In a pilot study, we showed that the intermittent administration of benznidazole in chronic Chagas disease patients resulted in a low rate of treatment suspension and therapeutic failure, as assessed by quantitative PCR (qPCR) at the end of treatment. Here, a 3-year posttreatment follow-up study of the same cohort of patients is presented. The treatment scheme consisted of 12 doses of benznidazole at 5 mg/kg of body weight/day in two daily doses every 5 days. Parasite load, Trypanosoma cruzi-specific antibodies, and serum chemokine levels were measured prior to treatment and after a median follow-up of 36 months posttreatment by DNA minicircle kinetoplastid and nuclear DNA satellite sequence qPCR methods, conventional serological techniques, a Luminex-based assay with recombinant T. cruzi proteins, and a cytometric bead array.
KEYWORDS: Trypanosoma cruzi, Chagas disease, benznidazole, intermittent treatment, chronic infection
ABSTRACT
In a pilot study, we showed that the intermittent administration of benznidazole in chronic Chagas disease patients resulted in a low rate of treatment suspension and therapeutic failure, as assessed by quantitative PCR (qPCR) at the end of treatment. Here, a 3-year posttreatment follow-up study of the same cohort of patients is presented. The treatment scheme consisted of 12 doses of benznidazole at 5 mg/kg of body weight/day in two daily doses every 5 days. Parasite load, Trypanosoma cruzi-specific antibodies, and serum chemokine levels were measured prior to treatment and after a median follow-up of 36 months posttreatment by DNA minicircle kinetoplastid and nuclear DNA satellite sequence qPCR methods, conventional serological techniques, a Luminex-based assay with recombinant T. cruzi proteins, and a cytometric bead array. At the end of follow-up, 14 of 17 (82%) patients had negative qPCR findings, whereas three of 17 (18%) had detectable nonquantifiable findings by at least one of the qPCR techniques. A decline in parasite-specific antibodies at 12 months posttreatment was confirmed by conventional serological tests and the Luminex assays. Monocyte chemoattractant protein 1 levels increased after treatment, whereas monokine induced by gamma interferon levels decreased. New posttreatment electrocardiographic abnormalities were observed in only one patient who had cardiomyopathy prior to treatment. Together, these data strengthen our previous findings by showing that the intermittent administration of benznidazole results in a low rate of treatment suspension, with treatment efficacy comparable to that of a daily dose of 5 mg/kg for 60 days.
INTRODUCTION
The primary clinical outcome of Trypanosoma cruzi infection is a chronic cardiomyopathy, which manifests in approximately 30% of infected individuals 10 to 20 years after the initial infection [World Health Organization, https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis)]. It is estimated that approximately 1.2 million individuals are living with heart disease, making Chagas disease the most frequent cause of infectious cardiomyopathy in the world (1). Although efforts have been made in previous decades, no new compounds have been approved for the treatment of Chagas disease, with benznidazole (BZ) and nifurtimox being the only two currently available medications (2–5). Adverse events are some of the main limitations of widely applicable therapies in the chronic phase in adults (6).
Although various guidelines based on randomized studies in children recommend the use of a 60-day treatment schedule with BZ in adults (7), several studies have suggested that treatment outcomes do not differ between 30 and 60 days of BZ administration (8–10). Currently, in the Benznidazole New Doses Improved Treatment and Associations (BENDITA) clinical trial, BZ-sparing regimens in monotherapy, including the 30-day versus 60-day schemes, are being evaluated (ClinicalTrials.gov registration no. NCT03378661).
Furthermore, pharmacokinetic studies have shown that BZ plasma concentrations in children are markedly lower than those reported in adults, whereas the therapeutic response is higher (11, 12), adding more unresolved issues in the treatment of chronic Chagas disease. Additionally, in a mouse model of chronic T. cruzi infection, reducing the overall dosage of BZ or nifurtimox using intermittent administration every 5 days cured the infection (13). As part of a pilot study, we previously showed that the intermittent administration of BZ resulted in a low rate of treatment suspension and therapeutic failure, as assessed by qPCR at the end of treatment (14). Here, this same cohort of patients was followed up with a median of 36 months post intermittent administration of BZ, in which the clinical status, parasite burden, T. cruzi-specific humoral responses, and serum levels of chemokines were assessed.
RESULTS
Clinical characteristics of patients in the study.
We previously published a pilot study to assess the safety and short-term efficacy of a scheme of intermittent administration of BZ in patients chronically infected with T. cruzi (14). Seventeen of the 20 patients recruited in our former study were monitored for a median period of 3 years (range, 12 to 48 months) after BZ administration. Eleven of the 17 (65%) patients recruited had no electrocardiographic or echocardiographic alterations at baseline (Table 1). Ten of the 11 patients without cardiomyopathy remained stable during follow-up, while the remaining patient (i.e., patient I14 in Table 1) showed basal inferior hypokinesis of the left ventricle at 36 months posttreatment without significant changes in the ECG. Five of the 17 (35%) patients showed mild cardiomyopathy prior to treatment (i.e., two subjects with conduction disturbances [i.e., patient I8 and I9 in Table 1], one subject with both conduction and rhythm disturbances [i.e., patient I4], and two subjects with ventricular arrhythmia [i.e., patient I3 and patient I10]). Of note, patient I10, who had arterial hypertension as a comorbidity, developed diastolic dysfunction, as assessed by echocardiography, during follow-up (Table 1). The remaining subject showed repolarization abnormalities of the inferior left ventricle wall (i.e., patient I20) not related to Chagas disease and presented a mild mitral insufficiency at the end of follow-up.
