The gentamicin drug product is a complex mixture of numerous components, many of which have not individually undergone safety and efficacy assessments. This is in contrast to the majority of medicines that require rigorous characterizations of trace impurities and are dosed as single components. In gentamicin, four components, known as gentamicin congeners C1, C1a, C2, and C2a, comprise the majority of the mixture. A liquid chromatography-mass spectroscopy analysis revealed that the relative abundances of each gentamicin congener in commercial formulations can vary up to 1.
KEYWORDS: aminoglycoside-modifying enzymes, aminoglycosides, gentamicin, nephrotoxicity, susceptibility testing
ABSTRACT
The gentamicin drug product is a complex mixture of numerous components, many of which have not individually undergone safety and efficacy assessments. This is in contrast to the majority of medicines that require rigorous characterizations of trace impurities and are dosed as single components. In gentamicin, four components, known as gentamicin congeners C1, C1a, C2, and C2a, comprise the majority of the mixture. A liquid chromatography-mass spectroscopy analysis revealed that the relative abundances of each gentamicin congener in commercial formulations can vary up to 1.9-fold depending on the commercial source of the gentamicin. To determine if the gentamicin used for antibiotic susceptibility testing (AST) would be predictive of the microbiological activity of the product used to dose patients, the relative abundances of the four congeners contained on commercial AST disks were measured. It was found that the congener abundances on the commercial AST disks varied up to 4.1-fold. After purification of the four gentamicin congeners, similar potencies against bacterial strains lacking aminoglycoside-modifying enzymes (AMEs) were observed. However, the potency of the congeners against strains harboring a common AME differed up to 128-fold. Nephrotoxicity of the individual gentamicin congeners also differed significantly in cell-based and repeat-dose rat nephrotoxicity studies. Variations in the composition of commercial gentamicin products combined with toxicity differences between gentamicin congeners suggest that some gentamicin formulations may be more nephrotoxic. Our results also raise the concern that gentamicin susceptibility test results may not be predictive of patient outcomes and could lead to unexpected clinical treatment failures.
TEXT
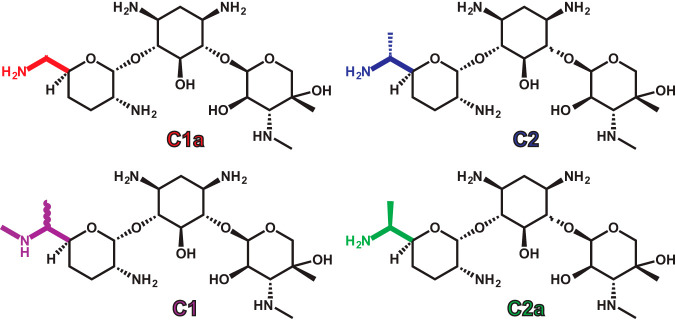
The aminoglycosides are a class of rapidly bactericidal antibiotics that are commonly used in the treatment of serious infections caused by Gram-negative bacteria. Aminoglycosides are also used in combination with other classes of antibiotics for certain infections caused by Gram-positive bacteria, such as infective endocarditis, and are an important option for treatment of multidrug-resistant tuberculosis. Since they are one of a few antimicrobial classes that have retained activity against multidrug-resistant bacteria, aminoglycosides are considered among the critically important antimicrobials by the World Health Organization (1). The aminoglycosides available for parenteral administration in the United States are amikacin, gentamicin, plazomicin, streptomycin, and tobramycin. While amikacin and plazomicin are synthetically derived, streptomycin, gentamicin, and tobramycin are products of fermentation and not pure compounds. Since streptomycin is produced by fermentation, formulations contain up to 16% impurities, including the less active streptomycin B congener (2). Tobramycin is also synthesized through fermentation, though as a finished product it contains <1% impurities (3). Gentamicin formulations are derived from Micromonospora purpurea and consist largely of 4 major gentamicin congeners, called C1, C1a, C2, and C2a (Fig. 1), and one minor congener, C2b (4). Within the gentamicin formulations there are also related aminoglycosides, such as sisomicin and garamine, which exist as minor components. The major gentamicin congeners differ only by methyl groups or hydrogen atoms at the 6′ position of the purpurosamine ring and have similar activities against wild-type bacterial strains. However, these minor structural differences have been suggested to alter cytotoxicity (5) and render some of the gentamicin congeners susceptible to inactivation by resistance enzymes (6, 7).
FIG 1.
Structures of the four major gentamicin congeners. Colors highlight the structural differences between the different congeners at the 6′ position of the purpurosamine ring.
Aminoglycoside-modifying enzymes (AMEs) are the most clinically common mechanism for aminoglycoside resistance and can be harbored by both Gram-negative and Gram-positive bacteria. AMEs modify specific sites on the aminoglycoside to inactivate them and are classified as acetyltransferases (AACs), nucleotidyltransferases (ANTs), or phosphotransferases (APHs). Since the gentamicin congeners structurally differ at the 6′ position, AMEs that target this position, such as AAC(6′)-Ib, have variable potentials to acetylate the individual gentamicin congeners. AAC(6′)-Ib is one of the most common and clinically relevant AMEs expressed by Enterobacteriaceae, and it catalyzes inactivation of some of the gentamicin congeners, but not C1 (6–10). These findings raise the possibility that resistance to gentamicin caused by the presence of AAC(6′)-Ib depends on the relative abundances of the individual gentamicin congeners within the overall gentamicin mixture. The U.S. Pharmacopeia (USP) states that gentamicin formulations must contain between 10 and 35% gentamicin C1a, 25 to 55% gentamicin C2 plus C2a, and 25 to 50% gentamicin C1 (11). Given the variability in the relative gentamicin congener abundances that is allowed by the USP standard, the antibacterial activities of different USP-conforming gentamicin formulations may vary, particularly against strains harboring the clinically common AAC(6′)-Ib AME.
In addition to differences in antibacterial activity, the gentamicin congeners have also been suggested to have different nephrotoxicity potentials (5, 12). Nephrotoxicity occurs in ∼10 to 20% of patients receiving an aminoglycoside and is an important dose-limiting factor (13, 14). Among aminoglycosides, gentamicin has been observed to cause nephrotoxicity more frequently (15). Gentamicin C2 has been claimed to be nonnephrotoxic, but it has not been studied at increasing doses or over prolonged periods (5). Since the amount of each gentamicin congener within the commercial formulation may differ between the manufacturers or lots, but still meet the USP standards, nephrotoxicity caused by gentamicin may also be unpredictable and variable.
