Presentation of Case
Dr. Meridale V. Baggett (Medicine): A 60-year-old woman presented to this hospital with altered mental status during the pandemic of coronavirus disease 2019 (Covid-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The patient had been in her usual state of health until 1 week before admission, when cough and headache developed. One day before admission, vomiting and diarrhea developed. That day, the patient was last seen by her husband at 3:00 p.m. before she went to sleep. The husband reported that the patient awoke screaming at 1:00 a.m. on the day of admission, and emergency medical services (EMS) were called. When EMS personnel arrived, the patient was reportedly lethargic and unable to answer questions. The blood glucose level, obtained by fingerstick testing, was 60 mg per deciliter (3.3 mmol per liter; reference range, 70 to 110 mg per deciliter [3.9 to 6.1 mmol per liter]). Intravenous dextrose was administered. A repeat fingerstick glucose measurement was 127 mg per deciliter (7.0 mmol per liter), and the lethargy abated. The patient was brought by EMS personnel to the emergency department of this hospital.
In the emergency department, the patient reported weakness on the left side of her body and persistent headache. She reported that when she had awoken at 1:00 a.m., she was unable to move or speak, was afraid she was going to die, and began screaming for help. She reported no aura, urinary or fecal incontinence, or neck pain. She had recently moved from Puerto Rico and lived in a suburb of Boston. She did not speak English and was marginally housed, sleeping in the living room of a friend’s apartment with her husband. Several people living in the apartment had had cough and fever.
The patient had a history of diabetes mellitus, hypertension, and schizophrenia. Medications included aspirin, glipizide, hydrochlorothiazide, lisinopril, and risperidone. She had no known drug allergies. She drank alcohol occasionally and did not smoke tobacco or use illicit drugs.
On examination, the temperature was 37.4°C, the blood pressure 114/62 mm Hg, the heart rate 94 beats per minute, the respiratory rate 18 breaths per minute, and the oxygen saturation 94% while the patient was breathing ambient air; the body-mass index (the weight in kilograms divided by the square of the height in meters) was 30.5. The patient was alert and interactive. She was oriented to person and place but not to time. The cranial-nerve examination was normal. Strength in the left arm and left leg was 4/5, and strength in the right arm and right leg was 5/5. She had difficulty manipulating small objects placed in the left hand. The left arm showed pronator drift and orbiting. Sensation to light touch was intact. The remainder of the physical examination was normal.
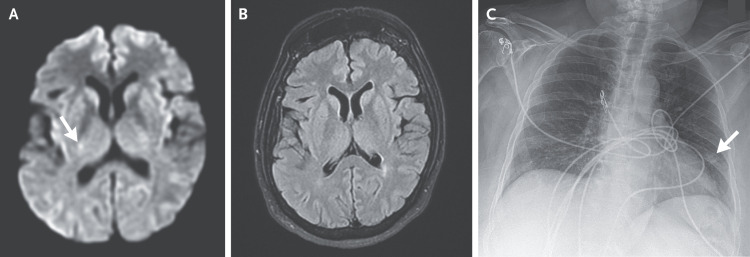
Dr. R. Gilberto Gonzalez: Magnetic resonance imaging (MRI) of the head was performed 2 hours after presentation. Diffusion-weighted images showed a punctate focus of restricted diffusion within the posterior limb of the right internal capsule; no corresponding abnormality was observed on fluid-attenuated inversion recovery images (Figure 1A and 1B). A chest radiograph (Figure 1C) obtained 2.5 hours after presentation showed a nodular focus in the left midlung field.
Figure 1. Imaging Studies of the Head and Chest.
MRI was performed 2 hours after presentation. A diffusion-weighted image (Panel A) shows a punctate focus of restricted diffusion within the posterior limb of the right internal capsule (arrow). No corresponding abnormality is observed on a T2-weighted fluid-attenuated inversion recovery image (Panel B). A chest radiograph (Panel C) obtained 2.5 hours after presentation shows a nodular focus in the left midlung field (arrow).
Dr. Baggett: A test of a nasopharyngeal swab for SARS-CoV-2 RNA was positive. Laboratory test results are shown in Table 1. The results of urine toxicologic screening were normal. Treatment decisions were made, and the patient was admitted to the hospital.
