The clinical situation for patients receiving extracorporeal membrane oxygenation (ECMO) is complex, and drug dosing is complicated by significant pharmacokinetic alterations. We sought to describe the frequency of achievement of therapeutic vancomycin concentrations in critically ill patients receiving ECMO with therapeutic drug monitoring (TDM). In this retrospective observational study, we included all critically ill patients receiving TDM for vancomycin while on ECMO. The primary outcome was the proportion of plasma vancomycin concentrations in the therapeutic range (15 to 20 mg/liter).
KEYWORDS: ECMO, TDM, therapeutic failure, toxicity, vancomycin
ABSTRACT
The clinical situation for patients receiving extracorporeal membrane oxygenation (ECMO) is complex, and drug dosing is complicated by significant pharmacokinetic alterations. We sought to describe the frequency of achievement of therapeutic vancomycin concentrations in critically ill patients receiving ECMO with therapeutic drug monitoring (TDM). In this retrospective observational study, we included all critically ill patients receiving TDM for vancomycin while on ECMO. The primary outcome was the proportion of plasma vancomycin concentrations in the therapeutic range (15 to 20 mg/liter). Factors associated with not achieving therapeutic concentrations were investigated, including ECMO duration and use of renal replacement therapies. Vancomycin TDM was performed for 77 of 116 (66%) patients on ECMO. Median (interquartile range) duration of ECMO support was 7 days (4 to 16 days). The proportion of measurements in the therapeutic range (15 to 20 mg/liter) was 24%, while 46% were subtherapeutic (<15 mg/liter) and 30% were supratherapeutic (>20 mg/liter). The proportion of measures in the therapeutic range was significantly higher on ECMO days for 6 to 13 (incidence rate ratio [IRR], 2.4; 95% confidence interval [CI], 1.2 to 4.6; P = 0.01). Supratherapeutic concentrations were more frequently observed in patients on renal replacement therapy (RRT) (IRR, 2.0; 95% CI, 1.3 to 3.1; P = 0.002). The vancomycin concentrations in patients did not vary with age, gender, or type of ECMO support. Patients receiving vancomycin had suboptimal concentrations early in the course of ECMO. Patients not receiving RRT and those with mild to moderate acute kidney injury (AKI) were at a risk of underdosing, while those with established AKI on RRT were likelier to experience supratherapeutic concentrations.
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is a life support system used in the treatment of patients with severe respiratory and/or cardiorespiratory failure (1, 2). ECMO is a supportive therapy, and its success depends on reversing the underlying disease process with disease-modifying therapies or bridging the patients to long-term mechanical support or transplant. It is an invasive intervention, with bleeding, clotting, and infection being a few of the most serious complications. Hence, optimal pharmacological management is critical to ensure that the underlying disease is reversed and complications minimized (3, 4). Patients requiring ECMO are critically ill, with various diagnoses, comorbidities, degrees of immunosuppression, ages, body sizes, and degrees of end organ dysfunction (5, 6) that predispose them to a heightened risk of infection (7). Optimal dosing is important because infections are common in patients on ECMO and result in substantial morbidity and mortality (8). According to the Extracorporeal Life Support Organization (ELSO), infection rates among all age groups are as high as 15 per 1,000 ECMO days. The mortality of patients on ECMO with reported infections ranges from 56% to 68% (9).
Vancomycin is a glycopeptide antibiotic used in patients receiving ECMO for treatment of infections caused by Gram-positive organisms, particularly methicillin-resistant Staphylococcus aureus (MRSA). The effectiveness of vancomycin is dependent on achieving and maintaining optimal plasma concentrations. In the critically ill, a trough concentration between 15 and 20 mg/liter is recommended by the Infectious Diseases Society of America (IDSA) guidelines for the treatment of MRSA infections (10). Some studies have proposed that ECMO is associated with altered pharmacokinetics (PK) of vancomycin, potentially caused by reduced drug elimination, increased volume of distribution, and sequestration within the ECMO circuit (11). Targeting troughs between 15 and 20 mg/liter increases the probability of achieving an AUC (area under the curve from 0 to 24 h)/MIC ratio target of ≥400, which has been advocated for clinical effectiveness with vancomycin (12).