Unfortunately, the variation in relative gentamicin congener abundances between commercial gentamicin, parenteral formulations, and antibiotic susceptibility testing (AST) devices is not well described. Further, the impact of various AMEs on the MIC of each individual gentamicin congener remains poorly defined. Lastly, the nephrotoxicities of gentamicin congeners C2 and C2a have never been thoroughly measured at escalating doses or directly compared in animal studies, which leaves the previous claims of nephrotoxicity differences between congeners incomplete. Here, we show for the first time substantial variation in the proportions of gentamicin congeners within commercial formulations of gentamicin and commercial AST devices. We also show the wide range of gentamicin congener activities against bacterial strains with various AMEs. Our findings raise the concern that unexpected treatment failures may occur when the ratio of gentamicin congeners in the commercial AST device, which clinicians use to make decisions about antibiotic selection, do not match the ratio in the formulation administered to the patient. Lastly, we observed differences in the nephrotoxicity potentials of gentamicin (consisting of a mixture of compounds as would be present in a typical clinical formulation) and two purified gentamicin congeners over 11 days in an in vivo rat study.
RESULTS
Characterization of gentamicin commercial formulations and commercial AST disks.
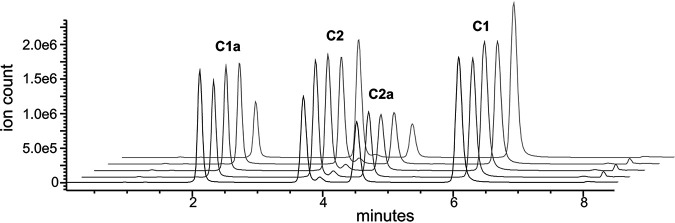
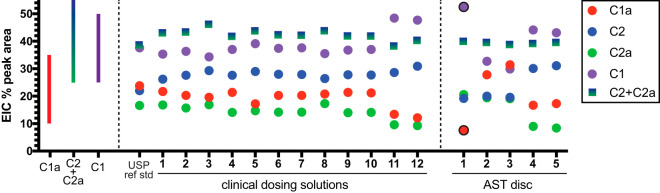
The gentamicin USP reference standard, 12 gentamicin clinical dosing formulations, and 5 commercial AST disks were analyzed by liquid chromatography-mass spectroscopy (LC-MS) to determine the proportions of gentamicin congeners C1, C1a, C2, and C2a in each product. The extracted ion counts (EIC) from the LC-MS analyses were used to compare the relative amounts of each of the 4 major gentamicin congeners, which maintained consistent retention times (Fig. 2; see also Fig. S1 and Table S1 in the supplemental material). The gentamicin congeners for the USP reference standard and 12 gentamicin clinical dosing formulations all fell within the permitted USP standard ranges (Fig. 3). Despite falling within the permitted ranges, the relative amounts of gentamicin congeners differed up to 1.9-fold between the different clinical dosing formulations. Among the 12 clinical gentamicin dosing formulations, gentamicin C1 ranged from 34.3 to 48.4%, C1a ranged from 12.1 to 21.7%, and C2 plus C2a ranged from 38.2 to 46.1%.
FIG 2.
Example LC-MS-extracted ion count traces of five different clinical lots of gentamicin. The ion counts shown are derived after extraction of molecular weights associated with the four major gentamicin congeners out of the overall total ion count spectra. Peaks for the four individual major gentamicin congeners are labeled. The minor peak between the C2 and C2a peaks is gentamicin C2b.
FIG 3.
Characterization of clinical lots and commercial AST disks of gentamicin by LC-MS. The percentage of each of the major gentamicin congeners was computed by the extracted ion count (EIC) based on the congener molecular weights from the mass spectroscopy signal. The bars on the left of the graph illustrate the range of the acceptance criteria for gentamicin clinical batches. Points that lie outside this range are outlined in black.
Relative amounts of each gentamicin congener varied even more among the commercial AST disks than for the parenteral formulations, with up to 4.1-fold differences observed. One of the commercial AST disks did not meet USP specifications. Specifically, commercial AST disk 1 contained 7.6% gentamicin C1a (USP reference range, 10 to 35%) and 52.5% gentamicin C1 (USP reference range, 25 to 50%), while the amount of gentamicin C2 plus C2a was within the specified range. Variability between the commercial AST disks and the clinical dosing formulations was also observed. Maximal differences were observed between commercial AST disk 3 and clinical dosing formulation 11 for gentamicin C1 (1.6-fold higher in dosing formulation), commercial AST disk 3 and clinical dosing formulation 12 for gentamicin C1a (2.6-fold higher in commercial AST disk), commercial AST disk 1 and clinical dosing formulation 12 for gentamicin C2 (1.6-fold higher for dosing formulation), and commercial AST disk 1 and clinical dosing formulation 12 for gentamicin C2a (2.2-fold higher for commercial AST disk).
Susceptibility testing of gentamicin congeners.
Gentamicin congeners C1, C1a, C2, and C2a were isolated from a laboratory-grade gentamicin powder, and their individual activities were tested against wild-type bacterial isolates and an isogenic Escherichia coli panel that harbored clinically relevant AMEs (Table 1). Against E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 wild-type isolates, gentamicin congener MICs all fell within 1 log2 dilution of the gentamicin mixture MIC, which is likely within the error of the broth microdilution MIC test. Against a wild-type Acinetobacter baumannii strain, gentamicin congeners C2, C2a, and C1 also all fell within 1 doubling dilution of the gentamicin mixture MIC. However, gentamicin C1a had an MIC that was 4-fold more potent than that of the gentamicin mixture, which was significantly different.
TABLE 1.
Microbiological activities of gentamicin congeners against Gram-negative wild-type strains and an isogenic E. coli panel that harbored key aminoglycoside-modifying enzymes
| Panel | Geometric mean MIC value (mg/liter)a |
||||
|---|---|---|---|---|---|
| Mixb | C1a | C2 | C2a | C1 | |
| Wild type | |||||
| E. coli ATCC 25922 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| P. aeruginosa ATCC 27853 | 0.5 | 0.25 | 0.25 | 0.25 | 1 |
| A. baumannii M2c | 1 | 0.25 | 0.5 | 0.5 | 2 |
| Isogenice | |||||
| E. coli ATCC 700926 + empty vectord | 0.25 | 0.125 | 0.125 | 0.125 | 0.125 |
| E. coli ATCC 700926 + aac(6′)-Ib | 1 | 4 | 0.5 | 32 | 0.25 |
| E. coli ATCC 700926 + aac(3)-III | 4 | 2 | 4 | 4 | 32 |
| E. coli ATCC 700926 + aph(3′)-Ia | 0.5 | 0.125 | 0.25 | 0.25 | 0.5 |
MIC values are the geometric mean of multiple independent measurements rounded to the nearest CLSI standard dilution increment.