Table 1. Laboratory Data.*.
| Variable | Reference Range, Adults† |
On Admission |
|---|---|---|
| Hematocrit (%) | 36–46 | 36.7 |
| Hemoglobin (g/dl) | 12–16 | 12.1 |
| White-cell count (per μl) | 4500–11,000 | 10,520 |
| Differential count (per μl) | ||
| Neutrophils | 1800–7700 | 9080 |
| Lymphocytes | 1000–4800 | 700 |
| Monocytes | 200–1200 | 700 |
| Eosinophils | 0–900 | 0 |
| Platelet count (per μl) | 150,000–400,000 | 279,000 |
| Prothrombin time (sec) | 11.5–14.5 | 13.0 |
| Prothrombin-time international normalized ratio | 0.9–1.1 | 1.0 |
| d-dimer (ng/ml) | <500 | 1263 |
| Sodium (mmol/liter) | 135–145 | 137 |
| Potassium (mmol/liter) | 3.4–4.8 | 4.2 |
| Chloride (mmol/liter) | 100–108 | 98 |
| Carbon dioxide (mmol/liter) | 23.0–31.9 | 24 |
| Urea nitrogen (mg/dl) | 8–25 | 56 |
| Creatinine (mg/dl) | 0.60–1.50 | 1.87 |
| Glucose (mg/dl) | 70–110 | 115 |
| Ferritin (μg/liter) | 20–300 | 450 |
| Lactate dehydrogenase (U/liter) | 110–210 | 278 |
| C-reactive protein (mg/liter) | <8 | 49.5 |
| Creatine kinase (U/liter) | 60–400 | 198 |
To convert the values for urea nitrogen to millimoles per liter, multiply by 0.357. To convert the values for creatinine to micromoles per liter, multiply by 88.4. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
Reference values are affected by many variables, including the patient population and the laboratory methods used. The ranges used at Massachusetts General Hospital are for adults who are not pregnant and do not have medical conditions that could affect the results. They may therefore not be appropriate for all patients.
Differential Diagnosis
Dr. Aneesh B. Singhal: This 60-year-old woman with hypertension, diabetes mellitus, and schizophrenia presented to this hospital with acute psychosis and focal neurologic deficits on the left side during the Covid-19 pandemic. One week earlier, she had begun to have headache and cough, and more recently, vomiting and diarrhea had developed; an initial evaluation confirmed the diagnosis of SARS-CoV-2 infection. Although infection with SARS-CoV-2 provides an explanation for her prodromal symptoms, could this patient’s acute psychosis and weakness on the left side also be associated with Covid-19? Because our understanding of the clinical manifestations of SARS-CoV-2 infection is still evolving, it is important to consider other causes that may be contributing to her presentation.
Brief Psychosis
Could this patient’s abrupt onset of screaming from the fear of dying and an inability to move indicate an episode of brief psychosis? Brief psychosis is usually precipitated by extreme life stress and can be a component of schizophrenia.1 In this patient with schizophrenia, factors such as social isolation, insomnia, a language barrier, fear of the development of Covid-19, and housing insecurity may have triggered psychosis.2 However, brief psychosis associated with schizophrenia typically lasts more than 1 day, so it is unlikely to account for her altered mental status.
Hypoglycemia, which can manifest as psychosis,3 should be considered in this case, given the patient’s development of lethargy and subsequent decrease in symptoms after the administration of dextrose. However, her glucose level was 60 mg per deciliter, and symptoms of hypoglycemia usually develop when the level drops below 45 mg per deciliter (2.5 mmol per liter). Other metabolic or toxic encephalopathies can be ruled out on the basis of her prompt cognitive improvement and negative results of toxicologic screening.
Seizures can cause psychosis and may resemble schizophrenia.4 However, this patient had no history of seizures, and Covid-19 does not appear to increase the risk of seizure.5-7
Neuropsychiatric Manifestations of Covid-19
Can this patient’s apparent episode of psychosis be a manifestation of encephalitis associated with SARS-CoV-2 infection, resulting either from direct infection or from an immune, inflammatory, cytokine, or neural receptor–mediated mechanism?8 Coronaviruses primarily affect the lungs but have widespread systemic and cerebral effects. Neuropsychiatric symptoms have been associated with other coronavirus infections; hence, an association with Covid-19 would not be surprising.6,9 There is a long association between viral pandemics and psychiatric symptoms such as acute psychosis, delirium, insomnia, mania, depression, and suicidality. The medical literature from the time of the Russian (1889–1890) and Spanish (1918–1920) influenza pandemics is replete with reports of fearful experiences (e.g., “psychoses of influenza” and “influenza nervosa”).10 Similarly, von Economo’s encephalitis (encephalitis lethargica) (1917–1928)11 was associated with several neuropsychiatric features. Encephalitis remains a possible cause of this patient’s brief psychosis, but I will also consider the diagnosis of stroke.
