CzIE strains (which are most frequently agrIII) are less often associated with multiple debridements.
Based on whole-genome sequencing studies, the most common sequence type among CzIE isolates was ST30, accounting for nearly half of all strains, which is consistent with work from South America (9). Interestingly, previous research examining all invasive MSSA in children found that ST30 strains only account for ∼10% of all disease (21). Taken together, these data suggest that strain background may be important in at least a subset of CzIE isolates. Notably, the overwhelming majority of ST30 CzIE isolates possessed type A β-lactamase, which is known to have the highest affinity for 1GCs (13, 14).
Additional limitations to this study should be acknowledged. Foremost, the retrospective nature of the study limits the degree to which conclusions can be made regarding therapeutic decisions and outcomes. The unequal contribution of cases by the two sites may have potentially introduced bias, and moreover, all MSSA osteomyelitis may not have been captured. Notably, the proportion of cases with CzIE was similar at both study institutions. The rate of chronic osteomyelitis in this study (5.2%) was higher than that reported in some centers but was overall consistent with the range reported across North American studies (20, 37); our study utilized a broad definition of chronic osteomyelitis that incorporated clinical, pathological, and radiographic criteria (24) to provide a thorough capture of sequelae. The high degree of surgical source control at the participating study sites may not accurately reflect wider pediatric practice and may have influenced the impact of CzIE on outcomes. Similarly, the fact that the majority of patients received a non-1GC regimen empirically may have impacted disease burden sufficiently to influence the observed relationship between CzIE, 1GC use, and outcome. It is difficult retrospectively to account for the potential impact of postdischarge medication adherence on outcomes. Furthermore, clinical microbiology laboratories do not routinely screen MSSA isolates for CzIE, limiting the impact our findings have on management at the bedside. The relatively labor-intensive nature of the broth macrodilution assay may make implementation difficult for many busy clinical laboratories. The study is underpowered to explore the relationship between dosing of 1GCs, CzIE, and outcome. Finally, as this study focused on AHO, the findings may not necessarily be extrapolated to other invasive MSSA infections in children.
In conclusion, CzIE is exhibited by 14% of MSSA isolates at 2 geographically distinct pediatric centers. CzIE is associated with clindamycin resistance as well as specific genotypes which include agrIII/ST30. CzIE, along with delayed source control and the need for multiple surgical procedures in MSSA AHO, is associated with progression to chronic osteomyelitis. Further work is needed to better understand the relationship between CzIE and negative outcomes, as well as how or if this phenomenon should impact therapeutic decisions.
MATERIALS AND METHODS
Isolates and patients were identified through two separate ongoing surveillance studies at Texas Children’s Hospital (TCH; affiliated with Baylor College of Medicine, Houston, TX, comprising 724 inpatient beds) (38) and St. Louis Children’s Hospital (SLCH; affiliated with Washington University School of Medicine, St. Louis, MO, with 390 inpatient beds) (39). At TCH, since 2001, all S. aureus isolates identified by the clinical microbiology laboratory in the routine course of care are subcultured and stored in horse blood at –80°C in the Edward O. Mason, Jr., Infectious Diseases Research Laboratory (IDRL), and basic clinical and demographic data are recorded on a standardized case report form (19). In previous studies at our institution, MSSA accounted for 43% of all osteomyelitis cases, with the surveillance study capturing >70% of these isolates (40). During the same time period, at SLCH, S. aureus isolates recovered from the blood, bone, or synovial fluid were obtained from the clinical microbiology laboratory and stored at –80°C, and similar clinical data were recorded. Only one isolate per patient per episode of infection was collected, and isolates were not serially passaged. For the purposes of this study, only patients with culture-confirmed MSSA AHO with or without concomitant septic arthritis identified from 1 January 1 2011 to 31 December 2018 were included. The diagnosis of AHO was defined by a constellation of physical examination, radiology, and microbiological findings as previously described (19, 40, 41). Patients with open or penetrating trauma, orthopedic hardware in situ, or osteomyelitis secondary to a contiguous focus or a surgical procedure (such as sternal osteomyelitis after cardiac surgery) were excluded. Data from a subset of these patients have been reported in other publications (19, 24, 39, 42). All medical records were reviewed from the time of initial hospital admission with AHO until time of last follow-up with infectious diseases or orthopedics. The institutional review boards of Baylor College of Medicine and Washington University School of Medicine approved this study. A full description of study definitions and the genomic and statistical analyses are provided in Appendix S1 in the supplemental material.
Microbiology studies.