“Mix” refers to batch of laboratory-grade gentamicin used to purify the gentamicin congeners.
Wild-type A. baumannii M2 has been previously characterized (34).
Parent E. coli strain containing the empty version of the pBBR1MCS-4 vector (35) used to carry the aminoglycoside-modifying enzyme genes in the isogenic panel.
Nine additional isogenic E. coli isolates with either aac(6′)-aph(2′′), aph(3′)-II, aph(3′)-III, aph(3′)-IV, aph(3′)-V, aph(3′)-VII, aac(2′)-I, aac(3)-I, or aac(3)-X were also tested, and the MICs for each congener fell within ±1 log2 dilution of the gentamicin “Mix” MIC.
Against E. coli ATCC 700926 with an empty plasmid vector, none of the congener MICs differed by more than 1 doubling dilution compared to the gentamicin mixture. Similarly, congener MICs fell within ±1 log2 dilution of the gentamicin mixture MIC for E. coli ATCC 700926 harboring either aac(6′)-aph(2′′), aph(3′)-II, aph(3′)-III, aph(3′)-IV, aph(3′)-V, aph(3′)-VII, aac(2′)-I, aac(3)-I, or aac(3)-X. When aac(6′)-Ib was added to E. coli ATCC 700926, gentamicin C2 MICs remained within 1 doubling dilution of the gentamicin mixture MIC, whereas C1a and C2a MICs were 4-fold and 32-fold higher (i.e., 4- and 32-fold less potent), respectively. Notably, the gentamicin C2a MIC against the aac(6′)-Ib strain was 256-fold higher than the C2a MIC for the isogenic strain without the AME. Gentamicin C1 was 4-fold more potent than the gentamicin mixture for the isogenic strain with aac(6′)-Ib. E. coli ATCC 700926 with aac(3)-III primarily affected gentamicin C1 MIC, which was 8-fold higher than the gentamicin mixture MIC and 256-fold higher than the gentamicin C1 MIC for the isogenic strain without an AME. The last notable difference in congener activity was for the isogenic strain with aph(3′)-Ia, for which the gentamicin C1a MIC was 4-fold lower than the gentamicin mixture MIC.
Two artificial mixtures of gentamicin congeners were created and their activity was determined against clinical carbapenem-resistant Enterobacteriaceae (CRE) isolates containing aac(6′)-Ib (Table 2). Though congener ratios in both mixtures were within USP allowable ranges, artificial mix 1 maximized the amounts of gentamicin C1 (50%) and C2 (40%), which are the most active congeners against isolates with aac(6′)-Ib. Artificial mix 2 minimized the amounts of C1 (25%) and C2 (0%). Artificial mix 1 had significantly greater activity than artificial mix 2 against 9 out of 10 CRE isolates. Gentamicin MICs were 4-fold lower for half of the tested isolates when artificial mix 1 was used.
TABLE 2.
Microbiological activities of laboratory-grade gentamicin compared to artificial mixtures of gentamicin congeners that maximized gentamicin C1 and C2 (artificial mix 1) or minimized gentamicin C1 and C2 (artificial mix 2) within USP allowable ranges
| Clinical isolate | Geometric mean MIC value (mg/liter)a |
||
|---|---|---|---|
| Laboratory-grade gentamicin mix | Artificial mix 1b | Artificial mix 2c | |
| K. pneumoniae CDC-AR-0003 | 1 | 1 | 2 |
| K. pneumoniae CDC-AR-0004 | 0.25 | 0.25 | 1 |
| K. pneumoniae CDC-AR-0005 | 0.5 | 0.25 | 1 |
| K. pneumoniae CDC-AR-0012 | 8 | 16 | 64 |
| K. pneumoniae CDC-AR-0047 | 0.5 | 0.25 | 1 |
| K. pneumoniae CDC-AR-0097 | 2 | 2 | 8 |
| K. pneumoniae CDC-AR-0129 | 0.5 | 0.5 | 1 |
| E. coli CDC-AR-0061 | 16 | 32 | 32 |
| Enterobacter cloacae CDC-AR-0002 | 2 | 2 | 4 |
| E. cloacae CDC-AR-0053 | 2 | 2 | 4 |
MIC values are the geometric means of multiple independent measurements rounded to the nearest CLSI standard dilution increment. Experiments were performed using a clinical panel of CRE that harbor aac(6′)-Ib.
Gentamicin artificial mixture 1 contained the following: C1, 50%; C1a, 10%; C2, 40%; and C2a, 0%.
Gentamicin artificial mixture 2 contained the following: C1, 25%; C1a, 20%; C2, 0%; and C2a, 55%.
In vitro gentamicin congener cytotoxicity.
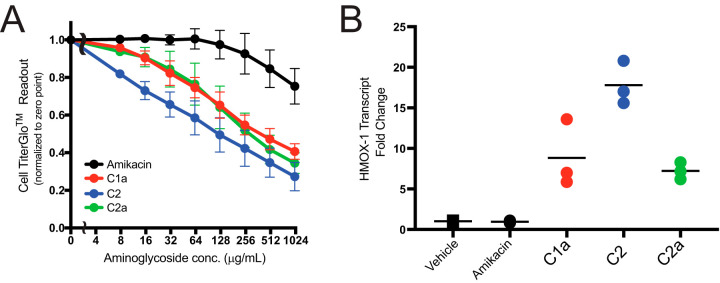
The cytotoxicities of purified gentamicin congeners C1a, C2, and C2a were compared to the cytotoxicity of amikacin on human renal proximal tubular cells (HK-2 cells) using a CellTiter-Glo luminescent cell viability assay (Fig. 4A). Unfortunately, gentamicin congener C1 was not isolated in sufficient quantity to allow for cytotoxicity testing. Relative Hmox-1 transcription levels, a previously identified toxicity biomarker (16), were also evaluated following exposure to the various gentamicin congeners and amikacin at 1 mg/ml for 6 h (Fig. 4B). All of the gentamicin congeners induced greater reductions in luminescence in the CellTiter-Glo cell viability assay than amikacin for concentrations >8 μg/ml. Minimal cytotoxicity was observed with clinically relevant amikacin concentrations (0 to 128 μg/ml). Gentamicin C2 was the most cytotoxic of the tested congeners. Gentamicin C2 also induced the highest change in Hmox-1 transcription levels, with a median 18-fold increase. Gentamicin C1a and C2a induced 8.8- and 7.2-fold increases in Hmox-1, respectively.