TABLE 1.
Clinical characteristics of the study population at baseline and following intermittent administration of BZ
| ID | Genderc | Age (yr) | ECGa |
Echocardiograma |
Cardiac disease progressionb | ||
|---|---|---|---|---|---|---|---|
| Basal | At the end of follow-up | Basal | At the end of follow-up | ||||
| I2 | F | 53 | Normal | Normal | Normal | Normal | No |
| I3 | F | 42 | VA | VA | LVSD 60/apical dyskinesis | LVSD 50/apical dyskinesis | No |
| I4 | F | 45 | LAFB/VA | LAFB/VA | Normal | Normal | No |
| I6 | F | 32 | Normal | Normal | Normal | Normal | No |
| I8 | M | 57 | RRBB | RRBB | Normal | Normal | No |
| I9 | M | 55 | RRBB/LAFB | RRBB/LAFB | Septal hypertrophy, LVH | Septal hypertrophy, LVH | No |
| I10 | F | 41 | VA | VA | MMI | MMI and diastolic dysfunction | Nod |
| I11 | F | 46 | Normal | Normal | Normal | Normal | Nod |
| I12 | F | 49 | Normal | Normal | Normal | Normal | Nod |
| I13 | M | 49 | Normal | Normal | Normal | Normal | No |
| I14 | M | 46 | Normal | Normal | Normal | Inferior basal hypokinesis | Yes (basal inferior hypokinesis) |
| I15 | M | 28 | Normal | Normal | Normal | Normal | No |
| I16 | F | 40 | Normal | Normal | Normal | Normal | No |
| I17 | M | 35 | Normal | Normal | Normal | Normal | No |
| I18 | M | 45 | Normal | Normal | Normal | Normal | No |
| I19 | F | 33 | Normal | Normal | Normal | Normal | No |
| I20 | M | 43 | Repolarization abnormalities | Repolarization abnormalities | Normal | MLAD | Yes (not related with Chagas disease) |
LAFB, left anterior fascicular block; LVH, left ventricular hypertrophy; LVSD, left ventricular systolic diameter; MMI, mild mitral insufficiency; MLAD, mild left auricular dilatation; RRBB, complete right bundle branch block; VA, ventricular extrasystoles.
Disease progression was defined by the development of new electrocardiographic or echocardiographic alterations related to Chagas disease.
F, female; M, male.
Patient with arterial hypertension prior to treatment.
PCR monitoring.
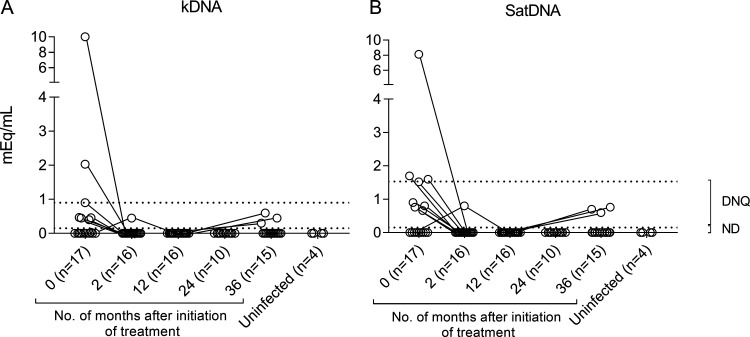
Fourteen of the 17 (82%) patients showed no detectable T. cruzi DNA either by DNA minicircle kinetoplastid (kDNA) (Fig. 1A) or nuclear DNA satellite sequence (SatDNA) (Fig. 1B) quantitative PCR (qPCR) methods at the end of follow-up, while three (18%) patients had positive qPCR results (Fig. 1A and B). The only patient who showed positive qPCR findings at the end of treatment had no detectable results by either qPCR method from 12 months until the end of posttreatment follow-up (Fig. 1A and B). During posttreatment follow-up, all positive qPCR samples gave parasitic loads below the limit of quantification for both qPCR methods. One of the seven patients with no detectable qPCR findings at baseline had positive results at the end of follow-up, while the remaining six patients showed no detectable qPCR results throughout the posttreatment follow-up. Fourteen out of the 15 subjects monitored until 36 months posttreatment had at least three samples tested during the follow-up period. As a whole, the proportion of subjects with positive kDNA/SatDNA qPCR of the total evaluated significantly decreased over time posttreatment (i.e., time zero [t0], 10/17, versus t2, 1/16, P = 0.0014; versus t12, 0/16, P= 0.0002; versus t24, 0/10, P < 0.0001; versus t36, 3/15, P = 0.025).
FIG 1.