Most available data suggest that the direct impact of ECMO on vancomycin PK is minimal and that dose recommendations for critically ill patients can also be applied to patients on ECMO to increase the likelihood of achieving therapeutic concentrations (13). In addition, therapeutic drug monitoring (TDM) is recommended to ensure optimal plasma drug concentrations are being achieved. TDM refers to the individualization of dosage by maintaining plasma or blood drug concentrations within a target range. Although TDM is a validated tool, it is not clear if doses can be predicted accurately throughout the course of ECMO. We hypothesized that, as critical illness resolves, there may be significant variability in predicted versus observed plasma vancomycin concentrations. Hence, we conducted this study of our adult patients on ECMO who received TDM-based vancomycin dosing. The objectives of this study were to describe the distribution of vancomycin concentrations based on TDM and investigate associations between patient factors, type and duration of ECMO, presence or absence of renal replacement therapy (RRT), and plasma vancomycin concentrations.
RESULTS
Of 116 patients who received vancomycin while on ECMO treatment during the study period, 77 (66%) had concentrations measured. Overall, 72 patients (62%) were male, the majority (57%) had cardiogenic shock, and a venoarterial (VA) system was used in 61%. Of the 115 patients with data, 41 (36%) died. Patients receiving renal replacement therapy (RRT) were more likely to have vancomycin concentrations monitored, but other variables did not differ significantly by whether any vancomycin concentration measures were recorded. Distribution of variables and vancomycin TDM measurements are summarized in Table 1.
TABLE 1.
Distribution of variables and vancomycin TDM measurementsa
| Variable | Category or measure | Value for vancomycin TDM status |
Total | P value | |
|---|---|---|---|---|---|
| Unmeasured | Measured | ||||
| All patients | No. | 39 | 77 | 116 | |
| Gender [no. (%)] | Female | 15 (38.5) | 29 (37.7) | 44 (37.9) | 0.93 |
| Male | 24 (61.5) | 48 (62.3) | 72 (62.1) | ||
| System [no. (%)] | VA | 25 (65.8) | 45 (58.4) | 70 (60.9) | 0.74 |
| VV | 12 (31.6) | 29 (37.7) | 41 (35.7) | ||
| Other | 1 (2.6) | 3 (3.9) | 4 (3.5) | ||
| Missing | 1 | ||||
| Diagnostic group [no. (%)] | Cardiac | 24 (63.2) | 42 (54.5) | 66 (57.4) | 0.48 |
| Respiratory | 12 (31.6) | 26 (33.8) | 38 (33) | ||
| Other/sepsis | 2 (5.3) | 9 (11.7) | 11 (9.6) | ||
| Missing | 1 | ||||
| Mortality (%) | No | 26 (68.4) | 48 (62.3) | 74 (64.3) | 0.52 |
| Yes | 12 (31.6) | 29 (37.7) | 41 (35.7) | ||
| Missing | 1 | 1 | |||
| Renal replacement therapy [no. (%)] | No | 24 (66.7) | 33 (43.4) | 57 (50.9) | 0.02 |
| Yes | 12 (33.3) | 43 (56.6) | 55 (49.1) | ||
| Missing | 4 | ||||
| Age (years) | Mean (SD) | 46.6 (15.0) | 43.4 (16.6) | 44.4 (16.1) | 0.31 |
| Wt (kg) | Mean (SD) | 82.3 (21.2) | 78.7(18.1) | 79.9 (19.2) | 0.35 |
| APACHE III score | Mean (SD) | 81.5 (34.8) | 86.5 (34.6) | 84.9 (34.6) | 0.47 |
| Total ECMO duration (days) | Median (IQR) | 6 (4–11) | 8 (5–16) | 7 (4–16) | 0.14 |
| ICU length of stay (days) | Median (IQR) | 17 (11–27) | 25 (11–39) | 21 (11–37) | 0.09 |
| Hospital length of stay (days) | Median (IQR) | 27 (17–43) | 35 (14–59) | 34 (15–51) | 0.21 |
SD, standard deviation; APACHE III, acute physiology and chronic evaluation III; IQR, interquartile range.