FIG 4.
Cytotoxicity results for gentamicin congeners C1a, C2, and C2a compared to amikacin. (A) CellTiter-Glo luminescent cell viability assay depicts the relative cytotoxicities of gentamicin congeners by quantitation of cellular viability based on ATP-induced luminescence in the presence of increasing aminoglycoside concentrations. (B) Induction of transcription of the Hmox-1 cytotoxicity marker for gentamicin congeners compared to amikacin.
In vivo gentamicin congener nephrotoxicity.
Nephrotoxicities of a commercial gentamicin mixture and gentamicin congeners C2 and C2a were evaluated in an 11-day repeat dose rat toxicity study (Fig. 5). Pharmacokinetic analysis of gentamicin C2 and C2a revealed similar exposures and plasma protein binding (Table 3). Therefore, any observed toxicity differences could not be attributed to differences in the in vivo concentration levels of the free, unbound gentamicins.
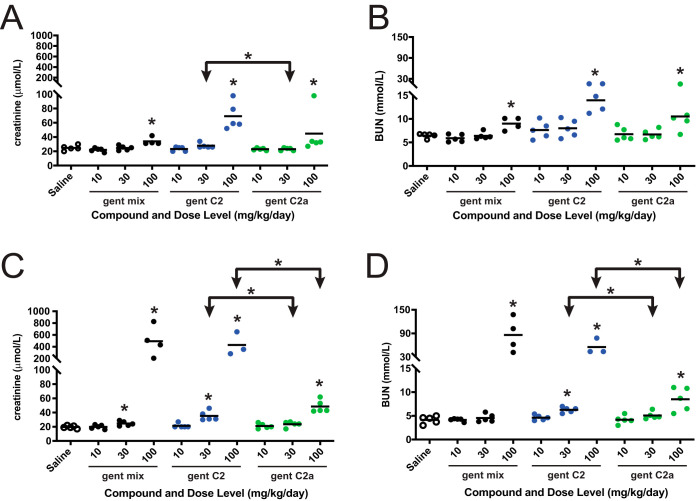
FIG 5.
Rat, 11-day repeat-dose toxicity of commercial gentamicin (gent mix; black) and gentamicin congeners C2 (gent C2; blue) and C2a (gent C2a; green). Toxicity was evaluated by measuring serum creatinine (A and C) and BUN (B and D) on day 6 (A and B) and day 11 (C and D). Creatinine and BUN values following administration of each compound were compared to the respective saline control values to identify significance by the Mann-Whitney U test as indicated by asterisks directly above the data points (*, P < 0.05). Cohorts receiving gent C2 and C2a were also compared to each other to identify significance by the Mann-Whitney U test as indicated by asterisks over the horizontal bars. Note that the number of data points reflects the surviving number of rats that were available for blood draws to be taken for the creatinine and BUN measurements reported here (n = 5 at the beginning of the study). The data for rat cohort receiving 100 mg/kg/day of gentamicin congener C2 were collected on day 10 before the rats were preterminally euthanized due to deteriorating health.
TABLE 3.
Pharmacokinetic data for gentamicin C2 and C2a in rata
| Dose (mg/kg) | AUC (mg/liter × h) |
|
|---|---|---|
| C2 | C2a | |
| 10 | 40 | 30 |
| 30 | 120 | 80 |
| 100 | 330 | 300 |
Repeat measurements were not made to enable a between-experiment statistical analysis, but typical fitting errors for an 8-time-point study to compute values for area under the concentration-time curve (AUC) are higher than the AUC differences observed between C2 and C2a. Plasma protein binding was 84% and 86% unbound for C2 and C2a, respectively.
To identify nephrotoxicity, analysis of clinical chemistry was performed on days 6 and 12 (i.e., 1 day after the end of dosing) except in the following 2 cases. First, one rat receiving 100 mg/kg (of body weight)/day of the gentamicin mixture was preterminally euthanized on day 10 due to deteriorating health. The clinical chemistry data from this rat were not included in the final analysis, as day 12 data from the remaining 4 rats of the cohort were sufficient for interpretation. Second, 2 rats receiving 100 mg/kg/day of gentamicin congener C2 were found dead on day 10 before blood draws could be taken for the analysis. Blood draws from the remaining 3 rats of the cohort were gathered and then the rats were preterminally euthanized on day 10 due to deteriorating health. Thus, the data shown in Fig. 5 for the remaining 3 rats of the 100-mg/kg/day gentamicin C2 cohort were gathered on day 10 instead of day 12 as originally intended. This difference is not expected to impact the interpretation of the relative toxicities of the gentamicin congeners discussed below.
On day 6, all three gentamicin compounds at the highest dose (100 mg/kg/day) significantly raised the levels of two common nephrotoxicity biomarkers (serum creatinine and blood urea nitrogen [BUN]) relative to the saline control (P < 0.05). Gentamicin congener C2 at 100 mg/kg/day had the highest average elevation in both creatinine and BUN levels at day 6, whereas the gentamicin mixture and gentamicin C2a had more similar levels. While neither gentamicin C2 nor C2a at 30 mg/kg/day was significantly different from the saline control, gentamicin C2 had significantly higher BUN levels than gentamicin C2a when dosed at 30 mg/kg/day.
In general, there was a trend toward increased creatinine and BUN on day 12 compared to day 6 for rats exposed to each of the gentamicin compounds. On day 12, the commercial gentamicin mixture and gentamicin C2 at 100 mg/kg/day increased serum creatinine >20-fold (P < 0.05) and BUN >10-fold (P < 0.05) versus the saline controls. Although gentamicin C2a at 100 mg/kg/day also significantly increased creatinine (P < 0.05) and BUN (P < 0.05), mean serum concentrations were only ∼10% those of the rats exposed to the commercial gentamicin mixture or gentamicin C2. At both the 30- and 100-mg/kg/day doses, gentamicin C2 elicited higher levels of both creatinine and BUN than gentamicin C2a by day 12.
Macro- and microscopic findings in the kidneys consistent with nephrotoxicity were observed in a dose-related pattern following administration of each compound for 11 days. The lowest severity and incidence of renal findings indicating toxicity were observed in rats exposed to gentamicin C2a, which is consistent with the serum creatinine and BUN findings. The highest severity and incidence of renal findings was observed in rats exposed to gentamicin C2, in which, among other findings, proteinaceous casts and tubular necrosis were observed at both the 30- and 100-mg/kg/day doses. Proteinaceous casts and tubular necrosis were only observed at the 100-mg/kg/day dose following administration of the commercial gentamicin mixture.