The clinical microbiology labs at TCH and SLCH performed initial isolate identification as well as susceptibility testing to oxacillin, vancomycin, and clindamycin in accordance with CLSI guidelines (43). Additional characterization of isolates as well as microbiology studies were performed in the IDRL at TCH. All isolates underwent testing for the presence of CzIE using paired cefazolin broth macrodilution assays with an inoculum of 105 CFU/ml (standard inoculum to confirm susceptibility) and 107 CFU/ml (high inoculum) (additional detail in Appendix S1). Current CLSI guidelines do not define 1GC breakpoints for S. aureus. CzIE was defined as a cefazolin MIC of ≥16 μg/ml using the 107 CFU/ml inoculum (9, 23); this value was regarded as a clinically significant MIC, as previous 2012 guidelines defined cefazolin susceptibility in S. aureus as an MIC of ≤8 μg/ml (28). All isolates exhibiting CzIE as well as an equivalent number of random non-CzIE isolates were also subjected to cephalexin MIC determinations using high and low inocula, as described above. Laboratory personnel performing CzIE testing were blind to all clinical data.
Supplementary Material
ACKNOWLEDGMENTS
S.F. was supported by the National Institutes of Health (NIH; UL1-RR024992, K23-AI091690, and R01-AI097434), the Agency for Healthcare Research and Quality (AHRQ; R01-HS021736 and R01-HS024269), and the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital. J.C.M. was supported by NIAID K23-AI099159, The Texas Children’s Hospital Pilot Research Fund, and AHRQ R01-HS026896. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or AHRQ.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.McMullan BJ, Bowen AC, Blyth CC, Van Hal S, Korman T, Buttery J, Voss L, Roberts S, Cooper C, Tong SY, Turnidge JD. 2016. The epidemiology and mortality of Staphylococcus aureus bacteremia in a prospective cohort of Australian and New Zealand children. JAMA Pediatr 170:979–986. doi: 10.1001/jamapediatrics.2016.1477. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics. 2018. Staphylococcus aureus, p 733–746. In Kimberlin DW, Brady MT, Jackson MA (ed), Redbook 2018 report of the committee on infectious diseases, 31 ed. American Academy of Pediatrics, Elk Grove Village, IL. [Google Scholar]
- 3.Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, Lewis JS. 2nd, 2014. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 58:5117–5124. doi: 10.1128/AAC.02800-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao SN, Rhodes NJ, Lee BJ, Scheetz MH, Hanson AP, Segreti J, Crank CW, Wang SK. 2015. Treatment outcomes with cefazolin versus oxacillin for deep-seated methicillin-susceptible Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 59:5232–5238. doi: 10.1128/AAC.04677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDanel JS, Perencevich EN, Diekema DJ, Herwaldt LA, Smith TC, Chrischilles EA, Dawson JD, Jiang L, Goto M, Schweizer ML. 2015. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis 61:361–367. doi: 10.1093/cid/civ308. [DOI] [PubMed] [Google Scholar]
- 6.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals From the American Heart Association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 7.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youngster I, Shenoy ES, Hooper DC, Nelson SB. 2014. Comparative evaluation of the tolerability of cefazolin and nafcillin for treatment of methicillin-susceptible Staphylococcus aureus infections in the outpatient setting. Clin Infect Dis 59:369–375. doi: 10.1093/cid/ciu301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller WR, Seas C, Carvajal LP, Diaz L, Echeverri AM, Ferro C, Rios R, Porras P, Luna C, Gotuzzo E, Munita JM, Nannini E, Carcamo C, Reyes J, Arias CA. 2018. The cefazolin inoculum effect is associated with increased mortality in methicillin-susceptible Staphylococcus aureus bacteremia. Open Forum Infect Dis 5:ofy123. doi: 10.1093/ofid/ofy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong YP, Park SJ, Kim ES, Bang KM, Kim MN, Kim SH, Lee SO, Choi SH, Jeong JY, Woo JH, Kim YS. 2015. Prevalence of blaZ gene types and the cefazolin inoculum effect among methicillin-susceptible Staphylococcus aureus blood isolates and their association with multilocus sequence types and clinical outcome. Eur J Clin Microbiol Infect Dis 34:349–355. doi: 10.1007/s10096-014-2241-5. [DOI] [PubMed] [Google Scholar]