Monitoring of parasitological response following intermittent administration of BZ. Blood samples collected prior to treatment and at several posttreatment time points were analyzed by T. cruzi kDNA and SatDNA qPCR assays. Blood samples of uninfected subjects were tested as controls. Each circle represents the maximum qPCR value for each patient at each time point. Dotted lines represent the limit of detection of each qPCR method, 0.90 and 1.53 par. eq./ml for kDNA and SatDNA qPCRs, respectively. ND, not detectable; DNQ, detectable but nonquantifiable.
T. cruzi-specific humoral response following intermittent administration of BZ.
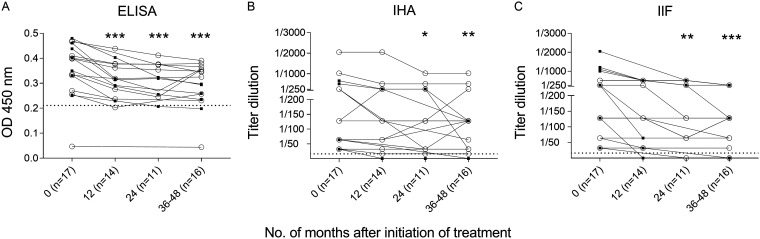
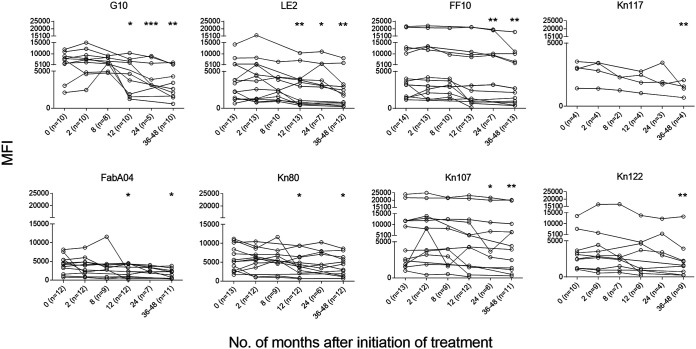
The levels of T. cruzi-specific antibodies measured by conventional techniques significantly declined 12 months following treatment by enzyme-linked immunosorbent assay (ELISA) (Fig. 2A) and at 24 months posttreatment by indirect hemagglutination assay (IHA) (Fig. 2B) and indirect immunofluorescence test (IIF) (Fig. 2C). As defined in Materials and Methods, on an individual basis, seven of 16 (43.75%) patients had a decrease in the levels of T. cruzi-specific antibodies by ELISA (Fig. 2A), four of 17 (23.53%) by IHA (Fig. 2B), and seven of 17 (42.18%) by IIF (Fig. 2C). The multiplex assay to measure antibodies against a set of 10 T. cruzi-derived recombinant proteins was conducted in 14 of the 17 patients treated with intermittent BZ. Twelve of the 14 (85.71%) patients with baseline reactive serum by the Luminex-based multiplex assay showed significant decreases in the reactivity to one or more proteins following intermittent administration of BZ (Fig. 3; see also Fig. S1 in the supplemental material).
FIG 2.
Monitoring of T. cruzi-specific antibodies by conventional serological tests following intermittent treatment with BZ. T. cruzi-specific antibodies, as determined by ELISA, IHA, and IIF, were measured prior to treatment and at different time points after completion of BZ administration. Each open circle represents the data for single subjects. Broken horizontal lines show the reactivity threshold for each serological test. ***, P < 0.001; **, P < 0.01; *, P < 0.05 versus pretreatment levels (time zero) by ANOVA for repeated measures after log transformation of the IHA and IIF data. Black square symbols indicate decreased reactivity compared with baseline reactivity, as defined in Materials and Methods. OD 450 nm, optical density at 450 nm.
FIG 3.
T. cruzi-specific humoral response measured by multiplex assay in chronic Chagas disease patients after intermittent administration of BZ. Plots exhibit representative data for single subjects for the different proteins assessed. Each point represents the mean fluorescence intensity (MFI) for reactive proteins out of 10 assessed, analyzed both prior to treatment (time zero) and at several posttreatment time points. ***, P < 0.001; **, P < 0.01; *, P < 0.05 versus pretreatment levels (time zero) by ANOVA for repeated measures.
Monitoring of inflammatory cytokines.
Serum levels of interleukin-8 (IL-8), gamma interferon (IFN-γ)-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), monokine induced by IFN-γ (MIG), and RANTES were measured prior to and after a median period of 36 months post intermittent administration with benznidazole. Prior to treatment, MCP-1 levels were increased, and MIG levels were decreased in T. cruzi-infected subjects compared with those of uninfected subjects (Fig. 4). These alterations in chemokine levels reverted following drug therapy, with an increase in MCP-1 levels and a decline in MIG levels. No significant changes were observed in serum levels of IL-8, IP-10, or RANTES (P > 0.05).
FIG 4.
Serum levels of MCP-1 and MIG in chronic Chagas disease patients treated with intermittent doses of BZ. A cytometric bead array was used to measure the concentrations of chemokines in the sera of subjects with chronic T. cruzi infection (circles) at different time points following intermittent administration of BZ and in uninfected subjects (triangles). Changes from baseline (time zero) were assessed using ANOVA for repeated measures. ** and ≠≠, P < 0.01 versus baseline (time zero); *, P < 0.05 versus baseline. The horizontal line in uninfected subjects shows the median values.
