Abstract
Purpose
The global coronavirus 2019 (COVID-19) pandemic has created unprecedented strains on healthcare systems around the world. Challenges surrounding an overwhelming influx of patients with COVID-19 and changes in care dynamics prompt the need for care models and processes that optimize care in this medically complex patient population. The purpose of this report is to describe our institution’s strategy to deploy pharmacy resources and standardize pharmacy processes to optimize the management of patients with COVID-19.
Methods
This retrospective, descriptive report characterizes documented pharmacy interventions in the acute care of patients admitted for COVID-19 during the period April 1 to April 15, 2020. Patient monitoring, interprofessional communication, and intervention documentation by pharmacy staff was facilitated through the development of a COVID-19–specific care bundle integrated into the electronic medical record.
Results
A total of 1,572 pharmacist interventions were documented in 197 patients who received a total of 15,818 medication days of therapy during the study period. The average number of interventions per patient was 8. The most common interventions were regimen simplification (15.9%), timing and dosing adjustments (15.4%), and antimicrobial therapy and COVID-19 treatment adjustments (15.2%). Patients who were admitted to an intensive care unit care at any point during their hospital stay accounted for 66.7% of all interventions documented.
Conclusion
A pharmacy department’s response to the COVID-19 pandemic was optimized through standardized processes. Pharmacists intervened to address a wide scope of medication-related issues, likely contributing to improved management of COVID-19 patients. Results of our analysis demonstrate the vital role pharmacists play as members of multidisciplinary teams during times of crisis.
Keywords: antimicrobial stewardship, care bundles, COVID-19, pandemics, pharmacists, organization & administration, pharmacy
KEY POINTS.
A pharmacy department’s response to the COVID-19 pandemic was optimized through standardized processes.
Results of a retrospective descriptive analysis show the quantity and scope of interventions clinical pharmacists are making in the care of patients with COVID-19.
The results demonstrate the vital role pharmacists play as members of multidisciplinary teams during times of crisis.
Since the first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was discovered in Wuhan, China, in December 2019, there has been an explosion of coronavirus disease 2019 (COVID-19) cases, leading to significant strains on healthcare systems and workers around the world.1 Hospitals have been forced to respond to a large influx of patients with COVID-19 while adjusting to staffing and medical supply shortages, including shortages of personal protective equipment (PPE) and critical medications such as antimicrobials, sedatives, and paralytics.2,3 The therapeutic management of patients with COVID-19 also constitutes a unique challenge. Patients can quickly decompensate, and unproven medical therapies are being used for treatment based on anecdotal or low-quality evidence.3,4 Many of these treatments also come with the potential for significant toxicity and a need for close monitoring, which requires the leadership of the pharmacist as a key part of the multidisciplinary team.
Pharmacists are well positioned to be a source of critical information and support during this public health crisis. Pharmacy services in critical care settings have been shown to reduce drug errors, adverse drug events (ADEs), morbidity and mortality rates, length of stay (LOS), and healthcare costs.6-10 Pharmacists also play a role in minimizing safety risks and conserving PPE by optimizing medication regimens to reduce entry to and exit from patient rooms.11 Managing and preventing drug shortages is a critical need during the COVID-19 pandemic, and pharmacists are well suited to meet this challenge.12,13
A pharmaceutical framework for the management of COVID-19 has been suggested by clinicians from around the world. This framework emphasizes pharmacist involvement in evidence-based decision-making regarding medications, assisting clinicians in formulating and adjusting drug regimens of patients with COVID-19, providing close monitoring of medication safety and efficacy, and managing drug interactions.2,14,15 Pharmacists have a history of responding similarly in previous outbreaks, such as the 2009 pandemic caused by influenza A virus subtype (H1N1), and national organizations such as the American Society of Health-System Pharmacists have issued guidance on the role of pharmacists in emergency preparedness.16-18 Although these concepts are familiar and part of the day-to-day fabric of pharmacy activities, the fluidity and rapidity of the spread of COVID-19 is unprecedented. Developing and implementing processes and interventions targeting specific disease syndromes has been shown to be an effective way to improve prescribing in the management of other infectious diseases, because messages can be focused, reinforced, and sustainable.19-22
Here we describe our experience and approach to management of patients with COVID-19 in a time when our state and healthcare organization were among those hit particularly hard by the pandemic. Utilizing aspects of the pharmaceutical framework for the management of COVID-19, our institution created a comprehensive care bundle within the electronic medical record (EMR). Decentralized clinical pharmacists were integrally involved in the care of patients with COVID-19 and used this comprehensive care bundle to help optimize patient care. The aim of this descriptive report is to share our experiences by describing pharmacy services at our institution during the COVID-19 pandemic.