- 11.Livorsi DJ, Crispell E, Satola SW, Burd EM, Jerris R, Wang YF, Farley MM. 2012. Prevalence of blaZ gene types and the inoculum effect with cefazolin among bloodstream isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 56:4474–4477. doi: 10.1128/AAC.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richmond MH. 1965. Wild-type variants of exopenicillinase from Staphylococcus aureus. Biochem J 94:584–593. doi: 10.1042/bj0940584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zygmunt DJ, Stratton CW, Kernodle DS. 1992. Characterization of four beta-lactamases produced by Staphylococcus aureus. Antimicrob Agents Chemother 36:440–445. doi: 10.1128/aac.36.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voladri RK, Kernodle DS. 1998. Characterization of a chromosomal gene encoding type B beta-lactamase in phage group II isolates of Staphylococcus aureus. Antimicrob Agents Chemother 42:3163–3168. doi: 10.1128/AAC.42.12.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn EL, Pohlod D, Madhavan T, Burch K, Fisher E, Cox F. 1973. Clinical experiences with cefazolin and other cephalosporins in bacterial endocarditis. J Infect Dis 128:S386–S391. doi: 10.1093/infdis/128.supplement_2.s386. [DOI] [PubMed] [Google Scholar]
- 16.Chapman SW, Steigbigel RT. 1983. Staphylococcal beta-lactamase and efficacy of beta-lactam antibiotics: in vitro and in vivo evaluation. J Infect Dis 147:1078–1089. doi: 10.1093/infdis/147.6.1078. [DOI] [PubMed] [Google Scholar]
- 17.Singh KV, Tran TT, Nannini EC, Tam VH, Arias CA, Murray BE. 2017. Efficacy of ceftaroline against methicillin-susceptible Staphylococcus aureus exhibiting the cefazolin high-inoculum effect in a rat model of endocarditis. Antimicrob Agents Chemother 61:e00324-17. doi: 10.1128/AAC.00324-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nannini EC, Stryjewski ME, Singh KV, Rude TH, Corey GR, Fowler VG Jr, Murray BE. 2010. Determination of an inoculum effect with various cephalosporins among clinical isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 54:2206–2208. doi: 10.1128/AAC.01325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kok EY, Vallejo JG, Sommer LM, Rosas L, Kaplan SL, Hulten KG, McNeil JC. 2018. Association of vancomycin mic and molecular characteristics with clinical outcomes in methicillin-susceptible Staphylococcus aureus acute hematogenous osteoarticular infections in children. Antimicrob Agents Chemother 62:e00084-18. doi: 10.1128/AAC.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold SR, Elias D, Buckingham SC, Thomas ED, Novais E, Arkader A, Howard C. 2006. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop 26:703–708. doi: 10.1097/01.bpo.0000242431.91489.b4. [DOI] [PubMed] [Google Scholar]
- 21.Hulten KG, Mason EO, Lamberth LB, Forbes AR, Revell PA, Kaplan SL. 2018. Analysis of invasive community-acquired methicillin-susceptible Staphylococcus aureus infections during a period of declining CA-MRSA infections at a large children's hospital. Pediatr Infect Dis J 37:235–241. doi: 10.1097/INF.0000000000001753. [DOI] [PubMed] [Google Scholar]
- 22.Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. 2016. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics 137:e20153099. doi: 10.1542/peds.2015-3099. [DOI] [PubMed] [Google Scholar]
- 23.Nannini EC, Stryjewski ME, Singh KV, Bourgogne A, Rude TH, Corey GR, Fowler VG Jr, Murray BE. 2009. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob Agents Chemother 53:3437–3441. doi: 10.1128/AAC.00317-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeil JC, Vallejo JG, Kok EY, Sommer LM, Hulten KG, Kaplan SL. 2019. Clinical and microbiologic variables predictive of orthopedic complications following S. aureus acute hematogenous osteoarticular infections in children. Clin Infect Dis 69:1955–1961. doi: 10.1093/cid/ciz109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood JB, Thomsen IP, Creech CB. 2016. Best practices for treatment of invasive methicillin-susceptible Staphylococcus aureus infections: the case for oxacillin. J Pediatric Infect Dis Soc 5:480–482. doi: 10.1093/jpids/piw052. [DOI] [PubMed] [Google Scholar]
- 26.Nannini EC, Singh KV, Arias CA, Murray BE. 2013. In vivo effects of cefazolin, daptomycin, and nafcillin in experimental endocarditis with a methicillin-susceptible Staphylococcus aureus strain showing an inoculum effect against cefazolin. Antimicrob Agents Chemother 57:4276–4281. doi: 10.1128/AAC.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song K-H, Jung S-I, Lee S, Park S, Kiem SM, Lee SH, Kwak YG, Kim YK, Jang H-C, Kim Y-S, Kim H-I, Kim CJ, Park K-H, Kim NJ, Oh M-D, Kim HB, Korea Infectious Diseases Study Group. 2017. Characteristics of cefazolin inoculum effect-positive methicillin-susceptible Staphylococcus aureus infection in a multicentre bacteraemia cohort. Eur J Clin Microbiol Infect Dis 36:285–294. doi: 10.1007/s10096-016-2799-1. [DOI] [PubMed] [Google Scholar]