Stroke
The neuropsychiatric consequences of stroke, such as depression, are common and well recognized. Acute stroke can unmask latent symptoms of schizophrenia and infrequently can trigger psychosis, mania, delirium, hallucinations, delusions, and other neuropsychiatric syndromes such as Anton’s syndrome, the Capgras syndrome, and the Cotard syndrome. Stroke-induced psychosis has been associated with lesions that affect the prefrontal and occipital cortexes and with disruption of neural networks resulting from subcortical lesions in the thalamus and basal ganglia (often on the right side),12 as well as the midbrain, pons, and cerebellum.
Both encephalitis and stroke are possible causes of the patient’s brief psychosis. Can the patient’s headache and focal neurologic deficits help narrow the differential diagnosis to the most likely diagnosis?
Headache and Focal Neurologic Deficits
In this patient with ongoing headache and focal neurologic deficits, we must consider the diagnosis of community-acquired bacterial meningitis.13 The absence of fever and of signs of meningeal irritation makes bacterial meningitis unlikely. Cerebrospinal fluid (CSF) analysis is required for diagnosis; however, lumbar puncture was not performed in this case, presumably because of the ability to promptly perform imaging of the head.13
Headache and cognitive changes are common in patients with viral encephalitis. Coronavirus infections, including SARS-CoV-2 infection, can cause meningitis, encephalitis, myelitis, and acute necrotizing encephalopathy, particularly in patients with moderate-to-severe illness.6,14
Embolic stroke (especially to the middle and posterior cerebral arteries), lacunar stroke, cerebral arteriopathies, venous sinus thrombosis, and brain hemorrhages are all associated with headache. The 1-week duration of the patient’s preceding headache with associated viral symptoms makes it unlikely that stroke was the cause of her headache. However, stroke cannot be ruled out as a cause of her focal neurologic symptoms. Headache disorders — such as migraine with aura, headache and neurologic deficits with CSF lymphocytosis (also known as the HaNDL syndrome), and familial hemiplegic migraine — are unlikely, given the absence of recurrent migraine headaches.15
Correlation of Clinical and Imaging Findings
Does the head imaging performed in the emergency department help us to determine the diagnosis in this case? MRI of the head revealed an acute brain lesion, effectively ruling out conditions that are often invoked in patients who previously had a stroke or psychiatric disease and now have new focal deficits (e.g., poststroke recrudescence [the reemergence of previous stroke-related deficits]16 and functional neurologic disorders17).
Brain lesions with restricted diffusion typically indicate stroke, but given this patient’s presentation, I will consider a broader differential diagnosis.18 Encephalitic and demyelinating lesions can have restricted diffusion, and demyelination has been reported in association with Covid-19–related encephalitis.6,14,19 However, demyelinating lesions are usually patchy and associated with leptomeningeal enhancement and moderate-to-severe respiratory illness, characteristics that are not consistent with those seen in this patient. Hypoglycemia can result in the development of lesions of diverse shapes, sizes, and locations that have restricted diffusion and may simulate lacunar infarction. However, lesions associated with hypoglycemia usually result in cerebral swelling, are reversible, and are most often associated with severe, prolonged hypoglycemia, which was not present in this patient.20 Lesions with restricted diffusion can occur with migraine disorders21; however, this patient’s headache shows no features of migraine,15 and lesions associated with migraine typically affect the posterior circulation and cross arterial territories. Seizure-induced lesions with restricted diffusion are unlikely in this patient, given that they usually develop after prolonged seizures or status epilepticus and typically appear in the cortical, hippocampal, or pulvinar region.22 Overall, the size and location of this patient’s lesion with restricted diffusion, combined with the abrupt onset of hemiparesis, strongly support a diagnosis of acute ischemic stroke.
Stroke Mechanism
This patient had pure motor hemiparesis, which is a classic lacunar stroke syndrome.23 The punctate size of the lesion and its location in the contralateral posterior limb of the internal capsule in this patient with chronic hypertension suggest small-vessel disease (lipohyalinosis) as the mechanism of the stroke.
