Abstract
Fibrous dysplasia (FD) is a benign bone disease characterized by expansile lesions that typically stabilize with age. Rarely, FD can undergo malignant transformation, presenting with atypical, rapid growth and destruction of adjacent bone. Other potential causes of rapid FD expansion include secondary lesions, such as aneurysmal bone cysts. We describe a case of an aggressive occipital lesion that presented with pain associated with diplopia and tinnitus, raising concern for malignant transformation. A massive intraosseous arteriovenous fistula was identified giving rise to an anomalous vein coursing to the cavernous sinus with compression of the abducens nerve. The vascular anomaly was mapped and after embolization symptoms resolved; a biopsy with extensive genetic analyses excluded malignancy. The differential diagnosis for expanding FD lesions includes aggressive FD, malignant transformation, and secondary vascular anomalies. In cases when traditional radiographic and histologic assessment are nondescript, use of additional radiographic modalities and genetic analyses are required to make an accurate diagnosis and guide treatment. When vascular anomalies are suspected, detailed angiography with embolization is necessary to define and treat the lesion. However, to rule out malignant transformation, genetic screening is recommended.
Keywords: Fibrous dysplasia, intraosseous arteriovenous fistula, malignant transformation, aneurysmal bone cyst
INTRODUCTION
Fibrous dysplasia (FD) is a disorder of fibro-osseous lesions, arising from gain-of-function mutations in the alpha subunit of the stimulatory G-protein transcript of GNAS, which is considered a weak oncogene due to its association with low grade and benign neoplasia (OMIM #174800).[1] These mutations result in ligand-independent Gs-protein-coupled receptor signaling, constitutive activation of adenyl cyclase, and increased cyclic adenosine monophosphate (cAMP) production. In bone marrow stromal cells (BMSCs), increased cAMP signaling alters differentiation and increases proliferation. Mutant BMSCs replace normal bone and marrow with woven bone and fibrotic stroma.
Typically, FD lesions expand slowing in childhood and stabilize during adulthood. However, rapidly enlarging lesions can occur as a result of malignant transformation or secondary benign lesions, such as aneurysmal bone cysts (ABCs). Clinical and radiographic findings in malignant transformation are often non-specific.[2] Therefore, biopsy with genetic and histological analyses are required for accurate diagnosis.
Here we present a case of an abnormally aggressive FD lesion within the occiput, presenting with pain, tinnitus, and diplopia. Ultimately, an intraosseous arteriovenous fistula (AVF) was identified, a rare finding not typically recognized in the phenotypic spectrum of FD. This case highlights the differential diagnosis of aggressive FD lesions and the necessity of a multi-disciplinary approach for diagnosis and treatment.
CASE PRESENTATION
A 48-year-old man with polyostotic FD presented to National Institutes of Health (NIH) as part of an FD natural history study (NCT00001727) with a new complaint of diplopia on rightward gaze for the past five days. He also reported a painful increase in the size of an established occipital FD lesion over the last six months and tinnitus in his right ear for a year. One year prior, he had been evaluated at an outside hospital after falling during a presumed seizure and sustaining a scapular fracture. At that time, magnetic resonance imaging (MRI) of the brain was obtained, which revealed a heterogeneously enhancing lesion with the occipital bone with a non-enhancing lesion within the clivus. The radiology report noted that differential considerations for these lesions included “vascular malformation, changes of chronic FD, malignant transformation, and metastatic disease.” A diagnostic angiogram was recommended, but was not obtained.
The patient was initially enrolled in the NIH study in 2001, and had been followed on an as-needed basis with evaluations every 1 to 5 ears. His medical history was significant for McCune-Alright Syndrome (MAS), a triad of FD, café-au-lait macules, and hyperactive endocrinopathies. He did not have any endocrine features of MAS, but had a single café-au-lait macule and FD involving the right lower extremity, bilateral upper extremities, several ribs, right scapula, cranial base, and occiput. His clinical course had been otherwise relatively benign until this point.