DISCUSSION
Clinical gentamicin formulations and commercial AST devices are composed of a mixture of gentamicin congeners and other minor components. The USP permits gentamicin formulations to contain different amounts of the major congeners (C1a, C2 plus C2a, and C1) as long as they fall within specified ranges. However, variations between different manufacturers’ formulations of gentamicin and their potential impact on the safety and activity of gentamicin are not well understood. Here, we have shown that there is substantial variation in the amounts of the gentamicin congeners within clinical formulations of gentamicin and commercially available gentamicin disks used to test antimicrobial susceptibility. Importantly, we also report that the activities of gentamicin congeners C2a and C1 were reduced in the presence of AMEs AAC(6′)-Ib and AAC(3)-III, respectively. Gentamicin C1 is also the most potent gentamicin congener in the presence of AAC(6′)-Ib and the least potent in the presence of AAC(3)-III. These findings raise the concern that gentamicin susceptibility in the clinical microbiology laboratory, particularly for bacteria with specific AMEs, may not accurately predict activity of the gentamicin formulation administered to patients and could lead to unexpected treatment failures. We also found that gentamicin C2a was significantly less cytotoxic and nephrotoxic than gentamicin C2. Toxicity differences observed between the gentamicin congeners coupled with the variation of the gentamicin formulations suggest that differences in gentamicin toxicity between different commercially available products are possible.
The compositions of the gentamicin clinical dosing formulations that we tested met USP specifications, but there were up to 1.9-fold differences in the amounts of the congeners among the various formulations. Stypulkowska et al. showed that for 3 European gentamicin products, the gentamicin congeners all fell within the European Pharmacopoeia’s requirements but that there was a 1.4-fold difference in the amounts of gentamicin C1a and smaller variations between the other congeners (4). Vydrin et al. also tested the compositions of 6 commercial gentamicin preparations from Europe and found that all met the European Pharmacopoeia’s requirements but 3 of the formulations had C1 concentrations that fell just below the USP lower limit of 25% (17). The authors also noted substantial variability of the congeners (up to 2.3-fold) within the permitted ranges, which is similar to the findings of our study.
Differences in the composition of the gentamicin formulations are not inherently problematic if all of the congeners have identical antibacterial activity and toxicity profiles. However, we show that the congeners do have different antibacterial activities in the presence of key AMEs, and they also have different toxicities (Table 1). In agreement with previous studies, our results suggest that gentamicin C1 is likely not a substrate of AAC(6′)-Ib (7, 10). The molecular basis by which gentamicin C1 evades the modification from AAC(6′)-Ib is likely due to the presence of the methyl group directly attached to the 6′ amine of the gentamicin. This methyl group presumably blocks acetylation at this position by the AME. More perplexing was the observation that gentamicin C2a appeared to be a better substrate for AAC(6′)-Ib than gentamicin C2. The molecular basis of this difference is unknown and was not further tested in this study. Nevertheless, a structure of AAC(6′)-Ib cocrystallized with kanamycin, an aminoglycoside that is structurally related to gentamicin and contains an identical 6′ amine (PDB code 2QIR), has been previously published (18) and does enable a speculative model that can account for the MIC differences between C2 and C2a against a strain harboring this AME (Fig. S2). Inspection of the active-site pocket with kanamycin reveals that the 6′ carbon of the aminoglycoside is oriented against the back wall of the pocket. If a methyl group were present on this 6′ carbon as it is on gentamicin C2 and C2a, its presence would sterically clash with the active-site pocket wall only in one of the two possible stereochemical configurations. Presumably, gentamicin C2 has the 6′ methyl group pointing in the direction of the active-site pocket wall, which results in a steric clash and a lack of binding to the AME, thus protecting it from modification. In contrast, gentamicin C2a is the stereoisomer that likely has its 6′ methyl group pointing away from the active-site pocket wall, and thus, a steric clash does not result, which enables the AME to modify the 6′ amine and render the gentamicin incapable of binding its ribosomal target. This model also predicts that gentamicin C1a, which does not contain any methyl groups on the 6′ carbon, would be a good substrate for AAC(6′)-Ib. Gentamicin C1a is less potent in the presence of AAC(6′)-Ib; however, the MIC did not shift as much as it did for C2a. This observation suggests that the presence of the methyl on gentamicin C2a makes it an even better substrate for AAC(6′)-Ib than if the methyl were absent. The molecular basis of this presumed effect cannot be readily explained by the structure that is currently available. Further structural and functional studies with AAC(6′)-Ib will be needed to fully elucidate the molecular details behind the MIC results. Similar studies will also be needed to understand the molecular basis for the differences in antibacterial activity among the gentamicin congeners against strains harboring AAC(3)-III, as the situation may be even more complex with this AME. To our knowledge, differences in the activity of gentamicin congeners against bacteria with AAC(3)-III have not previously been shown.
Employing our understanding of the potency of gentamicin C1 and C2 against aac(6′)-Ib, we created two artificial mixtures of the gentamicin congeners that represent best (artificial mix 1) and worst (artificial mix 2) case scenarios of gentamicin formulations that could be used against aac(6′)-Ib-harboring isolates. Worryingly, the activity of artificial mix 2 was roughly 2- to 4-fold less potent than that of artificial mix 1 (Table 2). One isolate, K. pneumoniae CDC-AR-0097, even had an MIC that went from being considered susceptible to intermediate based on Clinical and Laboratory Standards Institute (CLSI) breakpoints when switching from artificial mix 1 to artificial mix 2 (19). Three such categorical disagreements exist between the gentamicin mixtures when considering the EUCAST breakpoints for gentamicin (20). This potential variation in microbiological activity between gentamicin formulations is especially concerning since it is already difficult to attain the pharmacokinetic/pharmacodynamic target for gentamicin when the organism’s MIC is ≥2 mg/liter (21, 22). Thus, some patients may be treated with a commercial gentamicin formulation that is more or less active than gentamicin commercial AST disks used to define susceptibility.