Other mechanisms are also possible. This patient was taking risperidone, which has been associated with stroke.24 Primary angiitis of the central nervous system (CNS) and a reversible cerebral vasoconstriction syndrome are often invoked in patients with headaches and stroke; however, the clinical and imaging features of these entities were not observed in this patient.25 Finally, cardiac and artery-to-artery embolism cannot be firmly ruled out without appropriate testing, although both seem unlikely on the basis of the clinical and imaging characteristics favoring the presence of lipohyalinotic small-vessel disease.
Stroke and Covid-19
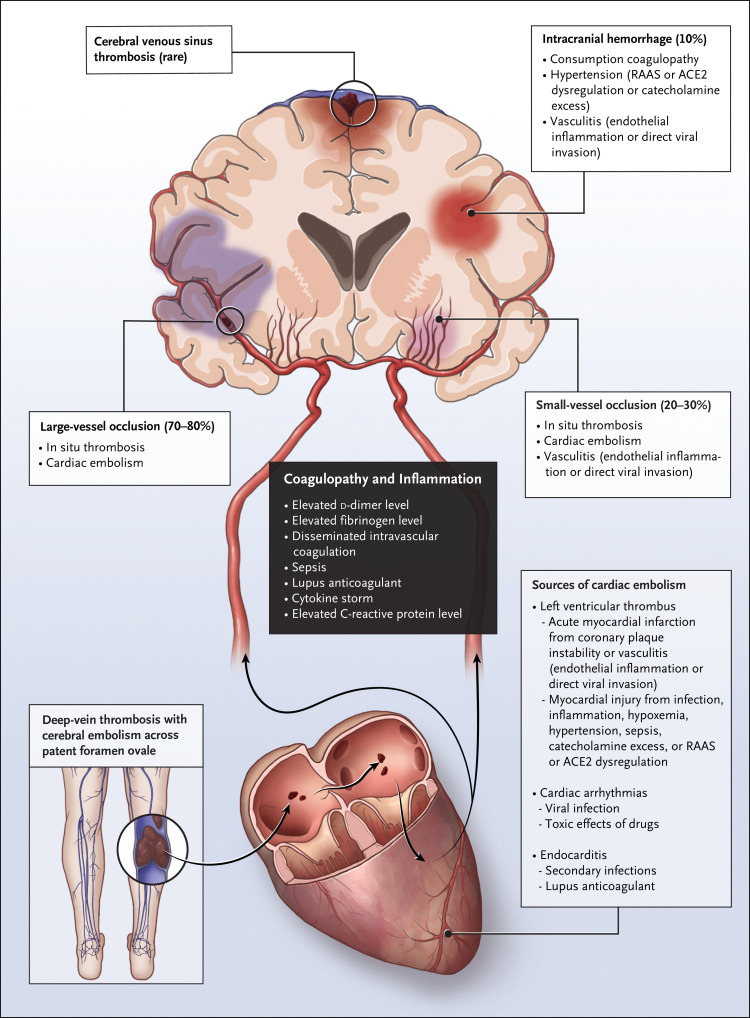
Is there a relationship between this patient’s stroke and Covid-19? Upper respiratory infections are known to trigger stroke; a case–crossover study showed an odds ratio of 2.88 (95% confidence interval, 1.86 to 4.47) for stroke occurring within 15 days after a hospital visit for an influenza-like illness, as compared with the same calendar period 1 or 2 years before the stroke.26 Emerging data suggest a much higher risk of stroke with Covid-19 than with influenza.27 Multiple studies have reported on the frequency and characteristics of stroke in patients with Covid-19.5,6,28 Ischemic stroke appears to affect men and older adults with cardiovascular risk factors most frequently. Potential mechanisms of stroke associated with Covid-19 include coagulopathy, cardiac embolism due to myocardial injury, and nonadherence to medication, all of which may be common during this pandemic (Figure 2). However, most patients with Covid-19 who had a stroke also had concurrent advanced systemic illness. This patient had mild viral illness at the time of stroke, and the d-dimer level at the time of admission was not markedly abnormal. It is conceivable that the small-vessel stroke resulted from viral endotheliitis or systemic inflammation, since her subacute headache and transient psychosis could be consistent with viral encephalitis. SARS-CoV-2 may invade the endothelium29; in addition, it is associated with vasculitis,30,31 and pathological evidence indicates that SARS-CoV-2 RNA may be present within brain tissue.32,33
Figure 2. Stroke Subtypes and Mechanisms in SARS-CoV-2 Infection.