Methods
The primary objectives of this analysis are to describe strategies used to standardize pharmacy processes to optimize the management of patients with COVID-19 and to quantify the volume and scope of pharmacist interventions during the peak of our pandemic response. Secondary objectives include describing clinical and medication use characteristics of patients with COVID-19 and analyzing documented interventions in patients receiving various medication therapies.
Workforce processes and bundle development.
Our hospital is a 537-bed community teaching hospital located in the state of Michigan. It has a central distribution pharmacy model and uses the Epic EMR (Epic Systems Corporation, Verona, WI). Clinical pharmacy services are provided by a team of clinical specialists (10.9 full-time equivalents), clinical pharmacists, and 4 postgraduate year 1 pharmacy residents. During the study time period, the number of confirmed COVID-19 cases in the state of Michigan rose from 9,334 to 28,059, with the number of reported deaths climbing from 337 to 1,921.23 The first patient with COVID-19 treated within our organization was admitted in mid-March 2020. The number of admitted patients with confirmed or suspected COVID-19 increased dramatically, with a proportionally greater increase in the intensive care unit (ICU) population. Elective procedures and surgeries were suspended. The ICU capacity of 36 beds was increased to 64 beds in order to house primarily patients with COVID-19. A 32-bed overflow special pathogens unit, previously equipped for management of critical care patients during a disaster response, was opened. The overall hospital census was below normal, so the clinical pharmacy coverage model was adjusted with a goal of 20 to 25 ICU patients per pharmacist and a maximum of 64 patients (to include 32 with COVID-19) for pharmacists covering intermediate care and general care patients. The pharmacy resident rotation schedule was revised so that residents could be part of the care teams during the crisis and still meet the requirements of the residency program. Pharmacists with previous critical care experience were oriented to COVID-19 protocols to ensure adequate clinical coverage for a potential ICU surge.
The institutional and department of pharmacy leaderships made it a priority to respect social distancing guidance and to protect the workforce. The department implemented strategies to limit healthcare worker exposure, both for immediate safety reasons and also to protect the ability to call upon those workers later for necessary in-house work and/or to provide relief for on-site pharmacists as the pandemic evolved. Therefore, as part of that overall strategy, many pharmacy staff members were authorized and encouraged to work from home prior to study initiation. Documentation fields for clinical pharmacy monitoring handoffs are built within our EMR, which facilitated a seamless transition to working from home. Daily interdisciplinary rounds for intermediate care and general care units were conducted via conference call, which allowed pharmacy staff members to participate. Pharmacists covering ICU patients attended rounds in person on the unit. Additional medication review was conducted through prospective audit and feedback mechanisms. Communication between pharmacy team members continued through phone calls, instant messaging, and secure text messaging.
Pharmacy personnel also led or supported organizational efforts in several ancillary ways. Pharmacy personnel collaborated to proactively develop strategies to mitigate resource limitations using a well-established process. The first step was identification of all available medications used for the treatment and management of COVID-19 (including sedatives, analgesics, paralytics, antimicrobials, continuous renal replacement therapy solutions, and immune modulators). We then worked with suppliers to obtain adequate stocks to meet the predicted pandemic surge. Daily shortage updates were disseminated to the pharmacy and hospital staffs. The most challenging medication shortages involved neuromuscular blockers and intravenous (i.v.) sedatives. The availability of products fluctuated, requiring careful oversight by pharmacists. In order to reduce PPE use by the pharmacy staff, the department leadership adjusted workflow in the i.v. room, consolidated medication deliveries to nursing units, and increased stocks in automated dispensing cabinets (ADCs) to reduce potential pharmacy technician exposure to SARS-CoV-2. Additionally, pharmacists adjusted medication orders and administration times during verification to help the nursing staff reduce trips into patient rooms.
