Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, endothelial injury, glycocalyx degradation, platelet adhesion, thrombosis
Objectives:
Coronavirus disease 2019 is caused by the novel severe acute respiratory syndrome coronavirus 2 virus. Patients admitted to the ICU suffer from microvascular thrombosis, which may contribute to mortality. Our aim was to profile plasma thrombotic factors and endothelial injury markers in critically ill coronavirus disease 2019 ICU patients to help understand their thrombotic mechanisms.
Design:
Daily blood coagulation and thrombotic factor profiling with immunoassays and in vitro experiments on human pulmonary microvascular endothelial cells.
Setting:
Tertiary care ICU and academic laboratory.
Subjects:
All patients admitted to the ICU suspected of being infected with severe acute respiratory syndrome coronavirus 2, using standardized hospital screening methodologies, had daily blood samples collected until testing was confirmed coronavirus disease 2019 negative on either ICU day 3 or ICU day 7 if the patient was coronavirus disease 2019 positive.
Interventions:
None.
Measurement and Main Results:
Age- and sex-matched healthy control subjects and ICU patients that were either coronavirus disease 2019 positive or coronavirus disease 2019 negative were enrolled. Cohorts were well balanced with the exception that coronavirus disease 2019 positive patients were more likely than coronavirus disease 2019 negative patients to suffer bilateral pneumonia. Mortality rate for coronavirus disease 2019 positive ICU patients was 40%. Compared with healthy control subjects, coronavirus disease 2019 positive patients had higher plasma von Willebrand factor (p < 0.001) and glycocalyx-degradation products (chondroitin sulfate and syndecan-1; p < 0.01). When compared with coronavirus disease 2019 negative patients, coronavirus disease 2019 positive patients had persistently higher soluble P-selectin, hyaluronic acid, and syndecan-1 (p < 0.05), particularly on ICU day 3 and thereafter. Thrombosis profiling on ICU days 1–3 predicted coronavirus disease 2019 status with 85% accuracy and patient mortality with 86% accuracy. Surface hyaluronic acid removal from human pulmonary microvascular endothelial cells with hyaluronidase treatment resulted in depressed nitric oxide, an instigating mechanism for platelet adhesion to the microvascular endothelium.
Conclusions:
Thrombosis profiling identified endothelial activation and glycocalyx degradation in coronavirus disease 2019 positive patients. Our data suggest that medications to protect and/or restore the endothelial glycocalyx, as well as platelet inhibitors, should be considered for further study.
Critically ill patients admitted to the ICU infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have an overall mortality rate of approximately 31% (1). A cytokine storm was suggested to underlie disease severity (2), and we have previously published that coronavirus disease 2019 (COVID19) is associated with a unique proinflammatory response in the critically ill ICU patients that is dominated by tumor necrosis factor, serine proteases (granzyme B and elastase 2), heat shock protein 70, interleukin 18, and interferon-gamma inducible protein 10 (3). Furthermore, the plasma proteome from critically ill COVID19 patients was dominated by interleukins, chemokines, and membrane receptors linked to lymphocyte-associated micro-particles and debris (4). In addition to persistent inflammation, a prothrombotic state has been suggested based on the changes in the plasma levels of d-dimers, fibrinogen-degradation products, and antithrombin (5), and an elevated d-dimer at hospital admission is associated with increased odds of in-hospital death (6). COVID19 patients have thrombotic complications and microthrombi in the pulmonary vasculature observed on autopsy (7, 8). The mechanisms underlying the thrombotic risk in COVID19 patients are unclear, but thrombosis develops in critically ill patients through the dysregulation of coagulation and/or endothelial injury (9, 10). Understanding the mechanisms of increased thrombotic risk in COVID19 is foundational to identifying potential treatments.
Activated protein C is a key anticoagulant, and it serves as a physiologically relevant modulator of the inflammatory response (11). Protein C levels are decreased in sepsis from different infectious causes, but therapy with exogenous activated protein C has generally not improved sepsis outcome (12). Alternatively, a disintegrin and metalloprotease with thrombospondin type 1 repeats-13 (ADAMTS13) is essential for cleaving von Willebrand factor (vWF), a large multimeric glycoprotein that mediates platelet adhesion to endothelium (13, 14). ADAMTS13 levels are also decreased in sepsis of various infectious etiologies and are suggested to increase platelet–vessel-wall interaction (9).
Microvascular endothelial cell injury also precipitates thrombosis (9), with or without coagulation abnormalities, particularly in the alveolar capillary where COVID19 pneumonia and lung injury are observed clinically (7). Finally, platelet adhesion to the microvasculature is largely inhibited by the glycocalyx, a gel-like substance that coats the luminal surface of endothelial cells (15, 16). Inflammation-induced degradation of the glycocalyx is thought to contribute to microvascular pathology and thrombosis formation in sepsis of various etiologies.
The overall aim of this hypothesis-generating study was to characterize the thrombotic profile of critically ill COVID19 patients over the first 7 days of ICU care to identify potential therapeutic targets. Our specific objectives were: 1) to determine the thrombotic factors and endothelial injury markers changing between coronavirus disease 2019 positive (COVID19+) ICU patients and healthy control subjects; 2) to determine the thrombotic factors and endothelial injury markers that differ between COVID19+ and coronavirus disease 2019 negative (COVID19–) ICU patients; 3) to determine the changes in relevant thrombotic factors and endothelial injury markers over time in COVID19+ ICU patients; and 4) to determine if the thrombotic profile in COVID19+ patients is associated with poor outcome.