Coagulopathy and inflammation (black box) are central to thrombosis within cerebral blood vessels and cardiac embolism, the two major mechanisms of ischemic stroke in SARS-CoV-2 infection. Other possible mechanisms of stroke and sources of cardiac embolism are listed in the lighter boxes. Patients with SARS-CoV-2 infection may have a higher risk of stroke because of coexisting factors such as advanced age, cardiovascular disease, nonadherence to medications, and cerebral microvascular dysfunction. ACE2 denotes angiotensin-converting enzyme 2, and RAAS renin–angiotensin–aldosterone system.
In conclusion, this patient’s brief psychosis was probably related to direct and indirect effects of SARS-CoV-2 infection, including the socioeconomic effect of Covid-19 in combination with underlying schizophrenia, and to the new infarct in the right hemisphere. The small-vessel stroke resulting from lipohyalinosis was probably triggered by Covid-19, although direct viral mechanisms cannot be ruled out.
Dr. Aneesh B. Singhal’s Diagnosis
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection resulting in acute psychosis and ischemic stroke.
Neurologic Illness Associated with Covid-19
Dr. Bart K. Chwalisz: High rates of neurologic dysfunction have been reported in retrospective case series of Covid-195,9,34 (Table 2). One can distinguish between minor neurologic symptoms that are important for diagnostic recognition and case finding and major neurologic disorders that can have an effect on a patient’s risk of death and complications. The severity of neurologic findings does not necessarily correlate well with the criteria used for the classification of severity of Covid-19, which are based on features of the respiratory illness.
Table 2. Reported Neurologic Manifestations of SARS-CoV-2 Infection.*.
| Manifestation | Percentage of Study Patients |
|---|---|
| Any neurologic manifestation |
|
| Decreased taste |
|
| Decreased smell |
|
| Muscle injury or myalgias |
|
| Dizziness |
|
| Headache |
|
| Neuropsychiatric disorder (delirium, agitation, or dysexecutive syndrome) or impaired consciousness |
|
| Stroke |
|
| Nerve pain | 2.3% (Mao et al.) |
| Vision disturbance or optic neuritis |
|
| Ataxia or movement disorder |
|
| Seizure |
|
| Guillain–Barré syndrome, Miller Fisher syndrome, or ophthalmoparesis |
|
| Acute necrotizing encephalopathy | Present in case report (Poyiadji et al.) |
| Meningoencephalitis | Present in case report (Moriguchi et al.) |
| Myelitis |
|
The study by Mao et al.5 (conducted in China) included 214 patients, Romero-Sánchez et al.34 841 patients, Giacomelli et al.35 (an inpatient study) 59 patients, Lechien et al.36 (an outpatient study) 417 patients, Goyal et al.37 393 patients, Wang et al.38 138 patients, Huang et al.39 41 patients, Helms et al.6 58 patients, Oxley et al.40 5 patients, Lu et al.7 304 patients, Zhao et al.41 1 patient, Toscano et al.42 5 patients, Gutiérrez-Ortiz et al.43 2 patients, Dinkin et al.44 2 patients, Poyiadji et al.45 1 patient, Moriguchi et al.46 1 patient, and Sotoca et al.47 1 patient.