Analysis of the rapidly evolving stream of primary literature pertaining to COVID-19 in order to contribute to the development of organizational guidelines was also a vital component of our response. This area of activity included incorporation of resident and student learners to maximize the review and evaluation process. Guidelines for COVID-19 management included a suite of recommendations emphasizing that standard therapy is supportive care and that antimicrobial use is guided by institutional antimicrobial stewardship principles. Additional recommendations included QTc monitoring (ie, cardiac monitoring of heart rate–corrected QT interval), and drug-drug interaction and laboratory monitoring targeted to COVID-19 therapies. Use of hydroxychloroquine was listed as a conditional “consider” recommendation, and we recommended avoidance of combined hydroxychloroquine and azithromycin therapy. All COVID-19 antimicrobial therapies and consideration of interleukin-6 inhibitor use required infectious diseases staff approval. Expanded access investigational new drug applications and clinical trial enrollment were also explored early in the pandemic response. This process was facilitated by redistribution of efforts by our oncology investigational research team and coordination between infectious diseases and pharmacy personnel and our investigational review board. During the latter half of the analysis, patient enrollment for an expanded-access trial of remdesivir (ClinicalTrials.gov trial identifier, NCT04323761) began.24 Qualifying patients were also subsequently enrolled in an expanded-access trial of convalescent plasma therapy (trial identifier, NCT04338360).25
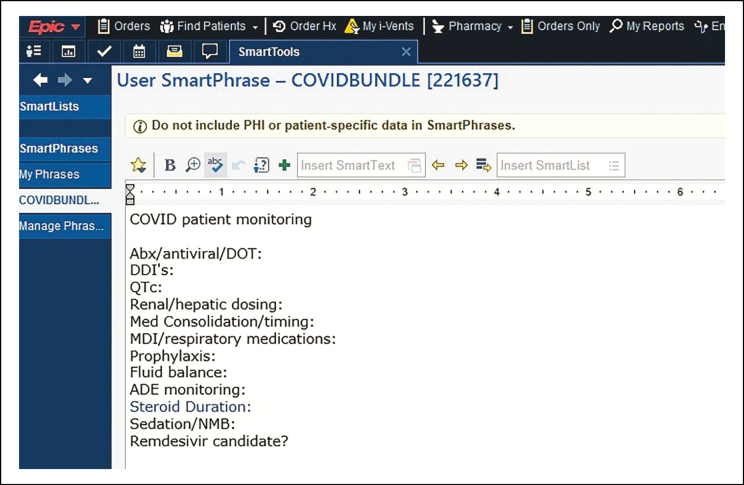
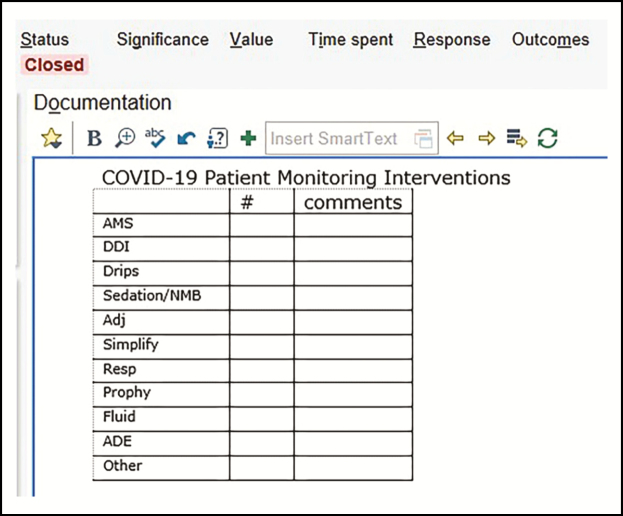
In order to standardize processes, the clinical pharmacy team developed a COVID-19 syndrome–specific intervention in the form of a standardized care bundle for management of patients with COVID-19. Elements of the bundle ensured that all aspects of care were addressed daily: optimization of medication therapy, streamlining of regimens for nursing workflow efficiency, and management of drug shortages. The components of the bundle were built in an EPIC SmartPhrase, which was loaded into the pharmacy handoff tool as a template for documentation (Figure 1). Interventions were captured using the EPIC iVent tool (Figure 2). The process for bundle review and documentation was not a unique concept for the pharmacy staff at our organization, as we use this strategy to target other high-impact infectious disease syndromes and the process is incorporated into the daily pharmacy workflow.20-22
Figure 1.