MATERIALS AND METHODS
Study Participants and Clinical Data
This study was approved by the Human Research Ethics Board, Western University (3, 4). We enrolled consecutive patients who were admitted to our level-3 academic ICUs at the London Health Sciences Centre (London, ON, Canada) and were suspected of having COVID19 based on the Centers for Disease Control and Prevention clinical screening criteria (17). We collected daily blood samples starting at admission and up to 3 days from COVID19– patients or 7 days from COVID19+ patients. COVID19 status was confirmed as part of standard hospital testing by the detection of two SARS-CoV-2 viral genes by polymerase chain reaction (18). Patient baseline characteristics were recorded at admission and included age, sex, comorbidities, medications, hematologic laboratories, creatinine, Pao2/Fio2 ratio, and chest radiograph findings. We calculated Multiple Organ Dysfunction Score (19) and Sequential Organ Failure Assessment score (20) for both COVID19+ and COVID19– patient groups to enable the objective comparison of their illness severity. We categorized both patient groups as having confirmed or suspected sepsis diagnosis using Sepsis 3.0 criteria (20). We also recorded clinical interventions received during the observation period including the use of antibiotics, antiviral agents, systemic corticosteroids, vasoactive medications, venous thromboembolism prophylaxis, antiplatelet or anticoagulation treatment, renal replacement therapy, high-flow oxygen therapy, and mechanical ventilation (invasive and noninvasive).
Once the first 10 COVID19+ patients were enrolled, 10 COVID19– patients were matched by age and sex only without the knowledge of other baseline characteristics. Healthy control subjects were previously banked in the Translational Research Centre, London, ON, Canada (https://translationalresearchcentre.com/) (21, 22).
Blood Draws
Standard operating procedures were used to ensure all samples were treated rapidly and equally. Blood was obtained from critically ill ICU patients via indwelling catheters daily in the morning and placed immediately on ice. Once transferred to a negative pressure hood, blood was centrifuged and plasma was isolated, aliquoted at 250 μL, and frozen at –80°C. All samples remained frozen until use and freeze/thaw cycles were minimized.
Enzyme-Linked Immunosorbent Assay
All plasma analytes were measured with immunoassays in duplicate as per the manufacturer’s recommendation. Analytes measured include ADAMTS13 (Abcam, Cambridge, MA; number ab234559, diluted 1:200), protein C (Assaypro, Saint Charles, MO; number EP1311-7, diluted 1:8), vWF (Thermo Fisher, Waltham, MA; number EHVWF, diluted 1:8,000), soluble platelet selectin (sP-selectin; Abcam number ab100631, diluted 1:50 or 1:20), heparan sulfate (TSZ ELISA; Biotang Inc., Lexington, MA; number HU8718, diluted 1:5), chondroitin sulfate (TSZ ELISA number HU8720, diluted 1:2), hyaluronic acid (R&D Systems, Minneapolis, MN; number DHYALO, diluted 1:20), and syndecan-1 (Abcam number ab46506, diluted 1:2).
Isolation and Culture of Human Pulmonary Microvascular Endothelial Cells
Human pulmonary microvascular endothelial cells (hPMVEC; a kind gift from Dr. S. Mehta) were isolated from resected human lung, as previously described (23, 24). Briefly, human peripheral lung tissue was finely minced and digested in 0.3% type II collagenase at 37°C. The digested suspension was filtered, centrifuged, and washed in phosphate buffered saline (PBS). Endothelial cells were then isolated using magnetic Dynabeads (Thermo Fisher; number 11155D) coated with antihuman CD31 antibody. Isolated cells were resuspended in endothelial growth media 2 (EGM-2) (Lonza, Morristown, NJ; CC-3162) with 10% fetal bovine serum and placed at 37°C in 5% co2 until 50% confluent, and then harvested and repurified using anti-CD31-coated magnetic microbeads as above. Pulmonary microvascular endothelial cell (PMVEC) were propagated in EGM-2 + 10% fetal bovine serum (FBS) and 20-mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) on fibronectin-coated flasks and passages 4–9 used.
Hyaluronidase Treatment of hPMVEC
PMVEC (2.5 × 104/well) were plated on the fibronectin-coated four-well plates in EGM-2 + 10% FBS and 20-mM HEPES. After 2 days, medium was changed to Hank’s balanced salt solution (+100-mM HEPES, no bicarbonate) + 0.01% bovine serum albumin and hPMVEC were treated for 1 hour with hyaluronidase (0.5 mg/mL; Sigma, St. Louis, MO; number H3506). Following the treatment, hPMVEC were loaded with a nitric oxide-sensitive fluorochrome (2-µM 4-Amino-5-Methylamino-2',7'-Difluorofluorescein Diacetate [DAF-FM DA]; Thermo Fisher; number D23844) for 1 hour before lysing with 0.5% sodium dodecylsulfate in PBS. After centrifugation for 10 minutes at 1 × 104 relative centrifugal force, the fluorescence of the triplicate aliquots of supernatant was measured using a Victor3 multilabel fluorescence microplate reader (Wallac; PerkinElmer, Waltham, MA) at 485/520 for DAF-FM. The nitric oxide donor 1,1-bis(2-aminoethyl)-2-hydroxy-3-oxotriazane (DETA NONOate) (20 µM; Cayman Chemical, Ann Arbor, MI; number 82120) was used as the positive control.
Population Statistics
Medians (interquartile ranges [IQRs]) and frequency (%) were used to report continuous and categorical variables, respectively. Continuous variables were compared using either the Mann-Whitney U test or the Kruskal-Wallis test, as appropriate, and categorical variables were compared using Fisher exact and chi-square tests. p values of less than 0.05 were considered statistically significant. All population statistics were conducted using SPSS Version 26 (IBM Corp., Armonk, NY). For data comparison that was nonsignificant, G*Power Version 3.1.9.4 (F. Faul, Universität Kiel, Germany) was used to determine the number of patients per cohort required to reach statistical significance potentially based on the measured values and 80% power (25).