On examination, a prominent occipital mass was noted, and an audible bruit was identified over the right lateral skull. Ophthalmologic evaluation revealed 16 prism diopter esotropia in right gaze, and 2 prism diopter esotropia in primary gaze, indicative of partial right abducens nerve palsy. Audiograms were normal. Cranial computed tomography (CT) demonstrated widespread FD involving the sphenoid, occiput, and right temporal bone. Multiple heterogeneous lytic/cystic areas were noted in the occiput, which had significantly expanded compared to the scan performed one year prior (Figure 1A–B). Full thickness cortical destruction was also seen. CT angiography revealed a tangle of vessels within the occipital FD with mixed extracranial and intracranial blood supply, including the occipital branches of the external carotid arteries, muscular branches of the vertebral arteries, and dural branches of the vertebral arteries (Supplemental Video 1). An intraosseous draining vein was identified, which coursed from the occiput, around the foramen magnum anteriorly, and into the cavernous sinus (Figure 1C). Two distal venous aneurysms were identified within the cavernous sinus, compressing the abducens nerve (Figure 1D). The patient was referred for cerebral arteriogram and transcatheter arterial embolization (TAE). Although the occipital expansion was likely due to the AVF, malignant transformation could not be excluded; therefore, a biopsy was planned after embolization to reduce the risk of hemorrhage.
Figure 1.
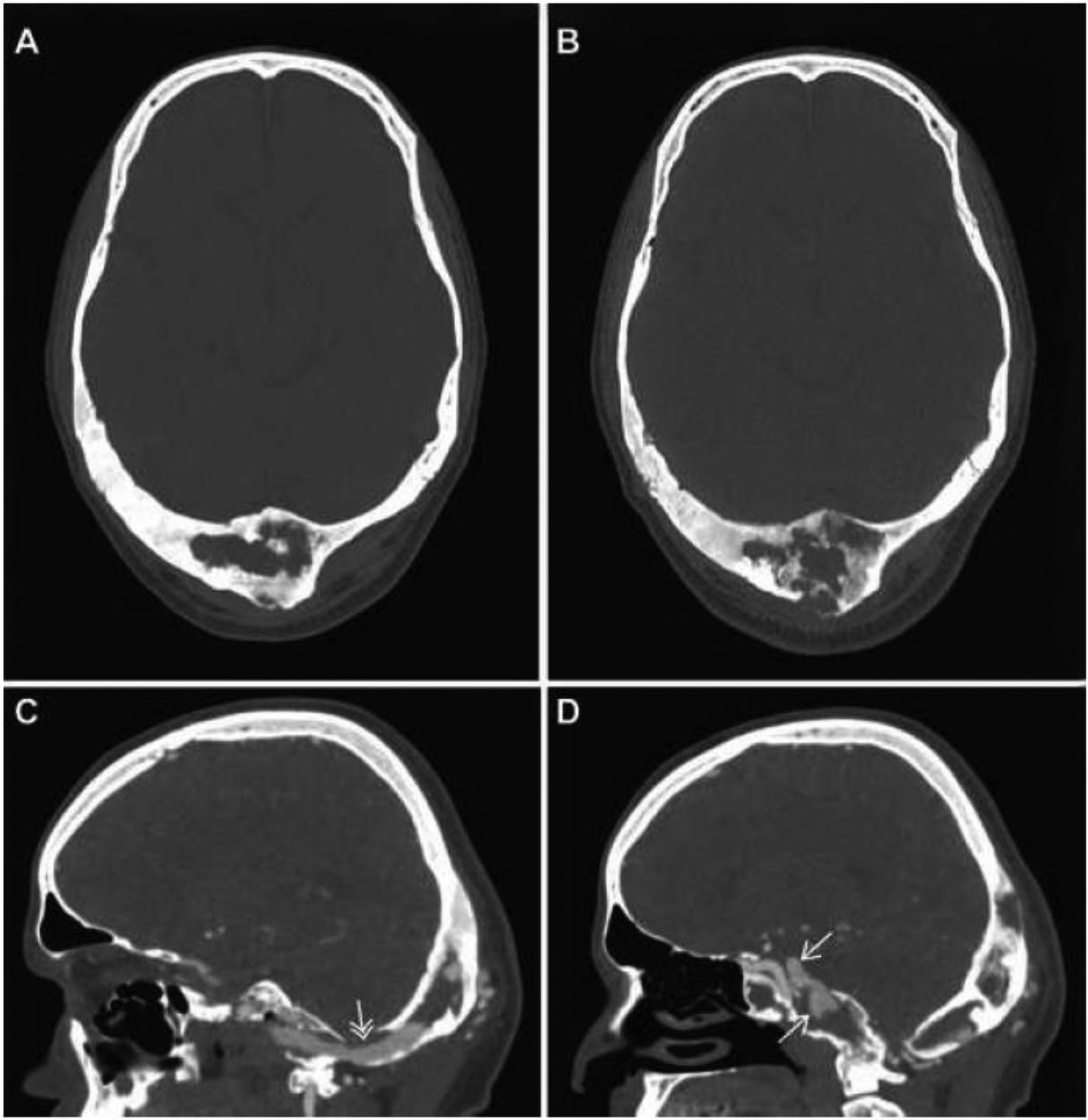
Computed tomography imaging and angiography: Axial plane images of the occipital FD lesion in 2017 (A) and 2018 (B) demonstrate significant growth and lytic changes with cortical destruction over the course of one year. Sagittal CT images of an angiographic study (C & D) performed concurrent with the 2018 image (B) demonstrated a large arteriovenous fistula with an intraosseous draining vein (C, white double arrow) and distal venous aneurysms (D, white arrows).