Screenshot of COVID-19 comprehensive care bundle SmartPhrase template within electronic medical record (Epic Systems Corporation, Verona, WI).
Figure 2.
Screenshot of field for documentation of pharmacists’ COVID-19–related interventions via COVID-19 iVent SmartPhrase within the electronic medical record (Epic Systems Corporation, Verona, WI).
Study design.
A retrospective, descriptive analysis covering the period April 1 through April 15, 2020, was conducted. Patients with COVID-19 were identified through review of EMR documentation. All patients during the study period with a documented pharmacy intervention and a positive SARS-CoV-2 test were included in the analysis. Positive tests were confirmed through reverse-transcriptase/polymerase chain reaction assay via nasopharyngeal swab. Medication utilization data were unavailable for 4 patients in our cohort, and these patients were excluded from medication utilization analyses. As patient admission may have occurred prior to the data collection period, total length of stay was calculated as the interval from admission to discharge or the end of the study period. Concurrent disease states was determined through EMR reporting. Medications were grouped for analysis according to therapeutic category. COVID-19 therapies were defined as hydroxychloroquine, tocilizumab, and remdesivir. Antipseudomonal antimicrobials were defined as any antimicrobial with activity against Pseudomonas aeruginosa. Medication days of therapy (DOT) was measured as the number of calendar days with any documented administration.
All pharmacy documentation was reviewed by research team members. Interventions were defined as any documentation of action by pharmacy personnel. Intervention types were classified according to predefined categorization built via SmartPhrase into iVent documentation. Research team members reviewed each intervention and categorically classified interventions based on written commentary by the clinical pharmacy team (if available). If commentary was unavailable, interventions were classified according to the designated category assigned by the pharmacist at the time of iVent documentation. Data analysis was performed using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA). The study was approved by the health system’s institutional review board.
Results
Clinical and demographic information on the 197 patients identified for inclusion in the analysis is presented in Table 1. Thirty-nine percent received care at any point in their stay in a designated ICU, and the average length of stay in the ICU during the study period was 7.9 days. At the end of the study period 47.2% of the patients remained admitted, 39.1% had been discharged home, and 11.7% had died.
Table 1.
Demographics and Clinical Characteristics of COVID-19 Cohort (n = 197)
| Variable | No. (%)a |
|---|---|
| Gender | |
| Male | 105 (53.3) |
| Female | 91 (46.2) |
| Age, mean (SD), y | 67 (16.7) |
| Length of stay through end of study period, d | 7.8 (5.6) |
| ICU stay, d | 75 (38.1) |
| ICU length of stay, mean (SD), d | 7.9 (4.7) |
| BMI ≥30 (kg/m2)b | 96 (49.5) |
| Concurrent disease states | |
| Hypertension | 133 (67.5) |
| Renal disease | 94 (47.7) |
| Diabetes | 62 (31.5) |
| Congestive heart failure | 33 (16.8) |
| Chronic obstructive pulmonary diseases | 29 (14.7) |
| Asthma | 18 (9.1) |
| Admission status during study period | |
| Admitted | 93 (47.2) |
| Discharged home | 77 (39.1) |
| Died | 23 (11.7) |
Abbreviations: BMI, body-mass index; ICU, intensive care unit.
aData are number (percentage) of patients unless otherwise specified.
bAnalysis included 194 patients with available data.
The 197 patients received a total of 15,818 medication DOT (Table 2). The average number of DOT per patient-day was 9.3, and patients received an average of 19.8 different medications. The most commonly received regimens included anticoagulation therapy included anticoagulation therapy (94.3%), electrolyte supplementation (83.9%), COVID-19-related treatment regimens (77.2%), and antimicrobials (69.2%). Excluding COVID-19–related treatments (eg, hydroxychloroquine), the average duration of antimicrobial therapy in patients treated for suspected or confirmed infection was 3.8 days. Antimicrobial use was generally directed towards community-acquired pathogens; however, 29% of patients received vancomycin and 34.7% received antipseudomonal coverage at any point during the study period. The average durations of vancomycin or antipseudomonal coverage were 2.1 and 3.0 days, respectively.
Table 2.