Machine Learning
Nonlinear dimensionality reduction on the full datasets to only two dimensions were completed using the t-distributed stochastic neighbor embedding (t-SNE) algorithm (26). For classification, we pooled analyte data across days 1–3 for each of the COVID19+ and COVID19– cohorts and normalized observations within analyte. A random forest classifier was trained on the variables to predict COVID status. In addition, another random forest classifier was trained on the pooled analyte data for COVID19+ patients for days 1–3 to predict patient mortality. A random forest is a set of decision trees that we can interrogate to identify the features with the highest predictive value. We limited the decision trees to a maximum depth of six levels and constrained the forest to 10 trees in order to avoid overfitting the small dataset. We trained and tested the classifier using a three-fold cross-validation approach.
RESULTS
We investigated 10 patients with a positive diagnosis of COVID19 (median age, 61.0 yr; IQR, 54.8–67.0 yr), 10 age- and sex-matched patients with a negative diagnosis of COVID19 (median age, 58.0 yr; IQR, 52.5–63.0 yr), and 10 age- and sex-matched healthy control subjects (median age, 57.5 yr; IQR, 52.8–62.8 yr; p = 0.686). Baseline demographic characteristics, comorbidities, laboratory values, and chest radiograph findings are reported in Table 1. COVID19+ patients relative to COVID19– patients were more likely to have bilateral pneumonia (p = 0.001). Pathogens were confirmed in only two of the COVID19– patients (p = 0.001). Although chest radiographs in three COVID19– patients were normal at ICU admission, all patients had respiratory insufficiency with associated hypoxemia. Both anticoagulation and antiplatelet medications were balanced between the patient cohorts. All other reported baseline measures were nonsignificant between the patients.
Table 1.
Subject Demographics and Clinical Data
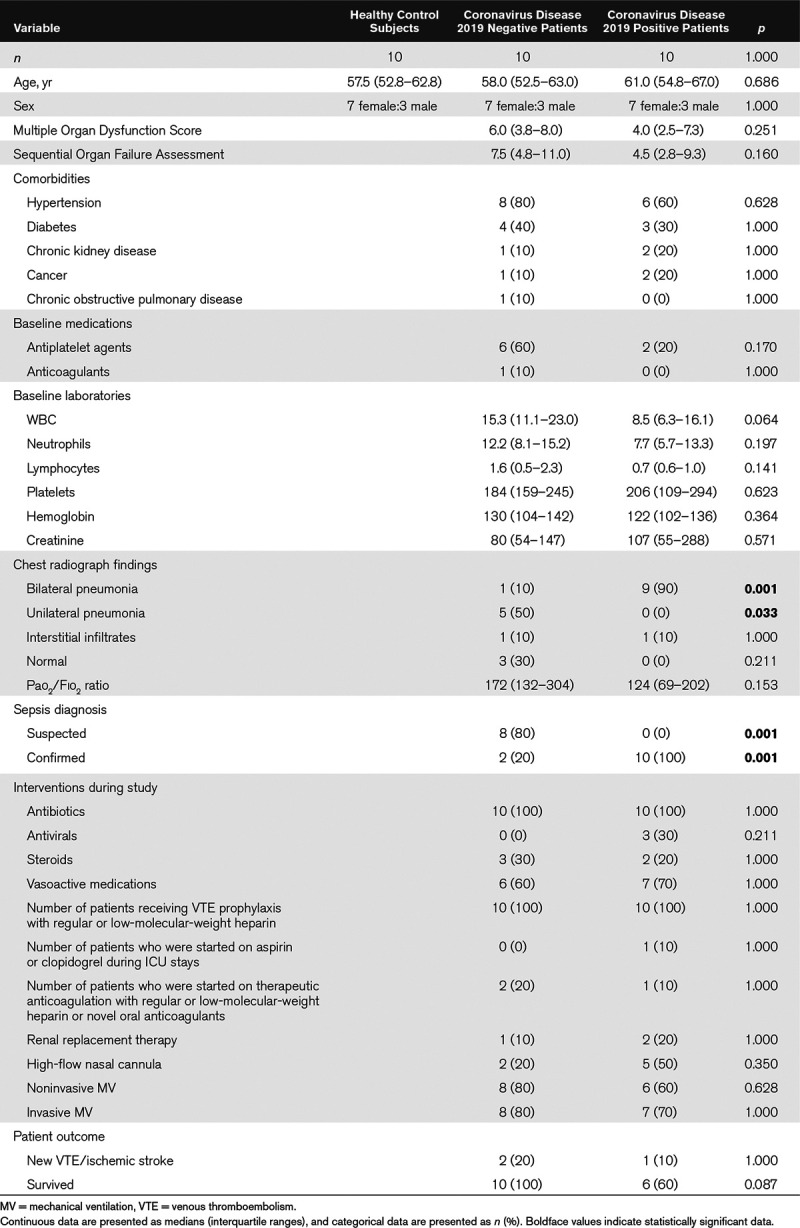
We measured three thrombosis factors and five endothelial cell injury markers in plasma using ELISAs. Table 2 shows that three markers (vWF, chondroitin sulfate, and syndecan-1) were significantly elevated in COVID19+ ICU patients relative to healthy control subjects. Table 3 lists the plasma measurements for eight markers between the COVID19+ and COVID19– patients on ICU days 1–3. Significant elevations were observed only in endothelial injury biomarkers, including sP-selectin (ICU day 3), heparan sulfate (ICU day 2), hyaluronic acid (ICU day 3), and syndecan-1 (ICU days 1–3).
Table 2.