- 28.CLSI. 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. CLSI M100-S22. CLSI, Wayne, PA. [Google Scholar]
- 29.Wang SK, Gilchrist A, Loukitcheva A, Plotkin BJ, Sigar IM, Gross AE, O'Donnell JN, Pettit N, Buros A, O'Driscoll T, Rhodes NJ, Bethel C, Segreti J, Charnot-Katsikas A, Singh K, Scheetz MH. 2018. Prevalence of a cefazolin inoculum effect associated with blaZ gene types among methicillin-susceptible Staphylococcus aureus Isolates from four major medical centers in Chicago. Antimicrob Agents Chemother 62:e00382-18. doi: 10.1128/AAC.00382-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wi YM, Park YK, Moon C, Ryu SY, Lee H, Ki HK, Cheong HS, Son JS, Lee JS, Kwon KT, Kim JM, Ha YE, Kang CI, Ko KS, Chung DR, Peck KR, Song JH. 2015. The cefazolin inoculum effect in methicillin-susceptible Staphylococcus aureus blood isolates: their association with dysfunctional accessory gene regulator (agr). Diagn Microbiol Infect Dis 83:286–291. doi: 10.1016/j.diagmicrobio.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Song KH, Jung SI, Park WB, Lee SH, Kim YS, Kwak YG, Kim YK, Kiem SM, Kim HI, Kim ES, Park KH, Kim NJ, Jang HC, Kim HB, Korea Infectious Diseases Study Group. 2018. Comparative outcomes of cefazolin versus nafcillin for methicillin-susceptible Staphylococcus aureus bacteraemia: a prospective multicentre cohort study in Korea. Clin Microbiol Infect 24:152–158. doi: 10.1016/j.cmi.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Miller WR, Singh KV, Arias CA, Murray BE. 2018. Adjunctive clavulanic acid abolishes the cefazolin inoculum effect in an experimental rat model of methicillin-sensitive Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 62:e01158-18. doi: 10.1128/AAC.01158-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tetzlaff TR, McCracken GH Jr, Nelson JD. 1978. Oral antibiotic therapy for skeletal infections of children. II. Therapy of osteomyelitis and suppurative arthritis. J Pediatr 92:485–490. doi: 10.1016/S0022-3476(78)80455-7. [DOI] [PubMed] [Google Scholar]
- 34.Ramchandar N, Arnold J, Cannavino C, Bradley JS. 2020. Frequency of dosing of cephalexin for oral step-down therapy of pediatric osteoarticular infections caused by methicillin-sensitive Staphylococcus aureus. Pediatr Infect Dis J 39:523–525. doi: 10.1097/INF.0000000000002661. [DOI] [PubMed] [Google Scholar]
- 35.Que Y-A, Moreillon P. 2010. Staphylococcus aureus (including staphylococcal toxic shock), p 2543–2578. In Mendell G, Bennett J, Dolin R (ed), Principles and practice of infectious diseases, 7 ed, vol 2 Churchill Livingstone Elsevier, Philadelphia, PA. [Google Scholar]
- 36.Suligoy CM, Lattar SM, Noto Llana M, Gonzalez CD, Alvarez LP, Robinson DA, Gomez MI, Buzzola FR, Sordelli DO. 2018. Mutation of Agr is associated with the adaptation of Staphylococcus aureus to the host during chronic osteomyelitis. Front Cell Infect Microbiol 8:18. doi: 10.3389/fcimb.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dich VQ, Nelson JD, Haltalin KC. 1975. Osteomyelitis in infants and children. A review of 163 cases. Am J Dis Child 129:1273–1278. doi: 10.1001/archpedi.1975.02120480007004. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan SL, Hulten KG, Gonzalez BE, Hammerman WA, Lamberth L, Versalovic J, Mason EO Jr. 2005. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis 40:1785–1791. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 39.Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Bubeck Wardenburg J, Hunstad DA. 2013. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 56:1554–1561. doi: 10.1093/cid/cit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNeil JC, Forbes AR, Vallejo JG, Flores AR, Hulten KG, Mason EO, Kaplan SL. 2016. Role of operative or interventional radiology-guided cultures for osteomyelitis. Pediatrics 137:e20154616. doi: 10.1542/peds.2015-4616. [DOI] [PubMed] [Google Scholar]
- 41.Williams DJ, Deis JN, Tardy J, Creech CB. 2011. Culture-negative osteoarticular infections in the era of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 30:523–525. doi: 10.1097/INF.0b013e318207a7a5. [DOI] [PubMed] [Google Scholar]
- 42.McNeil JC, Kaplan SL, Vallejo JG. 2017. The influence of the route of antibiotic administration, methicillin-susceptibility, vancomycin duration and serum trough concentration on outcomes of pediatric Staphylococcus aureus bacteremic osteoarticular infection. Pediatr Infect Dis J 36:572–577. doi: 10.1097/INF.0000000000001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CLSI. 2019. Performance standards for antimicrobial susceptibility testing, 29th ed CLSI, Wayne PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.