Variability in the composition of gentamicin commercial AST disks was even greater than the variability of the gentamicin clinical formulations. Our study is the first to examine the content of gentamicin commercial AST disks. The variations in gentamicin congeners may be important clinically when the commercial AST device has a different ratio of the congeners than the clinical dosing formulation. This would be particularly concerning when targeting bacterial isolates that express either AAC(6′)-Ib or AAC(3)-III, which we have shown inactivate some, but not all, of the gentamicin congeners. For example, if an E. coli strain expressing AAC(6′)-Ib is reported to be gentamicin susceptible based on testing with a product that has a high proportion of C1 (e.g., commercial AST disk 1) and the patient’s infection is treated with a clinical dosing formulation that has a lower proportion of C1 (i.e., clinical dosing formulation 3), then there may be an unexpected treatment failure. This is worrisome since aac(6′)-Ib is a prevalent AME gene among multidrug-resistant infections such as KPC-producing K. pneumoniae, in which it has been reported for ∼90% of isolates (23, 24).
Variations in the compositions of gentamicin commercial products are also concerning from a toxicity standpoint. Our experiments revealed that gentamicin C2 was more toxic than gentamicin C1a, gentamicin C2a, or amikacin. Since each model for nephrotoxicity has limitations in its ability to accurately predict toxicity in humans (25), we tested the gentamicin congeners in three established models. To further validate the ability for our data to be translated to patients, we included an amikacin control in two of the models (Fig. 4). Amikacin was shown to be less toxic than the gentamicin congeners in these in vitro tests, which is in agreement with clinical data (26, 27). Our observations were consistent across all three models, which suggests that differences in the nephrotoxicity between gentamicin congeners will likely translate to patients. This difference in toxicity between congeners is worrisome considering we observed variability in the amounts of gentamicin C2 (24.8% to 29.4%) and C2a (10.5% to 18.1%) in the commercial formulations of gentamicin. Two previous studies have attempted to test the relative toxicities of individual gentamicin components. First, Kohlhepp et al. found that gentamicin nephrotoxicity in a repeat-dose rat study was primarily caused by gentamicin C2 and/or C2a (12). However, the authors were unable to separate these two stereoisomers in order to analyze gentamicin C2 and C2a individually. More recently, Sandoval et al. compared the toxicity profiles of the different gentamicin congeners using in vitro cytotoxicity assays and then looked at the nephrotoxicity of C2 in a 5-day repeat-dose study in rats (5). The authors found that gentamicin C2 was the least cytotoxic congener in vitro, which does not agree with the findings of our study, in which gentamicin C2 was found to be more cytotoxic than C2a and C1a. There was also a difference in the observations from the repeat-dose rat studies in that Sandoval et al. report that gentamicin C2 was not nephrotoxic over 6 days, whereas we found that C2 was nephrotoxic at the same daily dose after 6 and 12 days. Unlike our study, the study by Sandoval et al. did not compare the nephrotoxicity of gentamicin C2a to that of gentamicin C2 in rats. The cause of this apparent discrepancy in nephrotoxicity remains uncertain but may be the result of a nomenclature difference for the congeners. However, our findings were consistent with those of Sandoval et al. in demonstrating that one of the gentamicin C2 congeners (i.e., C2a or C2) is less nephrotoxic than the overall commercial gentamicin mixture.
A few potential mechanisms may explain the observed differences in nephrotoxicity between gentamicin congeners. The first proposes that the less nephrotoxic gentamicin congener is a poorer substrate of a membrane transporter on the proximal tubule cell (PTC), where aminoglycoside nephrotoxicity occurs. The primary receptor that has been observed to mediate this specific uptake into the PTCs is megalin (28, 29). Thus, the position of the methyl group on the less nephrotoxic congener may limit its binding to megalin and subsequent uptake into the PTC. Alternatively, the less nephrotoxic congener may still enter the PTC but not cause the coalescing of the Golgi apparatus and lysosomes into myeloid bodies that have previously been associated with gentamicin nephrotoxicity (5, 30). However, it remains unclear precisely how the structure of the less nephrotoxic stereoisomer may evade activation of the intracellular pathways leading to formation of myeloid bodies. Regardless of the cellular mechanism of toxicity, electrostatic differences between the two gentamicin congeners may also play a role. The stereochemical position of the 6′ carbon methyl on the gentamicin congeners likely differentially affects the 6′ amine pKa. However, this potential electrostatic difference, which may be very subtle, remains to be measured.
In conclusion, there is a substantial amount of variability in the amount of each gentamicin congener present in clinical formulations of the antibiotic and commercial AST disks. Unexpected gentamicin treatment failure may occur if the ratios of gentamicin congeners present in the susceptibility testing product and the clinical dosing formulation are not the same, since microbiological activities of the congeners can differ. There is also variability in the nephrotoxicity of the gentamicin congeners, suggesting that certain gentamicin commercial formulations may be more nephrotoxic than others. Additional studies are warranted to determine if differences between clinical gentamicin dosing formulation affect patient outcomes due to the variation in activity and toxicity of the congeners.
MATERIALS AND METHODS
Characterization of gentamicin commercial formulations and commercial AST disks.
Ratios of the gentamicin congeners within each of the 12 commercial formulations and 5 commercial AST disks (identifiers [IDs] and batch numbers listed in Table S2) were quantified using LC-MS. All samples were injected at a concentration of 3 mg/ml. Analyses were performed on an Agilent Infinity II LC system connected to an Agilent 6130B single quadrupole mass spectrometer equipped with an electrospray ion source and operated in positive ion mode. Chromatography used an Agilent InfinityLab Poroshell HPH-C18 column, 2.1 by 50 mm, 1.9-μm particle size (part number 699675-702). A shallow gradient of acetonitrile (6 to 12%) was used for separation of the components. The aqueous mobile phase was 0.25 M ammonium hydroxide in water, while the organic mobile phase was acetonitrile without any modifiers added. The total run time was 20 min. To quantify how much of each gentamicin congener was present in each sample, we divided the peak area for the congener by the total peak areas for all four major congeners (C1, C1a, C2, and C2a). To test if peak area percentages were influenced by the detection method, samples were also analyzed using a liquid chromatography-evaporative light scattering detector under the same chromatography conditions as described above. No significant differences in the relative peak areas were observed compared to those obtained by the LC-MS method (Fig. S1B and Table S1).
Isolation of gentamicin congeners.