Comparison of Coronavirus Disease 2019 Positive Patients on ICU Day 1 to Healthy Age- and Sex-Matched Control Patients
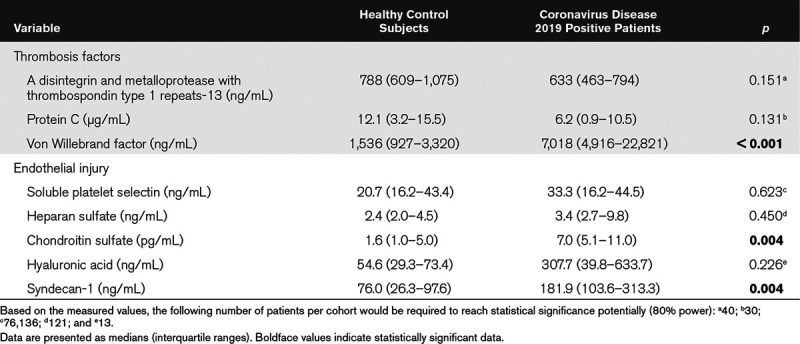
Table 3.
Comparison of Coronavirus Disease 2019 Negative and Coronavirus Disease 2019 Positive Patients on ICU Days 1–3
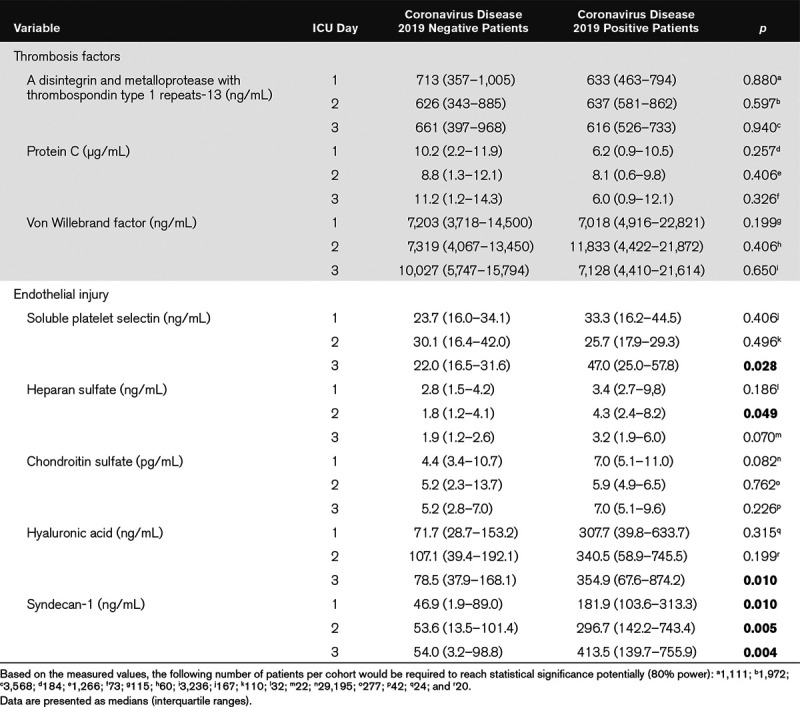
We then reduced the data to two dimensions using t-SNE to visualize the differences between the healthy control subjects and the COVID19+ patients (ICU days 1–3; Fig. 1A), as well as the COVID19– and COVID19+ patients (ICU days 1–3; Fig. 1B). In both cases, the COVID19+ patients were easily distinguishable from either healthy control subjects or COVID19– patients. We then trained and tested a random forest classifier that yielded a classifier accuracy, or the ability of the markers to predict COVID19 status, of 85% (five-fold cross-validation). To determine which of the eight markers were most informative for COVID19 status classification, we undertook feature selection with the random forest classifier. For ICU days 1–3, the top features in rank order were identified for the binary outcome of COVID19+ versus COVID19– as follows: syndecan-1 > hyaluronic acid > chondroitin sulfate > ADAMTS13 > heparan sulfate > protein C > sP-selectin > vWF. However, for ICU day 3 only, the top features in rank order were hyaluronic acid > sP-selectin > syndecan-1 >> ADAMTS13 > chondroitin sulfate = heparan sulfate > vWF > protein C.
Figure 1.
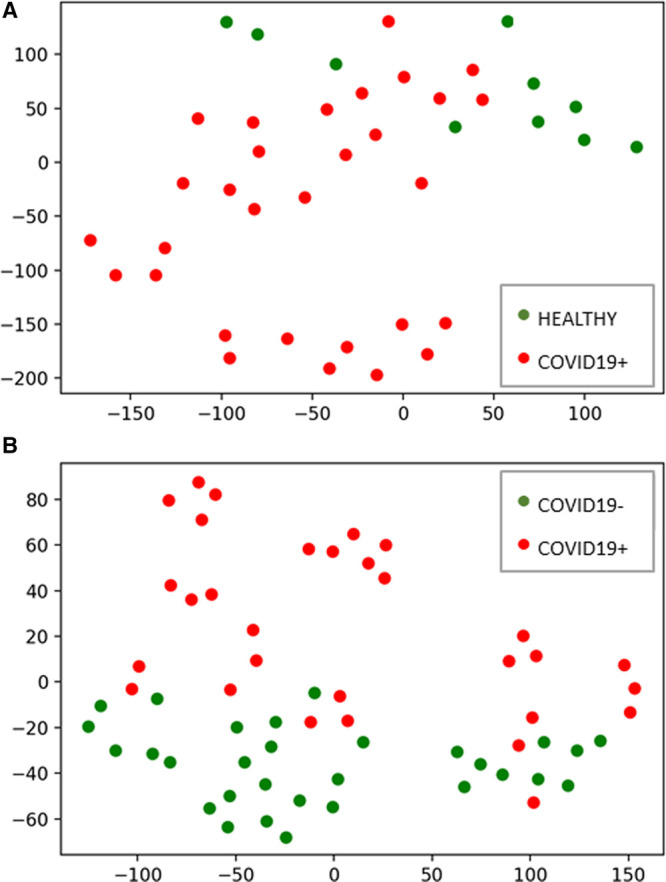
Thrombosis profiling with t-distributed stochastic neighbor embedding demonstrates that coronavirus disease 2019 positive (COVID19+) patients are distinct from either healthy control subjects or coronavirus disease 2019 negative (COVID19–) patients. A, Subjects plotted in two dimensions following dimensionality reduction by stochastic neighbor embedding. Red dots represent COVID19+ subjects (n = 10, days 1–3) and green dots healthy control subjects (n = 10). The dimensionality reduction shows that based on daily thrombotic factor and endothelial injury marker concentrations, the two cohorts are distinct and easily separable. The axes are dimensionless. B, ICU sepsis patients plotted in two dimensions following dimensionality reduction by stochastic neighbor embedding. Red dots represent COVID19+ subjects (n = 10, days 1–3) and green dots represent COVID19– subjects (n = 10, days 1–3). The dimensionality reduction shows that based on the daily thrombotic factor and endothelial injury marker concentrations, the two cohorts are distinct and easily separable. The axes are dimensionless.