Minor Neurologic Symptoms of Covid-19
Headache was one of the presenting symptoms in this patient. Minor neurologic symptoms — such as headache, anosmia, ageusia, dizziness, nausea and vomiting, and muscle aches — appear to be rather common, even in mild cases of Covid-19.34,37-39,48 The prevalence of neurologic symptoms was reported to be 36.4% in a retrospective series from China5 and 57.4% in a series from Spain.34 However, these rates appear to be underestimations; more recently, much higher rates of smell and taste disturbances have been reported from other countries (Table 2). Neurologic symptoms such as headache may be early manifestations of Covid-19 that develop before any respiratory symptoms or abnormalities on chest imaging are present.5
Smell and taste disturbances, which appear to be particularly characteristic of Covid-19, even among patients who report no nasal obstruction, include anosmia, ageusia, parosmia, and phantosmia. This patient did not report smell disturbances, but she may have had a decreased sense of smell at baseline, since odor discrimination can be decreased in patients with first-episode psychosis,49 as well as in those with chronic schizophrenia.50 In addition to the diagnostic importance of anosmia, the high rate of this disorder in Covid-19 has led to speculation that the first cranial nerve could be a portal of entry into the CNS. Of note, the olfactory bulb has direct axonal connections to the medial temporal lobe, an area of the brain associated with psychosis. However, it appears that, in most cases, anosmia is a transient symptom that resolves within a week.51 The rapid resolution suggests that anosmia may be mediated by a transient dysfunction of the olfactory epithelium rather than by neuronal destruction. Indeed, an analysis of gene expression patterns in various cell types of the upper respiratory epithelium showed that olfactory sensory neurons lack angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) — proteins that have been shown to facilitate SARS-CoV-2 infection. However, these proteins are expressed in supportive cells of the olfactory epithelium, such as basal cells, sustentacular cells, and secretory cells.52 Thus, the common occurrence of anosmia among patients with Covid-19 should not be taken as prima facie evidence of invasion of the CNS by SARS-CoV-2. However, in at least one case, radiologic changes in the olfactory bulb and gyri recti have been reported, findings that merit further study.53
Major Neurologic Symptoms of Covid-19
Major neurologic symptoms of Covid-19 include CNS disorders such as encephalopathy, stroke, and myelopathy, as well as severe neuromuscular disturbances such as rhabdomyolysis,54 the Guillain–Barré syndrome,55,56 and cranial-nerve dysfunction.43,44
In a retrospective series of patients with Covid-19, neurologic symptoms were reported in 45.5% of the patients with severe infection.5 As compared with patients who had milder illness, the patients with severe illness were older and more likely to have underlying disorders such as hypertension; they also had fewer typical symptoms of Covid-19, such as fever or cough, but were more likely to have multiorgan involvement such as liver-function abnormalities, impaired renal function, or elevated creatine kinase levels.5 In this series, impaired consciousness was present in 14.8% of the patients with severe Covid-19 and stroke in 5.7%.5
In a series of 58 consecutive patients with Covid-19 and acute respiratory distress syndrome in France, 14% had neurologic findings on admission, but 67% had neurologic findings when sedation and neuromuscular blockade were discontinued; findings included agitation, delirium, and corticospinal tract signs such as hyperreflexia, clonus, and extensor plantar reflexes.6 A dysexecutive syndrome was present in 33% of the patients at discharge.
Vascular dysfunction is prominent in severe Covid-19, as evidenced by elevated levels of d-dimer and procoagulant factors. Thromboembolic complications, including stroke, are common.57
There are case reports of additional clinically significant neurologic presentations in Covid-19, including a case of meningoencephalitis associated with Covid-19 (with the detection of SARS-CoV-2 nucleic acids in the CSF),46 a case of acute necrotizing encephalopathy,45 and two case reports of myelitis.41,47
Putative mechanisms of neurologic dysfunction in severe Covid-19 include direct neuroinvasion, endothelial dysfunction, and a neurotoxic effect from exuberant inflammation and cytokine release. Unfortunately, neuropathological confirmation of any of those mechanisms is currently lacking.
Discussion of Pathophysiology
Dr. Shibani S. Mukerji: Human coronaviruses are large, positive-sense, single-stranded RNA viruses, of which the betacoronavirus genus has been responsible for human disease outbreaks during the past 20 years.58 Although betacoronaviruses are known to cause neurologic illness in some people, there is limited understanding about the breadth of neurologic effects associated with SARS-CoV-2 and the potential entry of the virus into the CNS. In SARS-CoV infections, autopsy specimens of the brain show the presence of the SARS-CoV nucleocapsid protein in the cytoplasm of neurons in the hypothalamus and cortex on immunohistochemical analysis,59 and viral particles are seen on electron microscopy.60 Low levels of SARS-CoV-2 RNA were recovered from brains in two autopsy case series, although the presence of SARS-CoV-2 has not yet been identified in neuronal, glial, or endothelial cells in the CNS.32,33
SARS-CoV is structurally most similar to SARS-CoV-2, and both use the ACE2 receptor to gain entry into cells.58 In animal models, ACE2 protein is expressed in multiple tissue types, including that of the lung, gastrointestinal tract, and lymph nodes, as well as in endothelial and smooth-muscle cells. In murine brain tissue, basal ACE2 protein expression is widespread, and the highest levels of expression are found in the primary and secondary motor cortex, caudate nuclei, medullary nuclei, and circumventricular organs.61 Although ACE2 messenger RNA (mRNA) expression is low in the human brain relative to renal, gastrointestinal, and cardiovascular tissues, expression can be seen in the cerebellum, hippocampus, cortex, hypothalamus, substantia nigra, and medullar nuclei. Interestingly, mRNA has been identified in the choroid plexus and middle cerebral artery,62 and in an autopsy series of three cases in humans, ACE2 protein expression was limited to the endothelium and vascular smooth-muscle cells.63 Although SARS-CoV-2 uses the surface protease TMPRSS2 as a coreceptor for spike protein priming, TMPRSS2 has minimal expression in the brain.64 Given our limited understanding of SARS-CoV-2 in the CNS, including the mechanisms of neuronal infection, there is a critical need for integrating brain and spinal-cord autopsy findings with comprehensive clinical details to improve our understanding of virus–host interactions.