The average percentage of measurements in the therapeutic range was 24%, while 46% were low and 30% were high. Only 14 (18%) patients had ≥50% measures in the therapeutic range. The summary measures by percentage of vancomycin measures in the therapeutic range (<50% versus ≥50%) are shown in Table 2. Of patients with any vancomycin measures, 38% died; 43% of those died with <50% of vancomycin measures in the therapeutic range compared to 14% of those who had ≥50% of vancomycin measures in the therapeutic range (P = 0.05). The median percentage of measures in the therapeutic range was 26 (interquartile range [IQR], 3 to 47) in patients who survived compared to 9 (IQR, 0 to 29) in those who died.
TABLE 2.
Cross-tabulation of variables of interest by percentage of measures in the therapeutic rangea
| Variable | Category or measure | Value corresponding to proportion of vancomycin measures in therapeutic range |
P value | ||
|---|---|---|---|---|---|
| <50% (n = 63) | ≥50% (n = 14) | Total (n = 77) | |||
| Gender [no. (%)] | Female | 21 (33) | 8 (57) | 29 (38) | 0.10 |
| Male | 42 (67) | 6 (43) | 48 (62) | ||
| ECMO system [no. (%)] | VA | 39 (62) | 6 (43) | 45 (58) | 0.21 |
| VV | 21 (33) | 8 (57) | 29 (38) | ||
| Other | 3 (5) | 0 (0) | 3 (4) | ||
| Diagnosis [no. (%)] | Cardiac | 36 (57) | 6 (43) | 42 (55) | 0.62 |
| Respiratory | 20 (32) | 6 (43) | 26 (34) | ||
| Other/sepsis | 7 (11) | 2 (14) | 9 (12) | ||
| Mortality [no. (%)] | No | 36 (57) | 12 (86) | 48 (62) | 0.05 |
| Yes | 27 (43) | 2 (14) | 29 (38) | ||
| Renal replacement therapy [no. (%)] | No | 24 (39) | 9 (64) | 33 (43) | 0.08 |
| Yes | 38 (61) | 5 (36) | 43 (57) | ||
| Age (years) | Mean (SD) | 44 (16) | 39 (18) | 43 (17) | 0.33 |
| Weight (kg) | Mean (SD) | 80 (70–90) | 79 (69–80) | 80 (70–88) | 0.58 |
| APACHE III score | Mean (SD) | 88 (36) | 81 (26) | 87 (35) | 0.50 |
| Total ECMO duration (days) | Median (IQR) | 7 (4–17) | 9 (6–16) | 8 (5–16) | 0.34 |
| ICU length of stay (days) | Median (IQR) | 23 (9–39) | 32 (20–42) | 25 (11–39) | 0.30 |
| Hospital length of stay (days) | Median (IQR) | 34 (13–58) | 41 (20–63) | 35 (14–59) | 0.30 |
| No. of vancomycin doses | Median (IQR) | 7 (2–12) | 9 (4–11) | 7 (2–12) | 0.57 |
| No. of vancomycin measurements | Median (IQR) | 6 (2–9) | 4 (2–7) | 5 (2–9) | 0.32 |
| No. of measurements in therapeutic range | Median (IQR) | 1 (0–2) | 2 (1–5) | 1 (0–3) | |
VA, venoarterial; VV, venovenous; SD, standard deviation; APACHE III, acute physiology and chronic evaluation III; IQR, interquartile range.
The distribution of patient-level vancomycin concentrations by RRT is presented in Fig. 1. There was no evidence that the proportions therapeutic by week within patient differed by week on treatment. Patients on RRT had greater percentage of measurements in the supratherapeutic range.