Pharmacotherapy Regimens of COVID-19 Cohort
| Variable | No. (%)a |
|---|---|
| Total DOT | 15,818 |
| Patient-days | 1,589 |
| DOT per patient-day, mean | 9.3 (4.2) |
| Distinct medications per patient, mean (SD) | 19.8 (10.5) |
| Treatment regimens received | |
| Anticoagulation | 182 (94.3) |
| Electrolytes | 162 (83.9) |
| COVID-19 treatment | 150 (77.7) |
| Antimicrobials | 134 (69.4) |
| Antimicrobial duration, mean (SD), db | 3.8 (2.8) |
| Vancomycin | 56 (29) |
| Duration, mean (SD), db | 2.1 (1.6) |
| Antipseudomonal antimicrobial | 67 (34.7) |
| Duration, mean (SD), db | 3 (2.4) |
| Acid-suppressant medications | 108 (56) |
| Corticosteroids | 93 (48.2) |
| Glucose management agents | 72 (37.3) |
| Sedation with or without neuromuscular blockers | 61 (31.6) |
Abbreviations: DOT, days of therapy.
aData are number (percentage) of patients (n = 193) unless otherwise specified
bAnalysis included subset of patients who received therapy.
There were a total of 659 iVent entries documenting 1,572 interventions throughout the study period (Table 3). The average number of interventions per patient was 8, with an average of 1 intervention per patient-day. There were documented interventions involving all elements of the COVID-19 monitoring bundle. The most common pharmacy interventions were regimen simplification (15.9%), timing and dosing adjustments (15.4%), antimicrobial and COVID-19 treatment adjustments (15.2%), and nonspecific interventions classified as other (12.6%). Documented interventions for patients receiving sedation with or without neuromuscular blockers and patients receiving antimicrobial therapy and/or other COVID-19 treatments averaged 3.5 and 1.6 interventions per patient, respectively.
Table 3.
Documented Pharmacy Interventions in COVID-19 Cohort (n = 197)
| Variable | Value |
|---|---|
| Total interventions | 1,572 |
| Interventions per patient, mean (SD) | 8 (9) |
| Interventions per patient-day, mean | 1 |
| Intervention types, No. (%)a | |
| Regimen simplification | 250 (15.9) |
| Timing and dosing adjustments | 242 (15.4) |
| Antimicrobials and COVID-19 treatment | 239 (15.2) |
| Sedation and neuromuscular blockers | 226 (14.4) |
| Other | 198 (12.6) |
| Prophylaxis | 155 (9.9) |
| Electrolytes | 57 (3.6) |
| Fluid management | 50 (3.2) |
| Adverse drug event avoidance and management | 43 (2.7) |
| Drug-drug interactions | 32 (2) |
| Respiratory medications | 32 (2) |
| Intravenous drips | 29 (1.8) |
| Experimental agent trial enrollment | 19 (1.2) |
| Interventions per patient receiving therapy, mean (SD) | |
| Sedation and neuromuscular blockers | 3.7 (4.7) |
| Antimicrobials and COVID-19 treatment | 1.5 (2.2) |
| Respiratory medications | 1 (0.9) |
| Electrolytes | 0.3 (0.7) |
Abbreviations: DOT, days of therapy.
aData are number (percentage) of total interventions.
Seventy-five (38.9%) patients received care in an ICU at any point in their stay. This cohort of patients accounted for 66.7% of documented interventions. The top interventions among ICU patients were sedation and neuromuscular blockade (14.1%), antimicrobials and COVID-19 therapy (11.3%), and timing and dosing adjustments (8.7%). The top interventions for patients without an ICU stay were regimen simplification (9.2%), timing and dosing adjustments (6.7%), and antimicrobials and COVID-19 therapy (5.1%).
Discussion
The study quantified the volume and scope of interventions by clinical pharmacists in the care of hospitalized patients with COVID-19 at our institution. Intervention data were reflective of work done by clinical pharmacists who were rounding at the bedside within the institution and those working remotely. We feel the use of standardized processes for patient monitoring in the form of a care bundle for patients with COVID-19 was an effective method for directing the focus of the clinical pharmacy team as they managed complicated patients in a challenging environment. This tool was also beneficial to our department and the care of our patients, as some staff members were reassigned and were caring for patient populations outside of their usual scope of practice. In addition, the care bundle streamlined and facilitated the pharmacist handoff process during alternating shifts and across multiple pharmacists.