The arteriogram further characterized the vascular anatomy (Figure 2A–B); additional arterial supply was identified from the posterior branch of the right middle meningeal artery. TAE with N-butyl 2-cyanoacrylate glue blockade was performed. The dural and muscular branches of the right vertebral artery, branches of the right occipital artery, and the posterior branches of the right middle meningeal artery were embolized (Figure 2C–D). Post-embolization angiography demonstrated significant reduction in AVF flow (Supplemental Video 2). Immediately following the procedure, the patient reported resolution of his tinnitus, and within nine weeks his diplopia resolved. Three weeks after the procedure, the patient underwent an uncomplicated biopsy for histological and genetic analyses to rule out malignancy.
Figure 2.
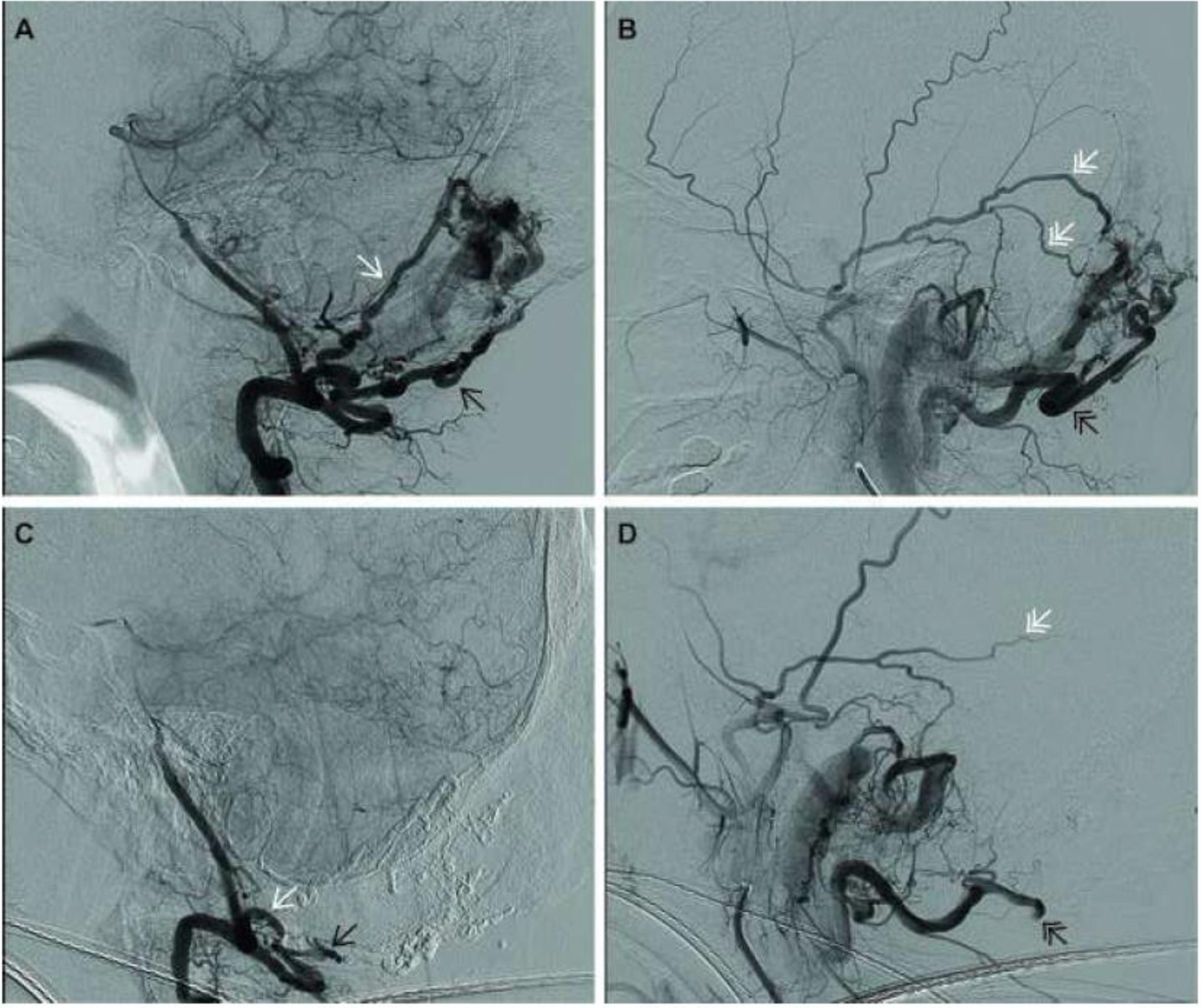
Pre- and post-embolization arteriograms: Angiographic images taken prior to embolization (A&B), demonstrate dural (white arrow) and muscular (black arrow) branches of the right vertebral artery (A), and posterior branches of the middle meningeal artery (double white arrows), and occipital artery (double black arrow) (B). The image in panel C, which was taken following embolization and during the injection of contrast in the right vertebral artery, clearly demonstrates loss of the dural and muscular branches that were present in panel A and had been feeding the plexus of vessels in the occipital bone. The image in panel D, which was taken after embolization and during the injection of the posterior branches of the middle meningeal and occipital arteries, demonstrates loss or truncation of the vessels that had also been feeding the occipital plexus.
METHODS