DISCUSSION
Most pharmacokinetic and pharmacodynamic studies have shown that patients with lower plasma BZ levels can achieve an appropriate parasitological response (15), even among those who cannot complete treatment due to adverse events (15, 16). Here, we report that the parasitological response in a group of patients treated with BZ administered every 5 days and monitored for a median period of 3 years was in the same range as that observed with the standard daily dose (4, 17, 18). In a 7-year follow-up study of chronic Chagas disease patients living in an area where T. cruzi is not endemic and treated with BZ as monotherapy, the rate of parasitological response measured by kDNA qPCR was 90% (18) measured 1 year after treatment. A lower rate of parasitological response was observed in the BENEFIT (Benznidazole Evaluation For Interrupting Trypanosomiasis Trial) clinical trial, in which chronic Chagas disease patients with cardiomyopathy received BZ monotherapy, with 55.4% of patients with negative qPCR findings at 2 years posttreatment and 46.7% at 5 or more years (17). In another study assessing the efficacy of three oral E1224 (a water-soluble ravuconazole prodrug) regimens and benznidazole versus placebo in adult chronic indeterminate Chagas disease, the rate of parasitological response with BZ was 82% at 12 months posttreatment by SatDNA qPCR (4).
In our study, one patient presented early therapeutic failure at the end of treatment but achieved no detectable PCR results at later time points. Of the remaining 16 patients, 13 had no detectable parasite DNA throughout the posttreatment follow-up period by either qPCR method, and three showed detectable PCR at the end of follow-up. These findings, and concordant results from other authors showing a high rate of undetectable levels by PCR after 1 year posttreatment (3, 4, 18), raise the question of the optimal length of time required to assess treatment efficacy. It is worth noting that, in addition to implementing a longer follow-up period, we applied two qPCR methods that use two different molecular targets, as recommended for confirmatory purposes (19, 20), and the sample analysis was performed in two different laboratories. We observed 6% discordancy between the two qPCR methods and 6% discordancy between the laboratories. Discordancy occurred between samples in which the parasitic loads were near the limit of detection of the corresponding methods. This can be expected, since qPCR precision diminishes at low parasitic loads (21). One limitation of this study is that the patients were not monitored during the same time interval, and as a consequence, the number of follow-up samples was different among patients.
Recently, nonreplicating intracellular T. cruzi amastigotes, considered dormant, were demonstrated to be resistant to extended drug treatment in vivo and in vitro and could reestablish a growing infection after drug exposure (22). Dormancy could be a factor for treatment failure, since BZ requires metabolic activation to exert its trypanocidal effect. Therefore, it is important to keep the plasma concentrations of the drug within the accepted therapeutic range and to guarantee that all dormant parasites are eliminated (12, 22). The fact that intermittent BZ administration allowed a 5-fold reduction of the standard daily dose of 5 mg/kg of body weight/day for 60 days (5) opens the possibility of extending the length of drug administration, eventually targeting dormant parasites that might reinitiate replication (22). It is also likely that the lower frequency of dosing every 5 days avoids the accumulation of toxic metabolites of BZ, thereby reducing the severity of adverse events (14).
The efficacy of intermittent BZ administration was also reflected by the decrease in T. cruzi-specific antibodies, either by conventional serologic tests or the multiplex assay, to the same extent as that observed in our previous study with daily BZ doses for 30 days (23). Although 36 months is a short period of time to assess disease progression, it is worth noting that the two patients who showed echocardiographic changes during follow-up had presented cardiac alterations prior to treatment. Of note, one of these patients had arterial hypertension, which is one of the most frequent comorbidities (24, 25), and a risk factor for heart failure (26), in chronic Chagas disease.
Chronic T. cruzi infection leads to an inflammatory process that keeps the parasite under control but can also induce tissue damage. Patients with chronic Chagas disease with cardiac involvement show a higher level of proinflammatory cytokines than patients without cardiac disease (27). Moreover, T. cruzi-infected patients with chronic heart disease and arterial hypertension have increased plasma levels of proinflammatory cytokines compared with those of patients without hypertension (28). Chemokines have been identified as regulators of leukocyte trafficking during the different phases of both innate and adaptive immune responses (29). MCP-1, which participates in the recruitment of monocytes, memory T cells, and dendritic cells, exerts its effects through binding to G-protein-coupled receptors on the surface of activated leukocytes. The decreased levels of MCP-1 found in untreated T. cruzi-infected subjects compared with uninfected controls may reflect an increased consumption of this chemokine during chronic infection (30), while the restoration of its levels following treatment reflects a decrease in leukocyte activation. In agreement with these findings, a decrease in macrophage activation following treatment with BZ has been reported (31, 32). In contrast, serum levels of MIG, which induces migration of activated T cells (33), decreased after the intermittent administration of BZ compared to pretreatment levels. This result is consistent with the early decrease of IFN-γ-producing cells observed in T. cruzi-infected subjects treated with the standard BZ scheme that can be followed by a reemergence of polyfunctional IFN-γ-producing cells (34–37). In summary, the findings of this pilot study provide a basis for further exploration of treatment schemes with intermittent administration of BZ.