Many pharmacy departments around the country will be faced with similar situations throughout the remainder of the COVID-19 pandemic as well as when planning for future disaster responses. Our descriptive analysis may help to guide allocation of pharmacy personnel in resource-limited settings by showing the quantity of interventions made during our experience responding to a surge in COVID-19 cases. The most common interventions during the study period were regimen simplification and adjustment of the timing or dosages of medications. Our clinical pharmacy department placed an emphasis on minimizing the bedside exposure of other healthcare workers, so efforts were made to adjust regimens so that medications administration times were consolidated and to discontinue unnecessary medications as part the bundle of care.
Antimicrobial stewardship–related interventions closely followed simplification- and dosing/timing-related interventions, with each of those 3 intervention categories accounting for approximately 15% of all documented interventions. Our organization maintains a robust antimicrobial stewardship program complete with well-established guidelines and treatment recommendations. Daily antimicrobial surveillance combined with prospective audit and feedback is a performance expectation for all clinical pharmacists. The influx of patients with COVID-19 presented a unique opportunity to reinforce established principles as well as relay emerging and evolving COVID-19 treatment recommendations in a hectic patient care environment. The high rate of antimicrobial-oriented interventions, results of review of documentation commentary, and relatively short durations of antimicrobial use (including vancomycin and antipseudomonal agents) suggest antimicrobial streamlining in areas such as avoidance of unneeded antimicrobial therapy and changing or escalating therapy when necessary. These practices were not dissimilar from routine antimicrobial stewardship efforts in our institution; however, the unfamiliar nature of the disease created unique opportunities to optimize antimicrobial therapy in a complex patient population.
The most common interventions for patients who received care in an ICU were related to sedation and/or neuromuscular blockade. Clinical pharmacists rounding in the ICU were relied upon by the care teams to guide therapy, given the profound shortages of first-line sedatives and neuromuscular blockers.3 Prevention of self-extubation in patients with COVID-19 requiring mechanical ventilation was also a priority for clinical pharmacists within the ICU, as evidenced by an average of 3.7 sedation interventions per patient. Emergent intubations pose a significant risk of exposure for the providers performing the procedure due to a high likelihood of viral load in the airway.26 Inadequate sedation is a significant risk factor for self-extubation, so vigilant monitoring and adjustment of therapy to maintain adequate sedation is essential.27
A significant limitation of our descriptive study was that patients were included in the analysis only if they had an intervention documented by a clinical pharmacist. Patients who were positive for SARS-CoV-2 without a documented intervention were not captured by the EMR reports used for data collection; this inherently biased the results for number of interventions per patient. However, the primary objective of the analysis was to quantify the type and scope of interventions, which we successfully did. It is also a limitation that we were unable to correlate interventions with clinical outcomes. However, previous studies have shown that prospective pharmacy involvement and interventions improve outcomes in critically ill patients and those with infectious diseases. A study by Leguelinel-Blache and colleagues28 found that bundled care services provided by ICU pharmacists to 1,164 critically ill patients led to decreases in ICU LOS and duration of mechanical ventilation of 1.2 days and 1.4 days, respectively, and decreased overall cost of care by €10,840.
A scoping review by Hammond and colleagues29 evaluated 93 studies of cost avoidance resulting from common pharmacy interventions in critically ill patients. Interventions were grouped into 6 overarching sections: ADE prevention, resource utilization, individualization of care, prophylaxis, hands-on care, and administrative tasks. Although such an evaluation was beyond the scope of our analysis, it is likely that the interventions made for COVID patients led to cost avoidance like that seen by Hammond and colleagues. Interventions evaluated in our analysis could also be grouped into these categories, as the scope of our work was not dissimilar from interventions normally made for critically ill patients. However, our experience caring for patients with COVID-19 differed from normal practice because of the increased quantity of critically ill patients, reassignment of other healthcare practitioners to critical care units outside of their usual scope of practice, and the rapidity with which treatment strategies evolved.