Given the significant elevation in plasma sP-selectin, hyaluronic acid, and syndecan-1 on ICU day 3, we continued daily plasma measurements until ICU day 7 (Fig. 2). For all three endothelial injury biomarkers, the plasma levels remained elevated, indicating ongoing glycocalyx degradation.
Figure 2.
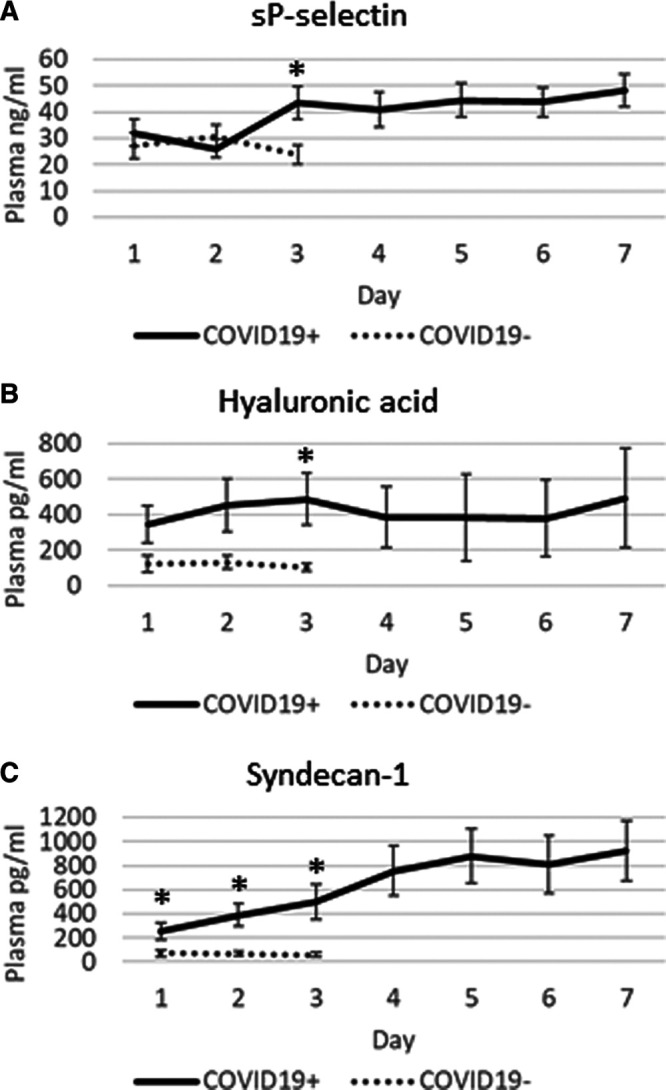
Time course for three endothelial injury markers between the coronavirus disease 2019 positive (COVID19+) and coronavirus disease 2019 negative (COVID19–) ICU patients. Soluble platelet selectin (sP-selectin), hyaluronic acid, and syndecan-1 remained elevated until the final plasma measurements on ICU day 7. Daily values are represented as means (± sem; *p < 0.05).
To determine a relationship between the thrombotic state and the outcome, we trained and tested a random forest classifier to determine the ability of the eight markers on ICU days 1–3 to predict mortality in COVID19+ patients. The thrombosis profile yielded a classifier accuracy, or the ability of the markers to predict mortality, of 86% (three-fold cross-validation).
Given the reliance of the classification accuracy on hyaluronic acid degradation and the reports of injury to the pulmonary endothelium with COVID19, we specifically removed hyaluronic acid from human hPMVEC with hyaluronidase treatment (Supplemental Fig. 1, http://links.lww.com/CCX/A291). Hyaluronidase treatment decreased basal intracellular nitric oxide production by 98% to 64 ± 87.5 relative fluorescence units, compared with untreated human PMVEC (p = 0.008, n = 5 separate experiments). To assure the reactivity/specificity of the cell membrane permeable nitric oxide-sensitive probe (DAF-FM-DA), naïve hPMVEC were treated with a nitric oxide donor (DETA NONOate; 20 μM) that appropriately increased the intracellular levels of nitric oxide by 16% compared with untreated cells (data not shown), thereby validating our in vitro nitric oxide experiments.
DISCUSSION
In this study, we measured three thrombotic factors and five endothelial cell injury markers in plasma obtained from ICU patients, both COVID19+ and COVID19–, as well as age- and sex-matched healthy control subjects. Our data indicate increased vWF in COVID19+ patients relative to healthy control subjects but, more importantly, exaggerated and persistent injury to the endothelium in COVID19+ patients, as shown by elevated sP-selectin and glycocalyx degradation. As a complimentary experiment, we reproduced glycocalyx degradation in hPMVEC to demonstrate that the cleavage of hyaluronic acid significantly decreased basal nitric oxide. These latter data represent a physiologic mechanism for platelet adhesion to the injured human pulmonary endothelium, as depressed nitric oxide is a necessary component for platelet attraction to the microvascular wall (16).