MATERIALS AND METHODS
Study population, etiological treatment, and clinical follow-up.
Seventeen adult patients with confirmed chronic Chagas disease (i.e., positive findings on at least two of the three serological tests, including enzyme-linked immunosorbent assay [ELISA], hemagglutination [IHA], and immunofluorescence [IIF]), aged between 28 and 57 (median, 45) years, were included (Table 1). All subjects recruited in the study provided informed consent. Patients with a history or laboratory findings compatible with liver or kidney disease, blood dyscrasia, or concomitant systemic illnesses were excluded from the study. Other exclusion criteria were previous etiological treatment, pregnancy or presumption of failure in contraception during the treatment period, and location of residence that could interfere with patient participation in the study. Since arterial hypertension is a very frequent comorbidity in patients with chronic Chagas disease in our health center, this factor was not considered an exclusion criterion. BZ was administered in intermittent doses of 5 mg/kg/day, divided into two daily doses every 5 days, with a total of 12 doses, as previously reported (14). A baseline electrocardiogram (ECG) and a two-dimensional echocardiogram were performed to stratify the patients according to the presence or absence of cardiomyopathy (Table 1). After treatment, ECG and echocardiogram were performed yearly. Blood samples were taken prior to treatment, 1 week after the end of treatment, at 12 months posttreatment, and yearly thereafter up to 48 months posttreatment follow-up. The median time of follow-up was 36 months (range, 12 to 48 months), and the median number of samples taken per patient was five (range, two to seven samples). The study was approved by the Committee for Research and Bioethics of the Hospital Eva Peron. The latter is enrolled in the Provincial Registry for Ethics Committees, accredited by the Central Ethics Committee, Ministry of Health, Buenos Aires, Argentina, dated 17 September 2010 under number 18/2010, page 54, of the Minutes Book No. 1.
Assessment of qPCR for T. cruzi.
Five milliliters of whole blood was mixed with an equal volume of 6 M guanidine hydrochloride buffer containing 0.2 M EDTA at pH 8.00 (GEB). After 48 to 72 h at room temperature, GEB samples were boiled at 100°C for 15 min and stored at 4°C until DNA extraction and subsequent analysis by qPCR. GEB samples were centralized, codified for distribution in aliquots, and sent to the two laboratories that performed the qPCR assay without knowledge of clinical data or sampling time. Each laboratory analyzed the samples in duplicate by two qPCR methods based on TaqMan technology, one directed to the conservative region of the DNA minicircle kinetoplastid (kDNA) and the other to the nuclear DNA satellite sequence (SatDNA). PCR findings were considered positive if T. cruzi DNA was detectable in both laboratories by at least one qPCR assay. DNA extraction and both qPCR methods were carried out as previously described (21). The limit of quantification was 0.90 par. eq./ml (parasite equivalents per milliliter of blood) and 1.53 par. eq./ml for the kDNA and SatDNA qPCRs, respectively (21). To minimize bias, blinded control samples from seropositive and seronegative subjects were run in parallel with the study samples.
Measurement of T. cruzi-specific antibodies.
Serum specimens were screened for the presence of T. cruzi-specific antibodies by conventional serological tests (38) and by a Luminex-based assay, as previously described (39). All samples were processed simultaneously by the same technician and with the same reagent lots. To determine the serological treatment response in individual patients, conversion to negative findings in at least 2 of 3 conventional serologic tests, a 30% reduction in ELISA titers, and a 2-fold dilution by IHA or IIF were considered significant declines in T. cruzi-specific antibodies, as previously reported (23, 34). Likewise, in the multiplex assay, the reduction of serological response to each individual T. cruzi protein was considered significant if the mean fluorescence intensity declined by 50% relative to that of a pretherapy sample assessed concurrently (35).
CBA.
Cytometric bead array (CBA) assays with serum samples were conducted for IL-8, IFN-γ-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), monokine induced by gamma interferon (MIG), and regulated on activation, normal T cell expressed and secreted (RANTES) according to the manufacturer’s instructions (BD Biosciences Franklin Lakes, NJ, USA). The samples were acquired on a FACSCalibur flow cytometer and were analyzed using FCAP Software v1.4 (BD, Franklin Lakes, NJ).
Statistical analysis.
The normality of the variable distribution was assessed using the Kolmogorov-Smirnov criterion. Data from descriptive statistics, such as the proportions of the total and percentages and medians, were determined as appropriate. Qualitative pre- and posttreatment PCR findings were compared by the McNemar test. Differences in chemokine levels between T. cruzi-infected and uninfected subjects were evaluated by the Mann-Whitney U-test. Changes in T. cruzi-specific antibodies and chemokine levels during posttreatment follow-up were evaluated with an analysis of variance (ANOVA) for repeated measures with the available data. To analyze changes in the levels of T. cruzi-specific antibodies measured by IHA and IIF, the ANOVA for repeated measures was performed after log transformation of the data. Statistical analysis was conducted using Statistix v8.0 (Analytical Software, Tallahassee, FL, USA) and GraphPad Prism v8.0.1 (GraphPad Software, San Diego, CA, USA).
