Abstract
Efforts toward the synthesis of the decalin ring system common to the hibarimicin shunt metabolite HMP-Y1 and parent aglycone hibarimicinone are reported herein. An intramolecular Diels–Alder cyclization rapidly generated the decalin framework. Two approaches toward completion of the AB decalin were vetted. Incorporation of a phenylsulfonyl leaving group β- to both a ketone and a γ-lactone followed by base-induced elimination of sulfinate led to the undesired α,β-unsaturated lactone. Methanolysis of the γ-lactone followed by elimination produced the unexpected bridged cyclic ether by way of an intramolecular oxy-Michael addition of the endo oriented C13 alcohol.
Keywords: Natural products, Diels–Alder cycloaddition, Michael addition, Stereoselective, Total synthesis
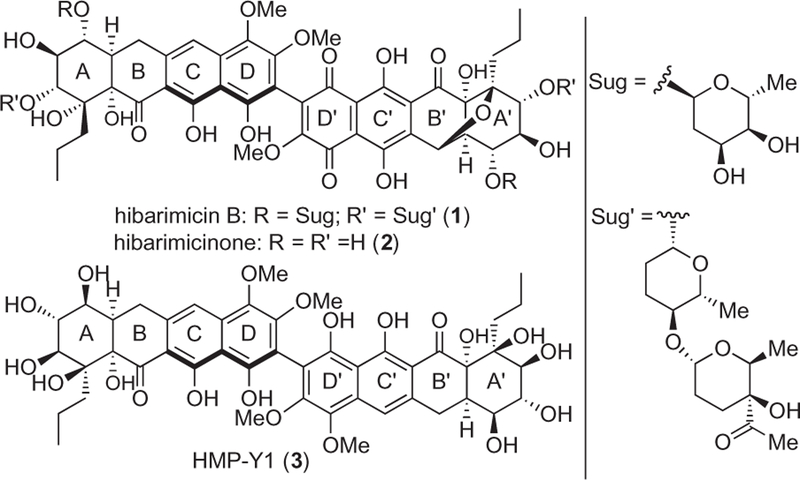
Hibarimicin B (1) was isolated from the soil bacterium Microbispora rosea in 1998, and its structure was proven identical to the previously reported angelmicin B (Fig. 1).1–4 The hibarimicins were reported to modestly inhibit src tyrosine kinase (hibarimicin A, IC50 = 5.8 µM, hibarimicin B, IC50 = 23 µM) and exhibited cytotoxicity against B16–F10 (murine melanoma) and HCT-116 (human colon carcinoma) cells at concentrations ranging from 0.41 to 1.1 µM. The 100-fold concentration difference between in vitro kinase inhibition and cancer cell growth inhibition (HL-60, IC50 = 57 nM) suggests further studies into their mode of action is warranted.5 Biosynthetic and structural studies demonstrated the aglycon hibarimicinone (2) to precede the hibarimicins, and arise from oxidation of a C2-symmetric precursor termed HMP-Y1 (3).6–8
Figure 1.
Hibarimicin B (1), hibarimicinone (2) and HMP-Y1 (3).
The symmetric structure of hibarimicinone is suggestive of a two-directional synthetic strategy, of which our laboratory9–12 and others13–16 have employed recently culminating in two total syntheses.17–19 With this strategy in mind we planned to utilize a biaryl D–D’ ring surrogate 4 of defined configuration in a bis-annulation reaction with a suitable unsaturated decalin 5 (Scheme 1). Furthermore, our ability to access 4 as a single atropisomer through our previously reported deracemization protocol avoids the necessity for separation of atropisomeric mixtures en route to the natural hibarimicinone isomer.12
Scheme 1.
Two-directional strategy and analysis of AB decalin 5.
Scheme 4.
Attempted B-ring enone formation through sulfone elimination.
At the time we initiated a synthetic route to the AB decalin 5 the absolute stereochemistry of hibarimicinone was unknown. Therefore, although 2 represents the now-defined absolute stereochemistry of hibarimicinone, we initially targeted the opposite absolute stereochemistry due to perceived ready access to the A-ring triol starting from methyl-α-d-gluco-pyranoside (8). Based on earlier studies of an intermolecular Diels–Alder approach10 to decalin 5 that failed to deliver the desired C9 ring fusion stereochemistry we elected to pursue an intramolecular Diels–Alder (IMDA) reaction employing 7 as the key Diels–Alder substrate. We anticipated triene 7 to be conveniently prepared starting from inexpensive methyl glucopyranoside 8.