Discussion of Management
Dr. Singhal: This patient had relative contraindications to treatment with intravenous tissue plasminogen activator and to mechanical thrombectomy (including mild deficit 10 hours after she was last seen well, and the potential for infection-related stroke). Because of her mild, stable symptoms and the challenges with performing imaging in the context of Covid-19, urgent computed tomography of the head was deferred and MRI of the head was performed within 2 hours after presentation. Once infarction was confirmed, treatment with clopidogrel was added to her current aspirin regimen on the basis of evidence that short-term dual antiplatelet treatment is beneficial in patients with minor stroke.65
Atorvastatin was initiated for stroke prevention. Despite theoretical concerns about the safety of ACE inhibitors in patients with Covid-19, treatment with lisinopril was continued in accordance with recent guidance.66 Risperidone treatment was continued, since the benefit outweighed known risks in patients who have had a stroke, and low-molecular-weight heparin was administered to prevent deep-vein thrombosis.
Follow-up
Dr. Baggett: On hospital day 5, tachypnea and fever developed in the patient. A chest radiograph showed bilateral multifocal patchy hazy and patchy consolidative opacities with peripheral predominance in the middle and lower lung zones. Ceftriaxone and azithromycin were administered. Supplemental oxygen was not initiated. The patient’s hospital course was further complicated by delirium, and she was treated with olanzapine and quetiapine. Her condition improved, and she was discharged to a rehabilitation facility on hospital day 14.
Final Diagnosis
Brief psychosis and small-vessel cerebral infarction, probably related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Disclosure Forms
Footnotes
This case was presented virtually at Neurology Grand Rounds during the Covid-19 pandemic.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Marder SR, Cannon TD. Schizophrenia. N Engl J Med 2019;381:1753-1761. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho PMM, Moreira MM, de Oliveira MNA, Landim JMM, Neto MLR. The psychiatric impact of the novel coronavirus outbreak. Psychiatry Res 2020;286:112902-112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lortie G, Laird DM. Hypoglycemia simulating psychoses. N Engl J Med 1957;256:1190-1192. [DOI] [PubMed] [Google Scholar]
- 4.Maguire M, Singh J, Marson A. Epilepsy and psychosis: a practical approach. Pract Neurol 2018;18:106-114. [DOI] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77(6):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020;382:2268-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu L, Xiong W, Liu D, et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia 2020;61(6):e49-e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun 2020;87:34-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020;7:611-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honigsbaum M. “An inexpressible dread”: psychoses of influenza at fin-de-siècle. Lancet 2013;381:988-989. [DOI] [PubMed] [Google Scholar]
- 11.Encephalitis lethargica, its sequelae and treatment. JAMA 1932;98:255-255. [Google Scholar]
- 12.McMurtray A, Tseng B, Diaz N, Chung J, Mehta B, Saito E. Acute psychosis associated with subcortical stroke: comparison between basal ganglia and mid-brain lesions. Case Rep Neurol Med 2014;2014:428425-428425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Beek D, de Gans J, Tunkel AR, Wijdicks EFM. Community-acquired bacterial meningitis in adults. N Engl J Med 2006;354:44-53. [DOI] [PubMed] [Google Scholar]
- 14.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol 2020. May 29 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia 2018;38:1-211. [DOI] [PubMed] [Google Scholar]
- 16.Topcuoglu MA, Saka E, Silverman SB, Schwamm LH, Singhal AB. Recrudescence of deficits after stroke: clinical and imaging phenotype, triggers, and risk factors. JAMA Neurol 2017;74:1048-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics 2018;59:358-368. [DOI] [PubMed] [Google Scholar]
- 18.Adam G, Ferrier M, Patsoura S, et al. Magnetic resonance imaging of arterial stroke mimics: a pictorial review. Insights Imaging 2018;9:815-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kremer S, Lersy F, de Sèze J, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology 2020. June 16 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yong AW, Morris Z, Shuler K, Smith C, Wardlaw J. Acute symptomatic hypoglycaemia mimicking ischaemic stroke on imaging: a systemic review. BMC Neurol 2012;12:139-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf ME, Szabo K, Griebe M, et al. Clinical and MRI characteristics of acute migrainous infarction. Neurology 2011;76:1911-1917. [DOI] [PubMed] [Google Scholar]