Supplementary Material
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.WHO. 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90:33–43. [PubMed] [Google Scholar]
- 2.Molina I, Goḿez I, Prat J, Salvador F, Treviño B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio L, Blanco-Grau A, Sánchez-Montalv́a A, Vidal X, Pahissa A. 2014. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med 370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 3.Morillo CA, Waskin H, Sosa-Estani S, del Carmen Bangher M, Cuneo C, Milesi R, Mallagray M, Apt W, Beloscar J, Gascon J, Molina I, Echeverria LE, Colombo H, Perez-Molina JA, Wyss F, Meeks B, Bonilla LR, Gao P, Wei B, McCarthy M, Yusuf S, Morillo C, Sosa-Estani S, Waskin H, Meeks B, Yusuf S, Diaz R, Acquatella H, Lazzari J, Roberts R, Traina M, Meeks B, Bonilla LR, Gao P, Taylor A, Holadyk-Gris I, Whalen L, Bangher MC, Romero MA, Prado N, Hernández Y, Fernandez M, Riarte A, Scollo K, Lopez-Albizu C, Cuneo CA, Gutiérrez NC, Milesi RR, Berli MA, Mallagray MH, Cáceres NE, Beloscar JS, Petrucci JM, Colombo H, Dellatorre M, Prado A, Apt W, Zulantay I, Echeverría LE, Isaza D, Reyes E, Wyss FS, Figueroa A, Guzmán Melgar I, Rodríguez E, Gascon J, Aldasoro E, Posada EJ, Serret N, Molina I, Sánchez-Montalvá A, Perez-Molina JA, López-Vélez R, Reyes-López PA. 2017. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers: the STOP-CHAGAS Trial. J Am Coll Cardiol 69:939–947. doi: 10.1016/j.jacc.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Torrico F, Gascon J, Ortiz L, Alonso-Vega C, Pinazo MJ, Schijman A, Almeida IC, Alves F, Strub-Wourgaft N, Ribeiro I, Santina G, Blum B, Correia E, Garcia-Bournisen F, Vaillant M, Morales JR, Pinto Rocha JJ, Rojas Delgadillo G, Magne Anzoleaga HR, Mendoza N, Quechover RC, Caballero MYE, Lozano Beltran DF, Zalabar AM, Rojas Panozo L, Palacios Lopez A, Torrico Terceros D, Fernandez Galvez VA, Cardozo L, Cuellar G, Vasco Arenas RN, Gonzales I, Hoyos Delfin CF, Garcia L, Parrado R, de la Barra A, Montano N, Villarroel S, Duffy T, Bisio M, Ramirez JC, Duncanson F, Everson M, Daniels A, Asada M, Cox E, Wesche D, Diderichsen PM, Marques AF, Izquierdo L, Sender SS, Reverter JC, Morales M, Jimenez W. 2018. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: a proof-of-concept, randomised, placebo-controlled trial. Lancet Infect Dis 18:419–430. doi: 10.1016/S1473-3099(17)30538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarim CB, Jornada DH, Machado MGM, Ferreira CMR, dos Santos JL, Chung MC. 2019. Thiazole, thio and semicarbazone derivatives against tropical infective diseases: Chagas disease, human African trypanosomiasis (HAT), leishmaniasis, and malaria. Eur J Med Chem 162:378–395. doi: 10.1016/j.ejmech.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Armenti A. 2009. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther 7:157–163. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- 7.Bern C, Montgomery SP, Herwaldt BL, Rassi A, Marin-Neto JA, Dantas RO, Maguire JH, Acquatella H, Morillo C, Kirchhoff LV, Gilman RH, Reyes PA, Salvatella R, Moore AC. 2007. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA 298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- 8.Barclay CA, Cerisola JA, Lugones H, Ledesma O, Lopez Silva JMG. 1978. Aspectos farmacológicos y resultados terapéuticos del benznidazol en el tratamiento de la infección chagásica. La Prensa Médica Argentina 65:239–244. [Google Scholar]
- 9.Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, Postan M, Armenti A. 2006. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med 144:724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 10.De Suasnábar DF, Arias E, Streiger M, Piacenza M, Ingaramo M, Del Barco M, Amicone N. 2000. Evolutive behavior towards cardiomyopathy of treated (nifurtimox or benznidazole) and untreated chronic chagasic patients. Rev Inst Med Trop Sao Paulo 42:99–109. doi: 10.1590/s0036-46652000000200007. [DOI] [PubMed] [Google Scholar]