Our synthesis of triene 7 proceeded by way of cyclohexenone 13 (Scheme 2). The multi-gram preparation of 13 started with a published five-step conversion of d-glucose 8 to the 6-iodo derivative 9.20,21 The latter was then subjected to a Vasella fragmentation by treatment with zinc dust in refluxing THF/H2O (Scheme 2).22 Due to instability upon concentration, aldehyde 10 was subjected without purification to a solution of vinylmagnesium bromide to afford an inconsequential mixture of secondary alcohols 11. Next, ring closing metathesis of 11 provided cyclohexenol 12 in good yield (46% over three steps). Finally, IBX oxidation of 12 in a solution of 1:1 DMSO/CH2Cl2 afforded enone 13 in 90% yield.
Scheme 2.
Preparation of cyclohexenone 13 from glucopyranoside 9.
Iodination of cyclohexenone 13 using the Johnson–Uskokovic protocol23 provided iodoenone 14, a key intermediate en route to IMDA substrate 7 incorporating an orthogonally protected C10 hydroxyl group, keto group poised for introduction of the C13 tertiary alcohol, and a C14 halide for vinylation. Suzuki cross coupling of 14 with the cyclic vinyl boronate provided dienone 15 in good yield (Scheme 3).24–26 We then turned our attention to installation of the C-13 stereocenter by addition of a 3-carbon allyl Grignard reagent to the keto group accompanied by C10 benzoate removal. Unfortunately, despite examination of numerous reaction conditions and reagents, this reaction suffered from low yield and diastereoselectivity, producing an optimized 2:1 mixture of inseparable diastereomers (16a and 16b) in 44% yield. The isomers were subject to esterification with β-phenylsulfonylacrylic acid, a dienophile chosen for its reactivity, and ready introduction of B ring unsaturation following base-mediated sulfinate elimination of the anticipated cycloadduct.27 To this end, Mukaiyama’s esterification conditions28 afforded ester 17 in 37% yield following separation of the minor diastereomeric ester derived from 16b. Upon heating a solution of 17 in toluene (0.02 M) over two days, we were pleased to observe clean conversion of 17 to decalin 18 as a single diastereomer. The assigned stereochemistry of 18 was based on the analysis of NOESY spectroscopy correlations.
Scheme 3.
Synthesis of decalin 18.
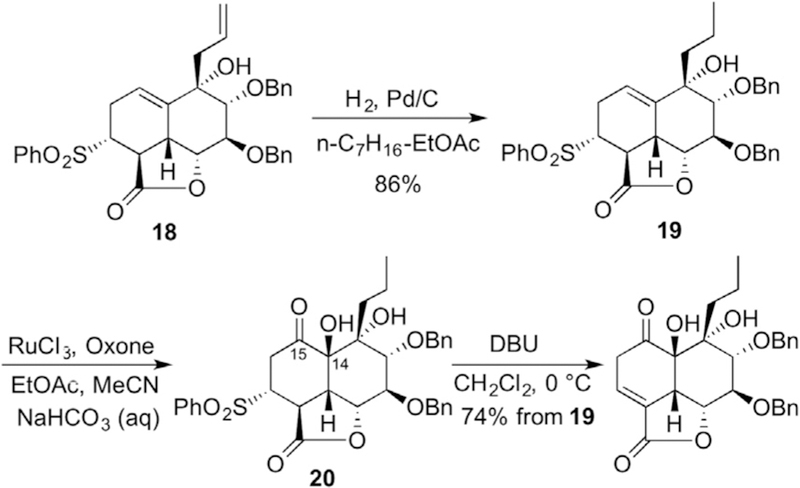
After screening various hydrogenation conditions we determined the allyl group of 18 could be selectively saturated under one atmosphere of hydrogen in a 3:2 mixture of heptane and ethyl acetate over 5% Pd/C (20 min) to provide 19 in 86% yield (Scheme 4). Ketohydroxylation of the C14–C15 alkene was accomplished using RuO4 in the presence of excess Oxone resulting in the stereoselective installation of the C14 