FIG 1.
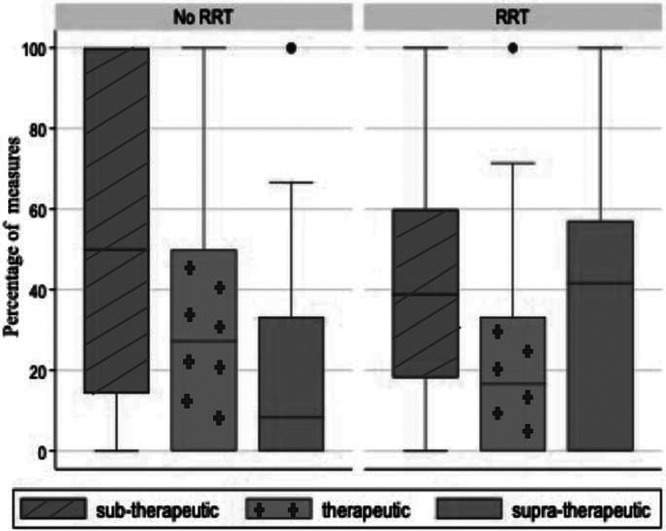
Box plots showing distribution of percentage of patient-level vancomycin concentrations by classification and RRT.
Results of Poisson regression modeling are shown in Table 3. Compared to patients not on RRT, the proportion of measurements in the therapeutic range was significantly lower in patients receiving RRT (incidence rate ratio [IRR], 0.6; 95% confidence interval [CI], 0.4 to 0.9; P = 0.01). Compared to patients on ECMO for <6 days, the proportion in the therapeutic range was significantly higher in those on ECMO for 6 to 13 days (IRR: 2.2; 95% CI: 1.2 to 4.0; P = 0.01). The proportion of measurements in the supratherapeutic range was significantly higher in patients on RRT and in patients with higher acute physiology and chronic health evaluation III (APACHE III) scores, although the latter did not remain significant in the adjusted model.
TABLE 3.
Results of Poisson regression testing for differences by weeka
| Wk | No. of patients with TDM | Median (IQR) vancomycin concn | IRR | 95% CI | P value |
|---|---|---|---|---|---|
| 1 | 70 | 15 (0–40) | Ref | 0.41 | |
| 2 | 31 | 0 (0–40) | 0.9 | 0.6–1.5 | 0.77 |
| 3 | 15 | 13 (0–60) | 0.9 | 0.5–1.8 | 0.83 |
| 4 | 8 | 50 (29–67) | 1.4 | 0.9–2.2 | 0.16 |
| Total | 77 | 20 (0–33) |
IQR, interquartile range; IRR, incidence rate ratio; 95% CI, 95% confidence interval; Ref, reference category.
DISCUSSION
To the best of our knowledge, this is the first study that evaluated the utility of TDM for vancomycin dosing in adult patients on ECMO. This study demonstrated that such patients have suboptimal concentrations, particularly early during ECMO treatment. This is concerning, because most antimicrobials that are used currently in ECMO patients do not have real-time dosing guidance.
The proportion of measurements in the therapeutic range was significantly higher in those on ECMO for 6 to 13 days than in those on ECMO for <6 days. The reasons for this could be multiple. The primary disease could be in the resolution phase, and return of capillary integrity and recovery of renal function over a period of time on ECMO may contribute to a requirement for more standard doses. Shekar et al. (14) demonstrated that there was no significant adsorption of vancomycin to ECMO circuits, and so this is unlikely to cause suboptimal drug exposures. In our study, the proportion of measures in the therapeutic range did not differ by type of ECMO. The proportion of measurements in the therapeutic range was significantly lower in patients who died than in those who survived. There was a trend toward higher mortality with subtherapeutic concentrations; however, this finding requires confirmation in prospective studies.