Bioptic tissue was fixed, embedded, and stained with hematoxylin and eosin (H&E) for transmitted histological study. Tumor profiling of formalin-fixed paraffin embedded (FFPE) tissue was performed at the NIH Clinical Center using the Ion Torrent™ Oncomine™ Comprehensive Assay v3, a next-generation sequencing multiple biomarker assay. The Oncomine™ panel covers 161 of the most relevant cancer driver genes, identifying hotspot genes, insertion-deletions, single nucleotide variations, and gene fusions. The tissue evaluated was dissected from unstained paraffin.
Primary BMSC cultures were established from fresh biopsy tissue. The sample was cut into pieces of approximately 2 mm3 while immersed in culture media. Culture media and tissue chips were then transferred to a flask and cultured as previously described.[3] BMSCs were cultured in α-MEM (Life Technologies, Grand Island, NY, USA; 12571), 20%, lot-selected, non–heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, USA; S11150) and 1% penicillin-streptomycin. For the first 10 days of culture, media was supplemented with the broad spectrum antimicrobial Primocin™ (Invivogen, San Diego, California). GNAS mutation burden was determined using the TaqMan™ Mutation Detection Assay (Thermo Fisher Scientific, Waltham, Massachusetts) with a competitive allele-specific TaqMan™ PCR-based assay designed to detect and measure MAS-specific GNAS R201 mutations. GNAS mutation burden was measured from DNA isolated from the cultured BMSCs at passage three with the Qiagen DNeasy Blood and Tissue kit.
RESULTS
H&E staining revealed typical features of benign fibrous dysplasia, including marrow devoid of hematopoiesis or adipocytes, fibro-osseous tissue with abundant collagen surrounding disconnected trabeculae composed of hyperosteocytic immature bone with expanded lacunae and abundant reversal lines. Osteoblasts associated with the bone surface lacked a typical cuboidal appearance and were associated with Sharpey collagen fibers. No evidence of malignancy was found in any of the biopsies (Figure 3). The only genetic finding identified on tumor profiling was a typical R201H mutation in GNAS with a remarkably high variant allele frequency of 46%. In cultured cells, mutant allele frequency was measured at 32%.
Figure 3.