- 22.Szabo K, Poepel A, Pohlmann-Eden B, et al. Diffusion-weighted and perfusion MRI demonstrates parenchymal changes in complex partial status epilepticus. Brain 2005;128:1369-1376. [DOI] [PubMed] [Google Scholar]
- 23.Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology 1965;15:774-784. [DOI] [PubMed] [Google Scholar]
- 24.Pasternak B, Svanström H, Ranthe MF, Melbye M, Hviid A. Atypical antipsychotics olanzapine, quetiapine, and risperidone and risk of acute major cardiovascular events in young and middle-aged adults: a nationwide register-based cohort study in Denmark. CNS Drugs 2014;28:963-973. [DOI] [PubMed] [Google Scholar]
- 25.Singhal AB, Topcuoglu MA, Fok JW, et al. Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol 2016;79:882-894. [DOI] [PubMed] [Google Scholar]
- 26.Boehme AK, Luna J, Kulick ER, Kamel H, Elkind MSV. Influenza-like illness as a trigger for ischemic stroke. Ann Clin Transl Neurol 2018;5:456-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with Covid-19 versus patients with influenza. May 21, 2020. (https://www.medrxiv.org/content/10.1101/2020.05.18.20105494v1). preprint. [Google Scholar]
- 28.Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke 2020;51:2002-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr 2020;10:537-540. [DOI] [PubMed] [Google Scholar]
- 31.Hanafi R, Roger P-A, Perin B, et al. COVID-19 neurologic complication with CNS vasculitis-like pattern. AJNR Am J Neuroradiol 2020. June 18 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020;383:590-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med. DOI: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology 2020. June 1 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis 2020. March 26 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020. April 6 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020;382:2372-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 2020;382(20):e60-e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Acute myelitis after SARS-CoV-2 infection: a case report. April 9, 2020. (https://www.medrxiv.org/content/10.1101/2020.03.16.20035105v2). preprint. [Google Scholar]
- 42.Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med 2020;382:2574-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 2020. April 17 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 44.Dinkin M, Gao V, Kahan J, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology 2020. May 1 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 45.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020. March 31 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis 2020;94:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sotoca J, Rodríguez-Álvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol Neuroimmunol Neuroinflamm 2020;7(5):e803-e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamath V, Lasutschinkow P, Ishizuka K, Sawa A. Olfactory functioning in first-episode psychosis. Schizophr Bull 2018;44:672-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moberg PJ, Kamath V, Marchetto DM, et al. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophr Bull 2014;40:50-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol 2020. April 12 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brann DH, Tsukahara T, Weinreb C, Logan DW, Datta SR. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. March 28, 2020. (https://www.biorxiv.org/content/10.1101/2020.03.25.009084v2). preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol 2020. May 29 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 54.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis 2020;26:1618-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alberti P, Beretta S, Piatti M, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm 2020;7(4):e741-e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci 2020;76:233-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry 2020. April 30 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 2020;5:562-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med 2005;202:415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis 2005;41:1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 2007;292:R373-R381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 2002;532:107-110. [DOI] [PubMed] [Google Scholar]
- 63.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol 2004;203:631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brain tissue expression of TMPRSS2. Human Protein Atlas (https://www.proteinatlas.org/ENSG00000184012-TMPRSS2/brain).
- 65.Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018;379:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382:1653-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.