Adult pharmacokinetic data have largely shown that ECMO does not significantly affect vancomycin volume of distribution and clearance (13, 15, 16). We also found that the proportion of supratherapeutic concentrations was higher in those patients receiving concomitant RRT and in patients with higher APACHE III scores. This reflects not only the severity of renal dysfunction but also the severity of primary disease and hence a potential for drug toxicity. A population pharmacokinetic study also showed that the total clearance of vancomycin during continuous renal replacement therapy (CRRT) was lower than in patients without CRRT (17). Previous studies reported that antibiotic dosing schedules were frequently insufficient due to hemodilution therapies such as CRRT, especially for hydrophilic antibiotics like vancomycin or meropenem (17, 18).
Hence, regular TDM could identify the occurrence of altered pharmacokinetics that manifest as subtherapeutic concentrations during the initial period on ECMO and minimize the risks of treatment failure. Equally, supratherapeutic concentrations are common in patients with acute kidney injury (AKI), including those on RRT, for whom dose adjustments should be considered.
The reasons for altered vancomycin pharmacokinetics during ECMO include the use of ECMO priming fluids, transfusion and hemodilution, concomitant administration of nephrotoxic drugs, and decreasing renal function (11). Hence, we emphasize the need for regular TDM-guided vancomycin dosing for patients on ECMO to prevent treatment failure.
In our study, the average percentage of measurements in the therapeutic range was 24%, while 46% were subtherapeutic and 30% were supratherapeutic. Park et al. (15) evaluated the appropriateness of the dosing strategy for vancomycin based on total body weight and creatinine clearance in adult patients on ECMO. Those authors observed that 95% of patients had a subtherapeutic concentration of vancomycin in the initial phase (mean initial trough concentration was <10 mg/liter), which was in keeping with our study.
Most previous studies used total body weight and renal functions for dosing vancomycin in critically ill patients receiving ECMO (15, 17). The current revised IDSA guidelines refer particularly to the general population and state that the preferred approach to monitor AUC involves the use of Bayesian software programs, embedded with a PK model based on richly sampled vancomycin data as the Bayesian prior, to optimize the delivery of vancomycin based on the collection of 1 or 2 vancomycin concentrations, with at least 1 trough (19). Despite extensive reporting, an optimal TDM approach for vancomycin has not been established for special populations, such as patients treated with ECMO (20, 21).
In this study, we explored the utility of TDM-guided vancomycin dosing in adult patients on ECMO. The strengths of this study include the comprehensive data collection utilizing more than one resource over an extended time frame, thus enhancing the reliability of the study. However, we used a retrospective design which is insufficiently powered to analyze any clinical outcomes. Vancomycin doses and timing of TDM were decided by the treating clinicians, and available measurements were assumed to be true trough concentrations for the purpose of analysis. Population PK studies are indicated to develop robust dosing guidelines.
In conclusion, we observed that patients on ECMO are more likely to experience subtherapeutic vancomycin concentrations in the initial phase, and caution needs to be exercised in treating those receiving concomitant RRT. We advocate TDM-based vancomycin dosing to monitor therapeutic efficacy in critically ill patients on ECMO.
MATERIALS AND METHODS
Study population.
This retrospective single-center study was approved by the Institutional Review Board of The Prince Charles Hospital and Human Research and Ethics Committee (HREC/17/QPCH/176). A consent waiver was granted owing to the retrospective nature of the study. The study included all the patients 18 years or older who received ECMO during the period of January 2012 to June 2017.
ECMO apparatus.
The ECMO system comprised a Rotaflow centrifugal pump and Cardiohelp system (Maquet, Germany). For the venovenous (VV) configuration, a 21-25 French (F) multistage access cannula and a 19-25 F single-stage return cannula were used. For peripheral VA configuration, a 21-25 F multistage access cannula and a 17-21 F return cannula were used. Additionally, an anterograde single-lumen 9 F catheter (Arrow Inc., PA, USA) was placed to prevent limb ischemia.
Data collection.