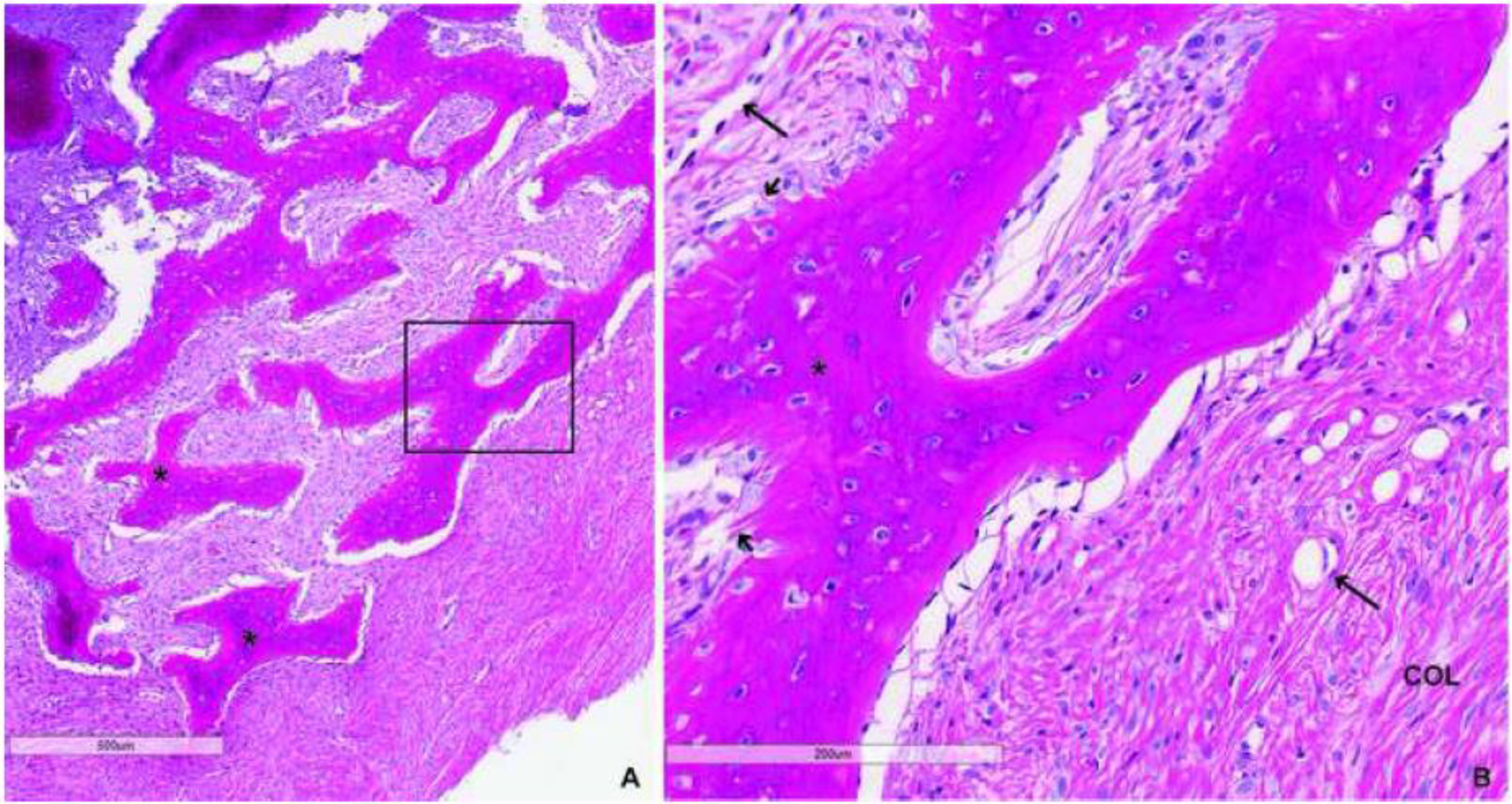
H&E staining of fibrous dysplasia (FD) biopsy: Low (A) and high power (B) images demonstrate typical features of FD including abnormal, hyperosteocytic bony trabeculae (*) with classic “Chinese-writing” appearance, cellular fibrotic stroma with prominence of vascular structures (long arrows), Sharpey fibers (short arrows), abundant collagen fibers (col) and an absence of hematopoietic marrow or adipocytes. Importantly, there was no histological evidence of malignancy.