Our COVID19+ ICU patients were similar to those reported in earlier cohorts from multiple countries with respect to age, comorbidities, and clinical presentation (6, 27–29). In contrast to COVID19– ICU patients, our COVID19+ ICU patients had a higher occurrence rate of bilateral pneumonia (3). The COVID19+ patients in our study appeared to have lower illness severity scores than the COVID19– patients, yet mortality was high at 40%. In contrast, all COVID19– ICU patients survived. Although these differences were not statistically significant, the findings suggest that acute respiratory distress syndrome in COVID19+ patients has worse outcomes, perhaps due to the persistently high levels of plasma serine proteases (3).
Only vWF was elevated in plasma from COVID19+ patients relative to healthy control subjects (30), whereas none of the three thrombotic factors (ADAMTS13, protein C, and vWF) measured differed significantly between the COVID19+ and COVID19– patients. Previous studies have demonstrated that ventilator strategies have no effect on vWF levels (31). Inflammation elevates vWF as an acute phase reactant, but elevated vWF levels also reflect endothelial activation and/or injury (13). Replenishment of ADAMTS13 via plasma administration has also been suggested as a potential treatment for thrombosis in COVID19 (32); however, plasma ADAMTS13 was not significantly different between the COVID19+ and COVID19– patients and plasma administration in sepsis patients is generally not recommended (33). Given its combined anticoagulant and anti-inflammatory properties, exogenous recombinant human activated protein C (rhAPC) might be a therapeutic option for COVID19. However, since we observed no difference in the degree of protein C reduction between the COVID19+ and COVID19– patients, and since rhAPC has demonstrated efficacy only in meningococcemia (34), but not in sepsis patients in general, rhAPC may be of limited benefit in COVID19 (12). Our power calculations (25) for both protein C and ADAMTS13 suggest much larger cohorts would be necessary to determine the differences between the cohorts, thereby questioning broad usage of these therapies.
Our data suggest that COVID19 results in endothelial injury and degradation of the endothelial glycocalyx. Specifically, sP-selectin, hyaluronic acid, and syndecan-1 were all significantly elevated by ICU day 3 in the plasma of COVID19+ patients and remained persistently elevated in plasma up to ICU day 7. The glycocalyx is a complex structure comprised of glycosaminoglycans (e.g., hyaluronic acid and chondroitin sulfate), proteoglycans (e.g., syndecan-1 and heparan sulfate), and various plasma proteins (e.g., albumin and antithrombin). Disturbance of the glycocalyx, often due to the increased expression and release of proteinases and glycosidases (e.g., hyaluronidases, sheddases, and matrix metalloproteinases), has profound consequences on vascular function (35). For example, loss of glycocalyx components decreases nitric oxide production and increases oxidant production, thereby facilitating ligand-receptor interactions and subsequent platelet recruitment to vascular endothelium (36). Loss of glycocalyx also facilitates the development of a prothrombotic phenotype due to the release of pathologic quantities of vWF (30), which promotes platelet aggregation and adhesion to the subendothelium and subsequent thrombi formation.
P-selectin is a cellular adhesion molecule stored in granules in both endothelial cells and platelets, which is quickly mobilized to the plasma membrane upon activation (37). During infection, P-selectin increases platelet aggregation and platelet–endothelial interactions. sP-selectin arises from an alternately spliced form in healthy individuals (38) and from the enzymatic shedding of mobilized surface P-selectin in inflammatory conditions (39). A procoagulant state results from mice engineered to express high levels of sP-selectin (40), although it appears dimerized sP-selectin (e.g., from microparticles or cell debris) is necessary to produce procoagulant effects (41). sP-selectin measurements from a plasma preparation will likely contain both dimerized and monomeric forms, somewhat confounding whether these measurements represent a causal agent or an effect of increased inflammation and coagulation (42). Although elevated plasma sP-selectin, together with increased plasma glycocalyx-degradation products, was highly suggestive of endothelial activation/injury, we cannot exclude platelet activation as an additional source of sP-selectin. Nevertheless, sP-selectin has been shown to be an important biomarker in several inflammatory/procoagulopathy diseases including systemic inflammatory response syndrome (37, 43, 44).
Hyaluronic acid is a long, unbranched, highly anionic disaccharide polymer able to interact with various cell-surface molecules, such as CD44 on endothelial cells. Hyaluronic acid is predominantly synthesized as a high-molecular-weight (HMW-hyaluronic acid; 1,000–6,000 kDa) polymer and under physiologic conditions, and HMW-hyaluronic acid offers anti-inflammatory, antiangiogenic, and immunosuppressive effects. In severe inflammation, the glycocalyx is shed and HMW-hyaluronic acid is released. This HMW-hyaluronic acid then binds fibrin and fibrinogen to increase clot formation (45).
Syndecan-1 is a proteoglycan containing both heparan- and chondroitin-sulfate chains that mediate cellular responses to signaling molecules as well as cell-cell and cell-matrix interactions (46). During inflammation, syndecan-1 functions to inhibit neutrophil adhesion and migration. Shedding of syndecan-1 from the cell surface is initiated by the heparanase-dependent removal of the heparan-sulfate side chains (47), thereby instigating subsequent cleavage of the core syndecan-1 protein by enzymes such as matrix metalloproteinases. Importantly, moderate syndecan-1 shedding is thought to aid in resolving inflammation; however, excessive shedding is likely pathogenic, as complete loss of syndecan-1 allows for increased leukocyte adhesion and recruitment across the endothelial monolayer, as well as enhanced platelet aggregation and adhesion.