hydroxyl group.29 The stereoselectivity of this oxidation presumably results from a combination of steric (convex approach) and electronic (allylic alcohol) effects.30,31 Despite examining various conditions, all attempts to purify ketone 20 resulted in decomposition. However, having finally arrived at an AB decalin incorporating all six stereocenters we subjected crude 20 to base-mediated (DBU, CH2Cl2) sulfinate elimination. Unfortunately, we observed exclusive formation of α,β-unsaturated lactone 21, an intermediate which proved unworkable in advancing to decalin 5. We reasoned the resistance of the double bond occupying the desired C16–C17 position arose from strain imparted from the fused γ-lactone, and thus we moved to relieve that strain prior to sulfinate elimination.32
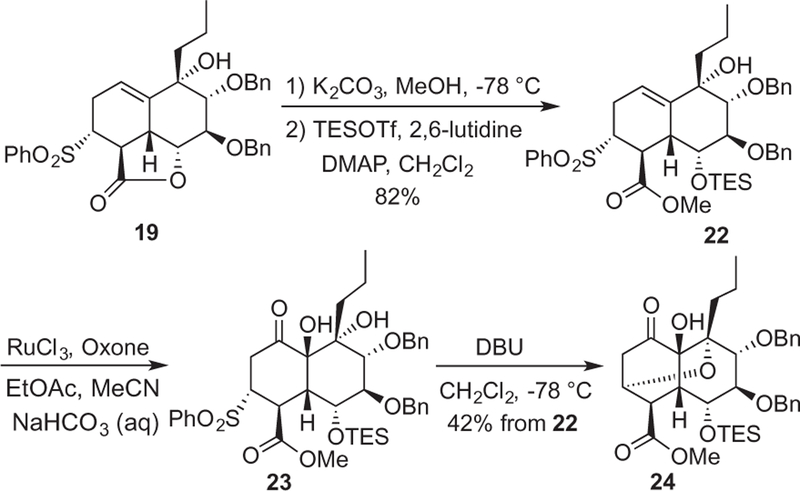
Treatment of lactone 19 with K2CO3 in methanol at a low temperature followed by TES protection of the released secondary alcohol afforded methyl ester 22 in 82% yield (Scheme 5). Oxidation of the C14–C15 trisubstituted olefin with RuO4 proceeded in good yield and stereoselectivity to afford α-hydroxyketone 23, a compound once again unstable to silica gel chromatography as experienced with α-hydroxyketone 20. When treated with DBU at a low temperature the former underwent sulfinate elimination and isomerization to bridged ether 24. This isomerization arises by way of base-mediated intramolecular Michael addition of the proximal C-13 tertiary alcohol. Notably, we observed no scrambling of the methyl ester stereocenter, possibly indicating the Michael addition perhaps proceeded by way of the desired α,β-unsaturated enone. Efforts to protect the C-13 alcohol and circumvent the undesired cyclization were unsuccessful.
Scheme 5.
Alternate route to B-ring enone leads to bridged ether formation.
In summary, during the course of our investigations to assemble an AB-decalin structure (5) to be employed in a two-directional approach to HMP-Y1, we encountered the unanticipated isomerization of 20–21 and enone interception of the C13 tertiary hydroxyl group (23–24). Workers successful in the total synthesis of HMP-Y1 and/or hibarimicinone avoided these obstacles by early protection of the C13 hydroxyl group. However, we demonstrated stereocontrolled decalin formation by way of a highly functionalized IMDA substrate (7), and we employed a novel method for installation of the essential b-hydroxyketone.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (CA 059515), and the Vanderbilt Institute of Chemical Biology.
Footnotes
Supplementary data
Supplementary data (experimental procedures and compound spectral data) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tetlet.2014.02.069.