DISCUSSION
This case of an occipital FD lesion with features concerning for malignant transformation and an atypical clinical presentation highlights a number of important considerations for FD management. Significant clinical findings (tinnitus, diplopia, and bruit) were explained by a complex intraosseous AVF with a large draining vein and distal venous aneurysms compressing the abducens nerve. Through effective coordination of a multidisciplinary team, the AVF was embolized, the FD lesion was biopsied, and malignant transformation excluded based on histological and genetic assessments.
Malignant transformation of FD is rare, but potentially devastating if not promptly diagnosed.[4–6] Malignant transformation typically presents with pain, swelling, and pathologic fracture ; however, insidious cases have also been reported.[4] Radiographic findings such as cortical destruction, soft tissue invasion, and periosteal scalloping, are non-specific.[2] Histological assessment is diagnostic if definitive features of malignancy are present, such as cytologic atypia and increased mitotic activity. However, in the absence of these features, malignancy cannot be excluded. Low grade central osteosarcoma is a distinct entity of slow growing osteosarcoma that is indistinguishable from FD on H&E staining. However, low grade central osteosarcoma is genetically characterized by MDM2 and CDK4 gene amplifications without mutations in GNAS, emphasizing the importance of genetic characterization to differentiate FD.[7]
ABCs are another sequela of FD that present with aggressive lesion expansion.[8, 9] Unlike malignant transformation, ABCs are easily diagnosed radiographically, by demonstrating characteristic multi-lobular cystic lesions with fluid-fluid lines. Primary ABCs exhibit cytological rearrangements of the USP6 gene, which are not present in secondary lesions.[10] Secondary ABCs are hypothesized to occur due to hemodynamic changes within abnormal, hypervascular bone.[11, 12] Evidence of arteriovenous shunting within ABCs suggests they likely develop from an inciting AVF.[13, 14] While AVFs are rare in FD,[15–17] dilated vascular channels and foci of interstitial and peritrabecular hemorrhage are common, and probably explain the propensity to develop vascular anomalies.[18] Mcintosh et al. examined circulatory changes in six patients with polyostotic FD, and found that the majority of patients had cardiomegaly, an elevated resting cardiac index, and narrow AV oxygen differences from selective venous sampling. Based on these findings, they concluded that osseous lesions in FD contain functioning AV fistulae that may cause high output heart failure.[16] However, only few studies have evaluated vascular changes in FD via angiography.[15, 17, 19] One other intraosseous AVF arising in craniofacial FD has been reported; this patient presented with a expansile frontal bone lesion with overlying dilated, pulsatile temporal vessels and an audible bruit.[15] In addition, there have been two reports of femoral AV malformations.[19, 20] All cases were successfully treated with intravascular embolization; however, definitive surgical resection was limited due to associated morbidity. When neither surgical resection nor complete fistula occlusion are possible, regular follow-up is recommended due to the potential risk of collateral vessel recruitment and recurrence associated with partial embolization.
FD generally follows a predictable natural history; lesions tend to grow during childhood and stabilize during adulthood.[21] FD stabilization correlates with a decline in the proportion of mutant alleles with age, and is hypothesized to represent apoptosis of mutation-bearing BMSCs that have expended their self-renewing capacity.[22] Therefore, expansion of FD lesions in adults raises concern for malignant transformation. Interestingly, in this case, increased GNAS mutant allele frequency in lesional tissue was found by two techniques—46% by oncogene profiling of fixed tissue and 32% from cultured cells. Allele frequency in a 48-year-old is usually ≤ 10%.[22] The association of increased GNAS mutation burden, lesion expansion, and vascular proliferation during adulthood in the absence of an oncogenic mutation, suggests an intermediate phase of pathogenicity, perhaps representing a premalignant state and dictating close follow-up. Recently, Corsi et al. described an aggressive FD lesion involving the mandible [23]. In that case, the local clinical aggressiveness was related to the occurrence of desmoplastic fibroma-like areas that had merged with typical fibrous dysplasia. Within the lesion, GNAS mutations were identified in both the typical FD tissue as well as the component of the lesion that histologically resembled a desmoplastic fibroma. Together, our findings demonstrate that multiple benign, and possibly pre-malignant, secondary changes may occur in FD, and further in-depth molecular studies are required to elucidate the pathogenesis of these changes.