Within the injured lung, platelet interaction with the pulmonary vasculature serves to augment inflammation and thrombi formation via the release of cytokines as well as procoagulation factors by the endothelial cells. Previous studies have demonstrated that pulmonary viral infections can drive platelet–endothelial interaction through the up-regulation of endothelial intracellular adhesion molecule 1, vWF, and fibronectin leading to ongoing lung injury (48). In addition, aggregation of activated platelets within the pulmonary microvasculature not only promotes lung inflammation and injury but also propagates viral pathogenesis (49). As such, platelet interaction with activated pulmonary endothelial cells, at least in part due to glycocalyx degradation and the subsequent decrease in nitric oxide production, promotes vascular occlusion, enhances inflammation, and drives viral pathogenesis. Inhibition of this interaction, through antiplatelet or thrombolytic therapies, could represent a potential therapeutic strategy (i.e., using reduced doses of recombinant tissue-type plasminogen activator over prolonged periods) for the treatment of severe viral infections, such as COVID19.
Our study has identified a unique prothrombotic state in the critically ill COVID19 patients that may be amenable to therapeutic targeting; however, our study has several limitations. First, we studied only critically ill patients and we cannot determine the thrombotic changes contributing to ICU admissions. Second, given the limited number of patients under study, we used two complimentary methods to analyze our data independently, and both methods arrived at similar conclusions. In addition, given the significant data, the paucity of information related to COVID19 illness, and the urgent need for novel therapeutics, our findings contribute critical understanding on the host response to SARS-CoV-2. These hypothesis-generating results are valuable for future studies on antithrombotic therapies, as well as clinical trials. Finally, we reported mortality as a clinical outcome in our COVID19+ patients; however, future studies with larger sample sizes can explore whether reported changes in thrombotic factors and endothelial injury markers correlate with additional clinical outcomes such as the duration of ICU and hospital stay or mortality.
Our study, taken in the context of the current literature, suggests that “not all coagulopathy is created equal.” Although an extreme prothrombotic state secondary to the development of anticardiolipin antibodies (50) and/or activated plasminogen (51) may develop in some COVID19 patients, others may be prothrombotic based on alveolar-capillary membrane denudation and exposure of tissue factor (52). Anticoagulants are one treatment strategy; however, low-molecular-weight heparin did not confer an overall survival advantage in COVID19 patients (53). The beneficial effects of specific therapeutic strategies may be diluted by patient and disease heterogeneity, suggesting that a personalized treatment approach is required. Our study revealed significant glycocalyx degradation in COVID19 patients, which can be measured in the patient’s plasma (hyaluronic acid and syndecan-1), suggesting that therapies to inhibit platelet adhesion (e.g., administration of nitric oxide via inhalation or by a donor [54]) and to protect/restore the glycocalyx may be therapeutically indicated. These latter interventions could include IV administration of sulodexide (55) and/or sphingosine-1-phosphate (56). Existing thrombus may also require a low-dose thrombolytic infusion (57).
ACKNOWLEDGMENTS
We thank the entire Lawson COVID19 Study Team for their support (Dr. Robert Arntfield, Dr. Ian Ball, Mr. Gordon Barkwell, Ms. Tracey Bentall, Dr. Karen Bosma, Ms. Saoirse Cameron, Ms. Eileen Campbell, Mr. David Carter, Dr. Carolina Gillio-Meina, Dr. Robert Hegele, Ms. Natalya Odoardi, Dr. Ram Singh, Dr. Kelly Summers, and Ms. Sue Tereschyn). We also thank for the enthusiastic assistance of the frontline Critical Care Nursing Staff at the London Health Sciences Centre.
Supplementary Material
Footnotes
The authors disclosed a patent pending (COVID-19 Treatment; #63044520).
We acknowledge funding from Western University (Research), the Departments of Medicine and Pediatrics at Western University, the Lawson Health Research Institute (https://www.lawsonresearch.ca/), the London Health Sciences Foundation (https://lhsf.ca/), and the AMOSO Innovation Fund.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
Collaborators: Robert Arntfield, Ian Ball, Gordon Barkwell, Tracey Bentall, Karen Bosma, Saoirse Cameron, Eileen Campbell, David Carter, Carolina Gillio-Meina, Robert Hegele, Natalya Odoardi, Ram Singh, Kelly Summers, and Sue Tereschyn
REFERENCES
- 1.Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020. May 26. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta P, McAuley DF, Brown M, et al. ; HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395:1033–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser DD, Cepinskas G, Slessarev M, et al. Inflammation profiling of critically ill coronavirus disease 2019 patients. Crit Care Explor. 2020; 2:e0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser DD, Cepinskas G, Patterson EK, et al. Novel outcome biomarkers identified with targeted proteomic analyses of plasma from critically ill coronavirus disease 2019 patients. Crit Care Expl. 2020; 2:e0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020; 191:148–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020; 220:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020; 383:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JC. Sepsis and septic shock: Endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 2019; 17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iba T, Levy JH, Levi M, et al. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Looney MR, Matthay MA. The role of protein C in sepsis. Curr Infect Dis Rep. 2007; 3:413–418 [PubMed] [Google Scholar]
- 12.Lai PS, Thompson BT. Why activated protein C was not successful in severe sepsis and septic shock: Are we still tilting at windmills? Curr Infect Dis Rep. 2013; 15:407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Chung DW. Inflammation, von Willebrand factor, and ADAMTS13. Blood. 2018; 132:141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levi M, Scully M, Singer M. The role of ADAMTS-13 in the coagulopathy of sepsis. J Thromb Haemost. 2018; 16:646–651 [DOI] [PubMed] [Google Scholar]
- 15.Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2019; 17:283–294 [DOI] [PubMed] [Google Scholar]
- 16.Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit Care. 2019; 23:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention: Overview of Testing for SARS-CoV-2. Available at: https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed August 18, 2020.