All the patients who were treated with vancomycin while receiving ECMO were identified from the department database registry, and an extensive chart review was performed using the clinical information system (CIS). Based on the institutional guideline, antibiotics were prescribed only when an infection was suspected, guided by microbiological sensitivities and antibiogram for the unit. The data extracted included age, gender, admission diagnosis, APACHE III score, type of ECMO, duration of ECMO, requirement for renal replacement therapy (RRT), length of stay, and mortality. The most common indication for VV ECMO was refractory hypoxemia secondary to either infective or noninfective etiology. VA ECMO was instituted for refractory cardiogenic shock following myocardial infarction, myocarditis, heart transplant, and cardiac surgery.
Vancomycin dosing and TDM.
Vancomycin, where indicated, was administered in a dose of 25 mg/kg (of actual body weight), as an intermittent infusion (infusion rate, typically 1,000 mg per 60 min). Subsequent maintenance doses of 15 mg/kg were administered every 12 h. The first trough concentration was measured prior to the fourth dose if renal functions were stable; in cases of unstable renal functions, earlier or more frequent TDM was performed. In patients with therapeutic concentrations, TDM was performed every 48 h, if vancomycin was continued. The trough vancomycin concentrations were collected by reviewing the laboratory reporting system for every patient. Therapeutic concentrations were defined as 15 to 20 mg/liter, subtherapeutic concentrations were below 15 mg/liter, and supratherapeutic concentrations were above 20 mg/liter.
Statistical analysis.
The percentages of subtherapeutic, supratherapeutic, and therapeutic concentrations by patient and by week on treatment within patients were determined. The main outcome of interest was the patient-level percentage of vancomycin concentrations in the therapeutic range. For descriptive purposes, patients were classified into two groups based on the percentage of vancomycin measurements in the therapeutic range (<50% or ≥50% of measures). Continuous variables were summarized using means (standard deviations) and tested between groups using Student's t test if normally distributed or summarized using medians (interquartile range) and tested using Wilcoxon’s rank-sum test if otherwise. Categorical variables were summarized using frequencies and percentages and tested between groups using Pearson’s chi-square test.
Associations between variables of interest (age, gender, ECMO system, APACHE III score, RRT, and duration of ECMO) and proportion of vancomycin measurements in the therapeutic range were explored using Poisson regression analyses. Analyses were performed using the Stata statistical software package (version 15), and a P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
Kiran Shekar acknowledges research fellowship support from Metro North Hospital and Health Service. Kiran Shekar has received grant funding from National Health and Medical Research Council, The Prince Charles Hospital Foundation, Intensive Care Foundation, Australia and New Zealand College of Anesthetists, Queensland Emergency Medicine Research Foundation and Defense Health Foundation, and the Extracorporeal Life Support Organization. Kiran Shekar’s institution received educational support from Abiomed. Jason Roberts acknowledges funding from the Australian National Health and Medical Research Council for a Centre of Research Excellence (APP1099452) and a Practitioner Fellowship (APP1117065).