CONCLUSIONS
The differential diagnosis for expanding FD lesions includes aggressive FD with benign secondary changes, malignant transformation, and vascular abnormalities. In cases when traditional radiographic and histologic assessments are nondescript, additional imaging modalities and genetic analyses are required for accurate diagnosis. When vascular anomalies are suspected, detailed angiography with embolization is necessary to define and treat the lesion. Furthermore, to rule out malignant transformation, genetic screening should be performed; with the increasing availability of commercial cancer gene panels, such genetic analyses can easily be incorporated into standard practice.
Supplementary Material
Supplemental Video 1. Animated 3D reconstruction of the cranium and vascular structures that demonstrate the arterial blood supply to the occipital arteriovenous fistula and the aneurysms that arose from the anomalous draining vein and were the cause of the abducens nerve palsy.
Supplemental Video 2. Serial pre- and post-embolization arteriographic images of the arterial blood supply to the occipital intraosseous arteriovenous fistula and the anomalous venous drainage and aneurysms. The first series depicts the external carotid artery injection that demonstrates the middle meningeal artery and posterior branches and their contribution to the arteriovenous fistula, followed by their disappearance or truncation after embolization. The second series is the pre- and post-injection images of the vertebral artery that demonstrates the dural and muscular branches and their contribution to the arteriovenous fistula, again followed by their disappearance or truncation after embolization.
Funding:
This research was funded by the Intramural Research Programs of the NIDCR.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest:
The authors do not have conflicts of interest to declare.
Ethics approval:
The study was approved by the NIDCR Institutional Review Board.
Consent to participate:
The patient was enrolled in the NIH FD/MAS natural history study (NCT00001727); Informed consent was obtained for participation in the study and for publication of results.
Availability of data and material:
Not applicable
Code availability:
Not applicable
REFERENCES
- 1.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. The New England journal of medicine. 1991;325(24):1688–95. doi: 10.1056/nejm199112123252403. [DOI] [PubMed] [Google Scholar]
- 2.Yao L, Eckardt JJ, Seeger LL. Fibrous dysplasia associated with cortical bony destruction: CT and MR findings. Journal of computer assisted tomography. 1994;18(1):91–4. [DOI] [PubMed] [Google Scholar]
- 3.Robey PG, Kuznetsov SA, Ren J, Klein HG, Sabatino M, Stroncek DF. Generation of clinical grade human bone marrow stromal cells for use in bone regeneration. Bone. 2015;70:87–92. doi: 10.1016/j.bone.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggieri P, Sim FH, Bond JR, Unni KK. Malignancies in fibrous dysplasia. Cancer. 1994;73(5):1411–24. [DOI] [PubMed] [Google Scholar]
- 5.Qu N, Yao W, Cui X, Zhang H. Malignant transformation in monostotic fibrous dysplasia: clinical features, imaging features, outcomes in 10 patients, and review. Medicine. 2015;94(3):e369. doi: 10.1097/md.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Raynald, Wang Z, Qian H. Malignant transformation of craniofacial fibrous dysplasia: a systematic review of overall survival. Neurosurgical review. 2019. doi: 10.1007/s10143-019-01089-1. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida A, Ushiku T, Motoi T, Shibata T, Beppu Y, Fukayama M et al. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2010;23(9):1279–88. doi: 10.1038/modpathol.2010.124. [DOI] [PubMed] [Google Scholar]
- 8.Buraczewski J, Dabska M. Pathogenesis of aneurysmal bone cyst. Relationship between the aneurysmal bone cyst and fibrous dysplasia of bone. Cancer. 1971;28(3):597–604. [DOI] [PubMed] [Google Scholar]
- 9.Boyce AM, Florenzano P, de Castro LF, Collins MT. Fibrous Dysplasia/McCune-Albright Syndrome In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K et al. , editors. GeneReviews((R)). Seattle, Washington: University of Washington; 1993. [PubMed] [Google Scholar]