The isolation of gentamicin congeners C1, C1a, C2, and C2a was achieved by purification of gentamicin sulfate using reverse-phase high-performance liquid chromatography. Gentamicin sulfate (CHEM-IMPEX International Inc.; catalog no. 00149 and lot no. 001204-15032206) was used as the source for congener isolation. A gradient of acetonitrile in water (modified with ammonium hydroxide to a concentration of 250 mM) from 8 to 16% acetonitrile and a flow rate of 250 ml/min were sufficient to separate the congeners. For injection, gentamicin sulfate was dissolved to 300 mg/ml in concentrated ammonium hydroxide, 3 ml of which was injected to the column (50 by 15 cm) packed with 3 kg of Waters XBridge Prep-C18 resin (10 μm; 130 Å). Depending on the amount of material needed, the size of the scale could be varied with the gradient conditions described above. Congeners were detected by UV light at 214 nm and eluted in the order as shown in the analytic chromatograms in Fig. 2 and Fig. S3. Solid powders of pure fractions were isolated by lyophilization. Prior to testing (or repurification of impure material), the isolated freebase was converted to the sulfate salt by titrating the aqueous solution until a steady pH less than 6.5 was maintained. In the case of impure material requiring repurification, the sulfate salt was preferred over the freebase because the presence of CO2 adducts and/or carbamates that formed during the lyophilization confound resolution. Congeners were routinely tested for purity using LC-MS (Fig. S3).
Susceptibility testing for gentamicin and congeners.
MICs were determined by broth microdilution according to guidelines established by the Clinical and Laboratory Standards Institute (31). Antibiotic stock solutions were prepared fresh prior to each experiment. Inocula were prepared from cells streaked onto Mueller-Hinton agar (MHA) and grown at 35°C overnight. Antibiotic solution was mixed with the bacterial inoculum in cation-adjusted Mueller-Hinton broth in 96-well assay plates, with a final inoculum of approximately 5 × 105 CFU/ml. After incubation of assay plates at 35°C for 18 to 20 h, the lowest concentration of antibiotic that prevented visible growth was recorded as the MIC.
In vitro gentamicin congener cytotoxicity.
The cytotoxicities of gentamicin congeners were measured using the CellTiter-Glo luminescent cell viability assay (Promega, Madison, WI) in 96-well, flat, clear-bottom, opaque-wall microplates according to the manufacturer’s protocol. Human renal proximal tubular cells (HK‐2 cells) from the ATCC (Manassas, VA) were seeded in 96-well plates (2 × 103 cells/well) in keratinocyte serum-free medium (SFM) plus supplements (human recombinant epidermal growth factor 1-53 [EGF 1-53] and bovine pituitary extract [BPE]). After an overnight incubation, cells were treated with three gentamicin congeners (C1a, C2, and C2a) and amikacin in eight concentrations (1,000.00 μg/ml to 7.8 μg/ml) in triplicates for 72 h. Luminescent signals were measured using an EnVision 2105 multimode plate reader (Perkin Elmer, Waltham, MA).
Gene-specific mRNA quantitation by quantitative PCR (qPCR) was performed to quantify the expression of the cell toxicity biomarker Hmox-1 by first extracting total RNA from HK-2 cells with the RNeasy minikit (Qiagen, Hilden, Germany) after the cells had been incubated with 1 mg/ml of test aminoglycoside for 6 h. Total RNA (1 μg) was reverse transcribed using oligonucleotide (random primer) and the QuantiTect reverse transcription kit (Qiagen). Real-time PCR was performed in a 96-well optical reaction plate using iQ SYBR green Supermix (Bio-Rad Laboratories, Hercules, CA). Real-time PCRs were performed on a CFX96 Touch real-time PCR detection system (Bio-Rad Laboratories). Assays used the following primer sets: Hmox-1, 5′-CAACAAAGTGCAAGATTCTG-3′ (forward) and 5′-TGCATTCACATGGCATAAAG-3′ (reverse), and GAPDH, 5′-ACAGTTGCCATGTAGACC-3′ (forward) and 5′-TTGAGCACAGGGTACTTTA-3′ (reverse). The relative gene expression of the RT-qPCR products was determined using the threshold cycle (ΔCT) method (32). This method calculates the relative gene expression using the following equation: fold induction = 2ΔCT, where CT is the threshold cycle and ΔCT = (CTHmox-1 of interest − CTGAPDH). Each sample was run in triplicate, and three independent experiments were performed. The mean CT was used in the ΔCT equation.
In vivo gentamicin congener nephrotoxicity.
The study followed appropriate standard operating procedures at the contract research organization (CRO) performing the study. All animals used on this study were cared for in accordance with the principles outlined in the current Guide for the Care and Use of Laboratory Animals (33). The test and vehicle control solutions were administered to cohorts of 8-week-old male Sprague-Dawley rats (Rattus norvegicus) once daily by subcutaneous injection administration for 11 consecutive days (exceptions due to deteriorating health conditions in some cohorts noted in the Results). The dose volume administered to each animal, including controls, was 1 ml/kg. The actual volume administered to each rat was calculated and adjusted based on the most recent practical body weight of each animal (i.e., body weights taken on days 1, 3, 7, and 11). All blood/urine sampling, hematology, clinical chemistry, and urinalysis were carried out according to the CRO’s standard operating procedures.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded in part by Achaogen, Inc. Z.P.B. was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), under grant KL2TR002002.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
None of the authors have current conflicts of interest to disclose. R.C., D.H., T.K., Z.R., K.W., M.P., and L.D.A. were all employees of Achaogen, Inc., during completion of the study.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2019. Critically important antimicrobials for human medicine, 6th revision. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Holzgrabe U, Nap CJ, Kunz N, Almeling S. 2011. Identification and control of impurities in streptomycin sulfate by high-performance liquid chromatography coupled with mass detection and corona charged-aerosol detection. J Pharm Biomed Anal 56:271–279. doi: 10.1016/j.jpba.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Hanko VP, Rohrer JS, Liu HH, Zheng C, Zhang S, Liu X, Tang X. 2008. Identification of tobramycin impurities for quality control process monitoring using high-performance anion-exchange chromatography with integrated pulsed amperometric detection. J Pharm Biomed Anal 47:828–833. doi: 10.1016/j.jpba.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Stypulkowska K, Blazewicz A, Fijalek Z, Sarna K. 2010. Determination of gentamicin sulphate composition and related substances in pharmaceutical preparations by LC with charged aerosol detection. Chromatographia 72:1225–1229. doi: 10.1365/s10337-010-1763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandoval RM, Reilly JP, Running W, Campos SB, Santos JR, Phillips CL, Molitoris BA. 2006. A non-nephrotoxic gentamicin congener that retains antimicrobial efficacy. J Am Soc Nephrol 17:2697–2705. doi: 10.1681/ASN.2005101124. [DOI] [PubMed] [Google Scholar]