REFERENCES
- 1.Bartlett R, Roloff D, Custer J, Younger J, Hirschl R. 2000. Extracorporeal life support. The University of Michigan experience. JAMA 283:904–908. doi: 10.1001/jama.283.7.904. [DOI] [PubMed] [Google Scholar]
- 2.Peek G, Killer H, Sosnowski A, Firmin R. 1998. Extracorporeal membrane oxygenation: potential for adults and children? Hosp Med (Lond) 59:304–308. [PubMed] [Google Scholar]
- 3.Shekar K, Mullany DV, Thomson B, Ziegenfuss M, Platts DG, Fraser JF. 2014. Extracorporeal life support devices and strategies for management of acute cardiorespiratory failure in adult patients: a comprehensive review. Crit Care 18:219. doi: 10.1186/cc13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser JF, Shekar K, Diab S, Dunster K, Foley SR, McDonald CI, Passmore M, Simonova G, Roberts JA, Platts DG, Mullany DV, Fung YL. 2012. ECMO–the clinician’s view. ISBT Sci Ser 7:82–88. doi: 10.1111/j.1751-2824.2012.01560.x. [DOI] [Google Scholar]
- 5.Allen S, Holena D, McCunn M, Kohl B, Sarani B. 2011. A review of the fundamental principles and evidence base in the use of extracorporeal membrane oxygenation (ECMO) in critically ill adult patients. J Intensive Care Med 26:13–26. doi: 10.1177/0885066610384061. [DOI] [PubMed] [Google Scholar]
- 6.Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. 2010. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2. Technical considerations. J Cardiothorac Vasc Anesth 24:164–172. doi: 10.1053/j.jvca.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Aubron C, Cheng AC, Pilcher D, Leong T, Magrin G, Cooper DJ, Scheinkestel C, Pellegrino V. 2013. Infections acquired by adults who receive extracorporeal membrane oxygenation: risk factors and outcome. Infect Control Hosp Epidemiol 34:24–30. doi: 10.1086/668439. [DOI] [PubMed] [Google Scholar]
- 8.Bizzaro MJ, Conrad SA, Kaufman DA, Rycus P. 2011. Extracorporeal Life Support Organization Task Force on Infections ECMO. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med 12:277–281. doi: 10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 9.Extracorporeal Life Support Organization Registry. 2016. Extracorporeal Life Support Organization Registry report: international summary. https://www.elso.org/Portals/0/Files/Infection-Control-and-Extracorporeal-Life-Support.pdf. Accessed 29 February 2016.
- 10.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 11.Shekar K, Fraser JF, Smith MT, Roberts JA. 2012. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care 27:741.e9–741.e18. doi: 10.1016/j.jcrc.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Mohr JF, Murray BE. 2007. Point: vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 44:1536–1542. doi: 10.1086/518451. [DOI] [PubMed] [Google Scholar]
- 13.Donadello K, Roberts JA, Cristallini S, Beumier M, Shekar K, Jacobs F, Belhaj A, Vincent J-L, de Backer D, Taccone FS. 2014. Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: a matched cohort study. Crit Care 18:632. doi: 10.1186/s13054-014-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shekar K, Roberts JA, Mcdonald CI, Fisquet S, Barnett AG, Mullany DV, Ghassabian S, Wallis SC, Fung YL, Smith MT, Fraser JF. 2012. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care 16:R194. doi: 10.1186/cc11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SJ, Yang JH, Park HJ, In YW, Lee YM, Cho YH, Chung CR, Park CM, Jeon K, Suh GY. 2015. Trough concentrations of vancomycin in patients undergoing extracorporeal membrane oxygenation. PLoS One 10:e0141016. doi: 10.1371/journal.pone.0141016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu CC, Shen LJ, Hsu LF, Ko WJ, Wu FL. 2016. Pharmacokinetics of vancomycin in adults receiving extracorporeal membrane oxygenation. J Formos Med Assoc 115:560–570. doi: 10.1016/j.jfma.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Covajes C, Scolletta S, Penaccini L, Ocampos-Martinez E, Abdelhadii A, Beumier M, Jacobs F, de Backer D, Vincent JL, Taccone FS. 2013. Continuous infusion of vancomycin in septic patients receiving continuous renal replacement therapy. Int J Antimicrob Agents 41:261–266. doi: 10.1016/j.ijantimicag.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Ocampos-Martinez E, Penaccini L, Scolletta S, Abdelhadii A, Devigili A, Cianferoni S, de Backer D, Jacobs F, Cotton F, Vincent JL, Taccone FS. 2012. Determinants of early inadequate vancomycin concentrations during continuous infusion in septic patients. Int J Antimicrob Agents 39:332–337. doi: 10.1016/j.ijantimicag.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro BM. 2020. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health-System Pharm 77:835–864. doi: 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 20.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 21.Ye ZK, Li C, Zhai SD. 2014. Guidelines for therapeutic drug monitoring of vancomycin: a systematic review. PLoS One 9:e99044. doi: 10.1371/journal.pone.0099044. [DOI] [PMC free article] [PubMed] [Google Scholar]