- 10.Oliveira AM, Perez-Atayde AR, Inwards CY, Medeiros F, Derr V, Hsi BL et al. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so-called secondary aneurysmal bone cysts. The American journal of pathology. 2004;165(5):1773–80. doi: 10.1016/s0002-9440(10)63432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtenstein L Aneurysmal bone cyst; observations on fifty cases. The Journal of bone and joint surgery American volume. 1957;39-a(4):873–82. [PubMed] [Google Scholar]
- 12.Rapp TB, Ward JP, Alaia MJ. Aneurysmal bone cyst. The Journal of the American Academy of Orthopaedic Surgeons. 2012;20(4):233–41. doi: 10.5435/jaaos-20-04-233. [DOI] [PubMed] [Google Scholar]
- 13.Lindbom A, Soderberg G, Spjut HJ, Sunnqvist O. Angiography of aneurysmal bone cyst. Acta radiologica. 1961;55:12–6. [DOI] [PubMed] [Google Scholar]
- 14.Biesecker JL, Marcove RC, Huvos AG, Mike V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26(3):615–25. [DOI] [PubMed] [Google Scholar]
- 15.Ishiguro S, Ikeda M, Shimizu H, Hayashi M. Arteriovenous fistula as an unusual complication of polyostotic fibrous dysplasia of the skull. Surg Neurol. 1985;24(6):681–4. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh HD MD, Gleason WL, Goldner JL. The circulatory dynamics of polyostotic fibrous dysplasia. The American Journal of Medicine. 1962;32(3):393–403. [Google Scholar]
- 17.Lin JP, Goodkin R, Chase NE, Kricheff II. The angiographic features of fibrous dysplasia of the skull. Radiology. 1969;92(6):1275–80. doi: 10.1148/92.6.1275. [DOI] [PubMed] [Google Scholar]
- 18.Ippolito E, Bray EW, Corsi A, De Maio F, Exner UG, Robey PG et al. Natural history and treatment of fibrous dysplasia of bone: a multicenter clinicopathologic study promoted by the European Pediatric Orthopaedic Society. Journal of pediatric orthopedics Part B. 2003;12(3):155–77. doi: 10.1097/01.bpb.0000064021.41829.94. [DOI] [PubMed] [Google Scholar]
- 19.MacErlean DP, Shanik DG, Martin EA. Transcatheter embolisation of bone tumour arteriovenous malformations. The British journal of radiology. 1978;51(606):414–9. doi: 10.1259/0007-1285-51-606-414. [DOI] [PubMed] [Google Scholar]
- 20.Ohshika S, Yanagisawa M, Tsushima F, Ishibashi Y. Diagnosis and conservative treatment of a rare case of femoral intraosseous arteriovenous malformation in a patient with polyostotic fibrous dysplasia: A case report. Molecular and clinical oncology. 2019;10(6):587–91. doi: 10.3892/mco.2019.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart ES, Kelly MH, Brillante B, Chen CC, Ziran N, Lee JS et al. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. J Bone Miner Res. 2007;22(9):1468–74. doi: 10.1359/jbmr.070511. [DOI] [PubMed] [Google Scholar]
- 22.Kuznetsov SA, Cherman N, Riminucci M, Collins MT, Robey PG, Bianco P. Age-dependent demise of GNAS-mutated skeletal stem cells and “normalization” of fibrous dysplasia of bone. J Bone Miner Res. 2008;23(11):1731–40. doi: 10.1359/jbmr.080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corsi A, Fadda MT, Terenzi V, Valentini V, Riminucci M. Secondary desmoplastic fibroma-like tissue changes in mandibular fibrous dysplasia: clinicopathological and molecular study of a case. The British journal of oral & maxillofacial surgery. 2020;58(1):96–8. doi: 10.1016/j.bjoms.2019.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1. Animated 3D reconstruction of the cranium and vascular structures that demonstrate the arterial blood supply to the occipital arteriovenous fistula and the aneurysms that arose from the anomalous draining vein and were the cause of the abducens nerve palsy.
Supplemental Video 2. Serial pre- and post-embolization arteriographic images of the arterial blood supply to the occipital intraosseous arteriovenous fistula and the anomalous venous drainage and aneurysms. The first series depicts the external carotid artery injection that demonstrates the middle meningeal artery and posterior branches and their contribution to the arteriovenous fistula, followed by their disappearance or truncation after embolization. The second series is the pre- and post-injection images of the vertebral artery that demonstrates the dural and muscular branches and their contribution to the arteriovenous fistula, again followed by their disappearance or truncation after embolization.