- 11.Altcheh J, Moscatelli G, Mastrantonio G, Moroni S, Giglio N, Marson ME, Ballering G, Bisio M, Koren G, García-Bournissen F. 2014. Population pharmacokinetic study of benznidazole in pediatric Chagas disease suggests efficacy despite lower plasma concentrations than in adults. PLoS Negl Trop Dis 8:e2907. doi: 10.1371/journal.pntd.0002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soy D, Aldasoro E, Guerrero L, Posada E, Serret N, Mejía T, Urbina JA, Gascón J. 2015. Population pharmacokinetics of benznidazole in adult patients with Chagas disease. Antimicrob Agents Chemother 59:3342–3349. doi: 10.1128/AAC.05018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bustamante JM, Craft JM, Crowe BD, Ketchie SA, Tarleton RL. 2014. New, combined, and reduced dosing treatment protocols cure Trypanosoma cruzi infection in mice. J Infect Dis 209:150–162. doi: 10.1093/infdis/jit420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Álvarez MG, Hernández Y, Bertocchi G, Fernández M, Lococo B, Ramírez JC, Cura C, Albizu CL, Schijman A, Abril M, Sosa-Estani S, Viotti R. 2016. New scheme of intermittent benznidazole administration in patients chronically infected with Trypanosoma cruzi: a pilot short-term follow-up study with adult patients. Antimicrob Agents Chemother 60:833–837. doi: 10.1128/AAC.00745-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández ML, Marson ME, Ramirez JC, Mastrantonio G, Schijman AG, Altcheh J, Riarte AR, Bournissen FG. 2016. Pharmacokinetic and pharmacodynamic responses in adult patients with Chagas disease treated with a new formulation of benznidazole. Mem Inst Oswaldo Cruz 111:218–221. doi: 10.1590/0074-02760150401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Álvarez MG, Vigliano C, Lococo B, Petti M, Bertocchi G, Viotti R. 2012. Seronegative conversion after incomplete benznidazole treatment in chronic Chagas disease. Trans R Soc Trop Med Hyg 106:636–638. doi: 10.1016/j.trstmh.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ, Yusuf S. 2015. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med 373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 18.Murcia L, Carrilero B, Ferrer F, Roig M, Franco F, Segovia M. 2016. Success of benznidazole chemotherapy in chronic Trypanosoma cruzi-infected patients with a sustained negative PCR result. Eur J Clin Microbiol Infect Dis 35:1819–1827. doi: 10.1007/s10096-016-2733-6. [DOI] [PubMed] [Google Scholar]
- 19.Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Mejia Jaramillo AM, Cura C, Auter F, Veron V, Qvarnstrom Y, Deborggraeve S, Hijar G, Zulantay I, Lucero RH, Velazquez E, Tellez T, Leon ZS, Galvão L, Nolder D, Rumi MM, Levi JE, Ramirez JD, Zorrilla P, Flores M, Jercic MI, Crisante G, Añez N, de Castro AM, Gonzalez CI, Viana KA, Yachelini P, Torrico F, Robello C, Diosque P, Chavez OT, Aznar C, Russomando G, Büscher P, Assal A, Guhl F, Estani SS, DaSilva A, Britto C, Luquetti A, Ladzins J. 2011. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis 5:e931. doi: 10.1371/journal.pntd.0000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cura CI, Ramírez JC, Rodríguez M, Lopez-Albízu C, Irazu L, Scollo K, Sosa-Estani S. 2017. Comparative study and analytical verification of PCR methods for the diagnosis of congenital Chagas disease. J Mol Diagn 19:673–681. doi: 10.1016/j.jmoldx.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Ramírez JC, Cura CI, Da Cruz Moreira O, Lages-Silva E, Juiz N, Velázquez E, Ramírez JD, Alberti A, Pavia P, Flores-Chávez MD, Muñoz-Calderón A, Pérez-Morales D, Santalla J, Marcos Da Matta Guedes P, Peneau J, Marcet P, Padilla C, Cruz-Robles D, Valencia E, Crisante GE, Greif G, Zulantay I, Costales JA, Alvarez-Martínez M, Martínez NE, Villarroel R, Villarroel S, Sánchez Z, Bisio M, Parrado R, Maria Da Cunha Galvão L, Da Câmara ACJ, Espinoza B, De Noya BA, Puerta C, Riarte A, Diosque P, Sosa-Estani S, Guhl F, Ribeiro I, Aznar C, Britto C, Yadón ZE, Schijman AG. 2015. Analytical validation of quantitative real-time PCR methods for quantification of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. J Mol Diagn 17:605–615. doi: 10.1016/j.jmoldx.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Valdéz FJ, Padilla A, Wang W, Orr D, Tarleton RL. 2018. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. Elife 7:e34039. doi: 10.7554/eLife.34039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viotti R, Vigliano C, Alvarez MG, Lococo B, Petti M, Bertocchi G, Armenti A, De Rissio AM, Cooley G, Tarleton R, Laucella S. 2011. Impact of aetiological treatment on conventional and multiplex serology in chronic Chagas disease. PLoS Negl Trop Dis 5:e1314. doi: 10.1371/journal.pntd.0001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alves R, Thomaz RP, De Almeida EA, Wanderley JDS, Guariento ME. 2009. Chagas’ disease and ageing: the coexistence of other chronic diseases with Chagas’ disease in elderly patients. Rev Soc Bras Med Trop 42:622–628. doi: 10.1590/s0037-86822009000600002. [DOI] [PubMed] [Google Scholar]