- 6.Shaw KJ, Rather PN, Hare RS, Miller GH. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev 57:138–163. doi: 10.1128/MMBR.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert T, Ploy MC, Courvalin P. 1994. A spontaneous point mutation in the aac(6′)-Ib′ gene results in altered substrate specificity of aminoglycoside 6′-N-acetyltransferase of a Pseudomonas fluorescens strain. FEMS Microbiol Lett 115:297–304. doi: 10.1111/j.1574-6968.1994.tb06654.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez MS, Tolmasky ME. 2010. Aminoglycoside modifying enzymes. Drug Resist Updat 13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castanheira M, Deshpande LM, Woosley LN, Serio AW, Krause KM, Flamm RK. 2018. Activity of plazomicin compared with other aminoglycosides against isolates from European and adjacent countries, including Enterobacteriaceae molecularly characterized for aminoglycoside-modifying enzymes and other resistance mechanisms. J Antimicrob Chemother 73:3346–3354. doi: 10.1093/jac/dky344. [DOI] [PubMed] [Google Scholar]
- 10.Rather PN, Munayyer H, Mann PA, Hare RS, Miller GH, Shaw KJ. 1992. Genetic analysis of bacterial acetyltransferases: identification of amino acids determining the specificities of the aminoglycoside 6′-N-acetyltransferase Ib and IIa proteins. J Bacteriol 174:3196–3203. doi: 10.1128/jb.174.10.3196-3203.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. 2016. WHO critically important antimicrobials for human medicine, 5th revision. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 12.Kohlhepp SJ, Loveless MO, Kohnen PW, Houghton DC, Bennett WM, Gilbert DN. 1984. Nephrotoxicity of the constituents of the gentamicin complex. J Infect Dis 149:605–614. doi: 10.1093/infdis/149.4.605. [DOI] [PubMed] [Google Scholar]
- 13.Moore RD, Smith CR, Lipsky JJ, Mellits ED, Lietman PS. 1984. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med 100:352–357. doi: 10.7326/0003-4819-100-3-352. [DOI] [PubMed] [Google Scholar]
- 14.Bell S, Davey P, Nathwani D, Marwick C, Vadiveloo T, Sneddon J, Patton A, Bennie M, Fleming S, Donnan PT. 2014. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 25:2625–2632. doi: 10.1681/ASN.2014010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humes HD. 1988. Aminoglycoside nephrotoxicity. Kidney Int 33:900–911. doi: 10.1038/ki.1988.83. [DOI] [PubMed] [Google Scholar]
- 16.Adler M, Ramm S, Hafner M, Muhlich JL, Gottwald EM, Weber E, Jaklic A, Ajay AK, Svoboda D, Auerbach S, Kelly EJ, Himmelfarb J, Vaidya VS. 2016. A quantitative approach to screen for nephrotoxic compounds in vitro. J Am Soc Nephrol 27:1015–1028. doi: 10.1681/ASN.2015010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vydrin A, Shikhaleev I, Makhortov V, Shcherenko N, Kolchanova N. 2003. Component composition of gentamicin sulfate preparations. Pharm Chem J 37:448–450. doi: 10.1023/A:1027372416983. [DOI] [Google Scholar]
- 18.Maurice F, Broutin I, Podglajen I, Benas P, Collatz E, Dardel F. 2008. Enzyme structural plasticity and the emergence of broad-spectrum antibiotic resistance. EMBO Rep 9:344–349. doi: 10.1038/embor.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing: 29th informational supplement M100-S29. CLSI, Wayne, PA. [Google Scholar]
- 20.European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters, version 9.0. http://www.eucast.org.
- 21.Zavascki AP, Klee BO, Bulitta JB. 2017. Aminoglycosides against CRE in the critically ill: the pitfalls of aminoglycoside susceptibility. Expert Rev Anti Infect Ther 15:519–526. doi: 10.1080/14787210.2017.1316193. [DOI] [PubMed] [Google Scholar]
- 22.Roger C, Nucci B, Molinari N, Bastide S, Saissi G, Pradel G, Barbar S, Aubert C, Lloret S, Elotmani L, Polge A, Lefrant JY, Roberts JA, Muller L. 2015. Standard dosing of amikacin and gentamicin in critically ill patients results in variable and subtherapeutic concentrations. Int J Antimicrob Agents 46:21–27. doi: 10.1016/j.ijantimicag.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, Kreiswirth B, Nguyen MH. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 58:4443–4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galani I, Nafplioti K, Adamou P, Karaiskos I, Giamarellou H, Souli M, Study Collaborators. 2019. Nationwide epidemiology of carbapenem resistant Klebsiella pneumoniae isolates from Greek hospitals, with regards to plazomicin and aminoglycoside resistance. BMC Infect Dis 19:167. doi: 10.1186/s12879-019-3854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soo JY, Jansen J, Masereeuw R, Little MH. 2018. Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat Rev Nephrol 14:378–393. doi: 10.1038/s41581-018-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerner SA, Schmitt BA, Seligsohn R, Matz GJ. 1986. Comparative study of ototoxicity and nephrotoxicity in patients randomly assigned to treatment with amikacin or gentamicin. Am J Med 80:98–104. doi: 10.1016/0002-9343(86)90486-9. [DOI] [PubMed] [Google Scholar]
- 27.Sweileh WM. 2009. A prospective comparative study of gentamicin- and amikacin-induced nephrotoxicity in patients with normal baseline renal function. Fundam Clin Pharmacol 23:515–520. doi: 10.1111/j.1472-8206.2009.00702.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz C, Hilpert J, Jacobsen C, Boensch C, Christensen EI, Luft FC, Willnow TE. 2002. Megalin deficiency offers protection from renal aminoglycoside accumulation. J Biol Chem 277:618–622. doi: 10.1074/jbc.M109959200. [DOI] [PubMed] [Google Scholar]
- 29.Nagai J, Takano M. 2004. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet 19:159–170. doi: 10.2133/dmpk.19.159. [DOI] [PubMed] [Google Scholar]
- 30.Beauchamp D, Gourde P, Bergeron MG. 1991. Subcellular distribution of gentamicin in proximal tubular cells, determined by immunogold labeling. Antimicrob Agents Chemother 35:2173–2179. doi: 10.1128/aac.35.11.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI document M07-A9 CLSI, Wayne, PA. [Google Scholar]
- 32.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 34.Niu C, Clemmer KM, Bonomo RA, Rather PN. 2008. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol 190:3386–3392. doi: 10.1128/JB.01929-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovach ME, Elzer PH, Steven Hill D, Robertson GT, Farris MA, Roop RM, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.