- 18.Centers for Disease Control and Prevention: CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. Available at: https://www.fda.gov/media/134922/download. Accessed August 18, 2020.
- 19.Priestap F, Kao R, Martin CM. External validation of a prognostic model for intensive care unit mortality: A retrospective study using the Ontario Critical Care Information System. Can J Anaesth. 2020; 67:981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brisson AR, Matsui D, Rieder MJ, et al. Translational research in pediatrics: Tissue sampling and biobanking. Pediatrics. 2012; 129:153–162 [DOI] [PubMed] [Google Scholar]
- 22.Gillio-Meina C, Cepinskas G, Cecchini EL, et al. Translational research in pediatrics II: Blood collection, processing, shipping, and storage. Pediatrics. 2013; 131:754–766 [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Chung J, Gill SE, et al. Quantification of adherens junction disruption and contiguous paracellular protein leak in human lung endothelial cells under septic conditions. Microcirculation. 2019; 26:e12528. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Mehta S, Ahmed Y, et al. Differential mechanisms of septic human pulmonary microvascular endothelial cell barrier dysfunction depending on the presence of neutrophils. Front Immunol. 2018; 9:1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009; 41:1149–1160 [DOI] [PubMed] [Google Scholar]
- 26.van der Maaten L, Hinton G. Visualizing data using t-SNE. J Mach Learn Res. 2008; 9:2579–2605 [Google Scholar]
- 27.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020; 180:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007; 120Suppl 1S5–S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricciuto DR, dos Santos CC, Hawkes M, et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med. 2011; 39:702–710 [DOI] [PubMed] [Google Scholar]
- 32.Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020; 7:e438–e440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rimmer E, Houston BL, Kumar A, et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: A systematic review and meta-analysis. Crit Care. 2014; 18:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alberio L, Lämmle B, Esmon CT. Protein C replacement in severe meningococcemia: Rationale and clinical experience. Clin Infect Dis. 2001; 32:1338–1346 [DOI] [PubMed] [Google Scholar]
- 35.Becker BF, Jacob M, Leipert S, et al. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br J Clin Pharmacol. 2015; 80:389–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilos M, Petousis S, Parthenakis F. Interaction between platelets and endothelium: From pathophysiology to new therapeutic options. Cardiovasc Diagn Ther. 2018; 8:568–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furie B, Furie BC. Role of platelet P-selectin and microparticle PSGL-1 in thrombus formation. Trends Mol Med. 2004; 10:171–178 [DOI] [PubMed] [Google Scholar]
- 38.Ishiwata N, Takio K, Katayama M, et al. Alternatively spliced isoform of P-selectin is present in vivo as a soluble molecule. J Biol Chem. 1994; 269:23708–23715 [PubMed] [Google Scholar]
- 39.Michelson AD, Barnard MR, Hechtman HB, et al. In vivo tracking of platelets: Circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci U S A. 1996; 93:11877–11882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.André P, Hartwell D, Hrachovinová I, et al. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci U S A. 2000; 97:13835–13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panicker SR, Mehta-D’souza P, Zhang N, et al. Circulating soluble P-selectin must dimerize to promote inflammation and coagulation in mice. Blood. 2017; 130:181–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gross PL. Soluble P-selectin is the smoke, not the fire. Blood. 2017; 130:101–102 [DOI] [PubMed] [Google Scholar]
- 43.Schrijver IT, Kemperman H, Roest M, et al. Soluble P-selectin as a biomarker for infection and survival in patients with a systemic inflammatory response syndrome on the intensive care unit. Biomark Insights. 2017; 12:1177271916684823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katayama M, Handa M, Araki Y, et al. Soluble P-selectin is present in normal circulation and its plasma level is elevated in patients with thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome. Br J Haematol. 1993; 84:702–710 [DOI] [PubMed] [Google Scholar]
- 45.LeBoeuf RD, Gregg RR, Weigel PH, et al. Effects of hyaluronic acid and other glycosaminoglycans on fibrin polymer formation. Biochemistry. 1987; 26:6052–6057 [DOI] [PubMed] [Google Scholar]
- 46.Teng YH, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012; 31:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fitzgerald ML, Wang Z, Park PW, et al. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000; 148:811–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugiyama MG, Gamage A, Zyla R, et al. Influenza virus infection induces platelet-endothelial adhesion which contributes to lung injury. J Virol. 2016; 90:1812–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rommel MGE, Milde C, Eberle R, et al. Endothelial-platelet interactions in influenza-induced pneumonia: A potential therapeutic target. 2019. Dec 2. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020; 382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji HL, Zhao R, Matalon S, et al. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev. 2020; 100:1065–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hofstra JJ, Haitsma JJ, Juffermans NP, et al. The role of bronchoalveolar hemostasis in the pathogenesis of acute lung injury. Semin Thromb Hemost. 2008; 34:475–484 [DOI] [PubMed] [Google Scholar]
- 53.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020; 18:1094–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martel J, Ko YF, Young JD, et al. Could nasal nitric oxide help to mitigate the severity of COVID-19? Microbes Infect. 2020; 22:168–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carroll BJ, Piazza G, Goldhaber SZ. Sulodexide in venous disease. J Thromb Haemost. 2019; 17:31–38 [DOI] [PubMed] [Google Scholar]
- 56.Zeng Y, Adamson RH, Curry FR, et al. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014; 306:H363–H372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Hajizadeh N, Moore EE, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J Thromb Haemost. 2020; 18:1752–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