- 25.Mordini OD, Bavio E, Beloscar J, Tognoni G, Sosa FJ, Reyes O, Pairone E, Lacunza D, Manzur R, Redondo M, Hernández D, Mujica H, Olavegogeoescoechea P, Antero A, María CA, Susana C, Lucía V, Mariana G, Beatriz JM, Patricia M, Verónica P, Janet G, Doris P, Carina A, Susana M, Belén M, Marcelo O, Georgina P, Emilia A, Lumila L, Osvaldo R, Del Carmen BM, Federico NB, Victorino F, Luis SJ, Martin V, Graciela S, Marcelo S, Héctor C, Luisa G, Jorge M, Karina P, Farez B, Angel AM, Antonio A, Virginia B, Néstor C, Fernando P, Jorge P, María B, Marianela G, Altina E, Pezzotto S. 2016. Chagas disease in Argentina. National Registry of Chagas Disease of the Federación Argentina de Cardiología. RENECH Study. Rev la Fed Argentina Cardiol 45:84–92. [Google Scholar]
- 26.Guariento ME, Ramos MC, Gontijo JA, Carvalhal SDS. 1993. Chagas disease and primary arterial hypertension. Arq Bras Cardiol 60:71–75. [PubMed] [Google Scholar]
- 27.Poveda C, Fresno M, Gironès N, Martins-Filho OA, Ramírez JD, Santi-Rocca J, Marin-Neto JA, Morillo CA, Rosas F, Guhl F. 2014. Cytokine profiling in Chagas disease: towards understanding the association with infecting Trypanosoma cruzi discrete typing units (a benefit trial sub-study). PLoS One 9:e91154. doi: 10.1371/journal.pone.0091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bestetti RB, Dellalibera-Joviliano R, Lopes GS, Faria-Jr M, Furlan-Daniel R, Lopes KC, Batista DR. 2019. Determination of the Th1, Th2, Th17, and Treg cytokine profile in patients with chronic Chagas heart disease and systemic arterial hypertension. Heart Vessels 34:123–133. doi: 10.1007/s00380-018-1228-z. [DOI] [PubMed] [Google Scholar]
- 29.Ridiandries A, Tan JTM, Bursill CA. 2018. The role of chemokines in wound healing. Int J Mol Sci 19:3217. doi: 10.3390/ijms19103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav A, Saini V, Arora S. 2010. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta 411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Marañón C, Egui A, Fernández-Villegas A, Carrilero B, Thomas MC, Segovia M, López MC. 2013. Benznidazole treatment reduces the induction of indoleamine 2,3-dioxygenase (IDO) enzymatic activity in Chagas disease symptomatic patients. Parasite Immunol 35:180–187. doi: 10.1111/pim.12030. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Mazliah DE, Alvarez MG, Cooley G, Lococo BE, Bertocchi G, Petti M, Albareda MC, Armenti AH, Tarleton RL, Laucella SA, Viotti R. 2013. Sequential combined treatment with allopurinol and benznidazole in the chronic phase of Trypanosoma cruzi infection: a pilot study. J Antimicrob Chemother 68:424–437. doi: 10.1093/jac/dks390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groom JR, Luster AD. 2011. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albareda MC, Natale MA, De Rissio AM, Fernandez M, Serjan A, Alvarez MG, Cooley G, Shen H, Viotti R, Bua J, Castro Eiro MD, Nuñez M, Fichera LE, Lococo B, Scollo K, Tarleton RL, Laucella SA. 2018. Distinct treatment outcomes of antiparasitic therapy in Trypanosoma cruzi-infected children are associated with early changes in cytokines, chemokines, and T-cell phenotypes. Front Immunol 9:1958. doi: 10.3389/fimmu.2018.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laucella SA, Mazliah DP, Bertocchi G, Alvarez MG, Cooley G, Viotti R, Albareda MC, Lococo B, Postan M, Armenti A, Tarleton RL. 2009. Changes in Trypanosoma cruzi-specific immune responses after treatment: surrogate markers of treatment efficacy. Clin Infect Dis 49:1675–1684. doi: 10.1086/648072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez MG, Bertocchi GL, Cooley G, Albareda MC, Viotti R, Perez-Mazliah DE, Lococo B, Castro Eiro M, Laucella SA, Tarleton RL. 2016. Treatment success in Trypanosoma cruzi infection is predicted by early changes in serially monitored parasite-specific T and B cell responses. PLoS Negl Trop Dis 10:e0004657. doi: 10.1371/journal.pntd.0004657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mateus J, Pérez-Antón E, Lasso P, Egui A, Roa N, Carrilero B, González JM, Thomas MC, Puerta CJ, López MC, Cuéllar A. 2017. Antiparasitic treatment induces an improved CD8+ T cell response in chronic Chagasic patients. J Immunol 198:3170–3180. doi: 10.4049/jimmunol.1602095. [DOI] [PubMed] [Google Scholar]
- 38.WHO. 2012. Research priorities for Chagas disease, human African trypanosomiasis and leishmaniasis. World Health Organ Tech Rep Ser 975:1–100. [PubMed] [Google Scholar]
- 39.Cooley G, Etheridge RD, Boehlke C, Bundy B, Weatherly DB, Minning T, Haney M, Postan M, Laucella S, Tarleton RL. 2008. High throughput selection of effective serodiagnostics for Trypanosoma cruzi infection. PLoS Negl Trop Dis 2:e316. doi: 10.1371/journal.pntd.0000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.