References and notes
- 1.Kajiura T; Furumai T; Igarashi Y; Hori H; Higashi K; Ishiyama T; Uramoto M; Uehara Y; Oki T J. Antibiot 1998, 51, 394–401. [DOI] [PubMed] [Google Scholar]
- 2.Hori H; Igarashi Y; Kajiura T; Furumai T; Higashi K; Ishiyama T; Uramoto M; Uehara Y; Oki T J. Antibiot 1998, 51, 402–417. [DOI] [PubMed] [Google Scholar]
- 3.Uehara Y; Li P-M; Fukazawa H; Mizuno S; Nihei Y; Nishio M; Hanada M; Yamamoto C; Furumai T; Oki T J. Antibiot 1993, 46, 1306–1308. [DOI] [PubMed] [Google Scholar]
- 4.Hori H; Higashi K; Ishiyama T; Uramoto M; Uehara Y; Oki T Tetrahedron Lett 1996, 37, 2785–2788. [Google Scholar]
- 5.Yokoyama A; Okabe-Kado J; Uehara Y; Oki T; Tomoyasu S; Tsuroka N; Honma Y Leukemia Res 1996, 20, 491–497. [DOI] [PubMed] [Google Scholar]
- 6.Hori H; Kajiura T; Igarashi Y; Furumai T; Higashi K; Ishiyama T; Uramoto M; Uehara Y; Oki T J. Antibiot 2002, 55, 46–52. [DOI] [PubMed] [Google Scholar]
- 7.Kajiura T; Furumai T; Igarashi Y; Hori H; Higashi K; Ishiyama T; Uramoto M; Uehara Y; Oki T J. Antibiot 2002, 55, 53–60. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi Y; Kajiura T; Furumai T; Hori H; Higashi K; Ishiyama T; Uramoto M; Uehara Y; Oki T J. Antibiot 2002, 55, 61–70. [DOI] [PubMed] [Google Scholar]
- 9.Lee C-S; Audelo MQ; Reibenpies J; Sulikowski GA Tetrahedron 2002, 58, 4403–4409. [Google Scholar]
- 10.Lee WD; Kim K; Sulikowski GA Org. Lett 2005, 7, 1687–1689. [DOI] [PubMed] [Google Scholar]
- 11.Kim K; Mararoof USM; Raushel J; Sulikowski GA Org. Lett 2003, 5, 2777–2780. [DOI] [PubMed] [Google Scholar]
- 12.Romaine IM; Hempel JE; Shanmugam G; Hori H; Igarashi Y; Polavarapu PL; Sulikowski GA Org. Lett 2011, 13, 4538–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert WT; Roush WR Org. Lett 2005, 7, 5501–5504. [DOI] [PubMed] [Google Scholar]
- 14.Li J; Todaro LJ; Mootoo DR Org. Lett 2008, 10, 1337–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J; Todaro L; Mootoo DR Eur. J. Org. Chem 2011, 6281–6287. [DOI] [PMC free article] [PubMed]
- 16.Li J; Mootoo DR Synthesis 2013, 45, 2287–2293. [Google Scholar]
- 17.Tatsuta K; Fukuda T; Ishimori T; Yachi R; Yoshida S; Hashimoto H; Hosokawa S Tetrahedron Lett 2012, 53, 422–425. [Google Scholar]
- 18.Milgram BC; Liau BB; Shair MD Org. Lett 2011, 13, 6436–6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liau BB; Milgram BC; Shair MD J. Am. Chem. Soc 2012, 134, 16765–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sletten EM; Liotta LJ J. Org. Chem 2006, 71, 1335–1343. [DOI] [PubMed] [Google Scholar]
- 21.Hyldtoft L; Madsen R J. Am. Chem. Soc 2000, 122, 8444–8452. [Google Scholar]
- 22.Bernet B; Vasella A Helv. Chim. Acta 1984, 67, 1328–1347. [Google Scholar]
- 23.Johnson CR; Adams JP; Braun MP; Senanayake CBW; Wovkulich PM; Uskokovic MR Tetrahedron Lett 1992, 33, 917–918. [Google Scholar]
- 24.Kerins F; O’Shea DF J. Org. Chem 2002, 67, 4968–4971. [DOI] [PubMed] [Google Scholar]
- 25.Esteban J; Costa AM; Vilarrasa J Org. Lett 2008, 10, 4843–4846. [DOI] [PubMed] [Google Scholar]
- 26.Dai C; Fu GC J. Am. Chem. Soc 2001, 123, 2719–2724. [DOI] [PubMed] [Google Scholar]
- 27.Buser S; Vasella A Helv. Chim. Acta 2005, 88, 3151–3173. [Google Scholar]
- 28.Mukaiyama T; Usui M; Shimada E; Saigo K Chem. Lett 1975, 1045–1048.
- 29.Plietker B J. Org. Chem 2004, 69, 8287–8296. [DOI] [PubMed] [Google Scholar]
- 30.Cha JK; Christ WJ; Kishi Y Tetrahedron 1984, 40, 2247–2255. [Google Scholar]
- 31.Stork G; Kahn M Tetrahedron Lett 1983, 24, 3951–3954. [Google Scholar]
- 32.Ziegler FE; Jaynes BH; Saindane MT Tetrahedron Lett 1985, 26, 3307–3310. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.