In contrast to usual practice, during the study period pharmacists dealt with widespread shortages of medications commonly used in critically ill patients. Medical teams relied heavily on pharmacists to combat these unprecedented shortages by using therapeutic strategies they otherwise might have been unfamiliar with. For example, many critically ill patients were switched to sedation with ketamine or high-dose enteral opioids in the absence of more familiar agents like propofol and fentanyl. Pharmacists also had an unusually heightened awareness of the need to conserve medications and PPE, which was likely reflected in the pattern of interventions reported. Pharmacists also played an important role in patient care in the ICU population due to the number of physicians, mid-level practitioners, and nurses reassigned to critical care units outside of their usual practice areas. In many cases the pharmacist was the only experienced ICU clinician and took a lead role in formulating therapeutic plans. This situation was unique to the COVID-19 pandemic response; under usual circumstances, critically ill patients are exclusively cared for by experienced ICU providers. Treatment strategies also evolved quickly with the release of new primary literature and guidelines during the onset of our health system’s pandemic response. Pharmacists took the lead in evaluating this literature and formulating treatment strategies that they disseminated to medical providers and the rest of the patient care team. This surge of evolving treatment strategies was unprecedented, and pharmacists were essential in ensuring safe usage and monitoring. A new role for many pharmacists at our institution was aiding in the identification of patients who may qualify for clinical trial enrollment. A monitoring reminder in the COVID-19 bundle facilitated a daily review of patient-specific criteria. Identified patients were referred to research team members for further review.
Our descriptive study was conducted at a single institution and captured pharmacy interventions during only 2 weeks of what we hope proves to be the peak phase of the COVID-19 pandemic at our institution. Therefore, the study results may not be generalizable to all institutions, but the study provided a good framework for clinical pharmacy involvement in response to a pandemic. When developing and implementing the bundle, we educated clinical pharmacy staff on the bundle elements to standardize documentation of interventions. However, there were 16 clinical pharmacists who documented interventions, so there was likely variability in reporting and categorization. Our analysis did not assess the acceptance rate for each of the evaluated interventions or the exact details of all interventions made.
Pharmacists have a history of responding during times of need such as the H1N1 influenza pandemic. Pharmacists around the world cared for critically ill H1N1-infected patients as well as serving on the front lines to educate communities and administer vaccinations. A study by Miller and colleagues30 showed an increase in the proportion of patients willing to receive the H1N1 vaccine from 69.3% to 81.4% following intervention by student pharmacists. The value of pharmacists in response to a pandemic has also been highlighted by influential organizations like the American College of Chest Physicians, which has pointed out that “during a disaster or pandemic, serious medication errors are likely to increase . . . . Pharmacists play an important role on interdisciplinary ICU teams as medication safety experts.” 31 Pharmacists take an oath to “devote myself to a lifetime of service to others” and to “consider the welfare of humanity and relief of suffering my primary concerns.” 32 Pharmacists at our institution and around the world are fulfilling this oath during the COVID-19 pandemic.
Results of our analysis show the quantity and scope of interventions clinical pharmacists are making in the care of patients with COVID-19. Our institution is not unique. We are confident this analysis is representative of the impact pharmacists are having as they work tirelessly to improve the treatment and management of patients with COVID-19 in healthcare systems and across the continuum of care around the world.
Conclusion
A pharmacy department’s response to the COVID-19 pandemic was optimized through standardization of clinical monitoring and documentation processes. Pharmacists intervened to address a wide scope of medication-related issues, likely contributing to improved management of patients with COVID-19.
Acknowledgments
The authors acknowledge the immense contributions of the entire department of pharmacy staff at the study institution, not only their participation in the study through dutiful documentation of efforts but also their extraordinary care and self-sacrifice provided during the pandemic response.
Disclosures
Dr. Collins reports a consulting arrangement with ASHP Consulting. The other authors have declared no potential conflicts of interest.
References
- 1. Han Q, Lin Q, Jin S, You L. Coronavirus 2019-nCoV: A brief perspective from the front line. J Infect. 2020;80:373-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu S, Luo P, Tang M, et al. . Providing pharmacy services during the coronavirus pandemic. Int J Clin Pharm. 2020. doi: 10.1007/s11096020010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. US Food & Drug Administration. FDA drug shortages.www.accessdata.fda.gov/scripts/drugshortages/default.cfm. Accessed April 20, 2020.
- 4. Bhimraj A, Morgan RL, Hirsch Shumaker A, et al. . Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Published April 11, 2020. Accessed April 20, 2020.
- 5. Alhazzani W, Møller MH, Arabi YM, et al. . Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020. doi. 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MacLaren R, Bond CA, Martan SJ, Fike D. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 2008;36:3184-3189. [DOI] [PubMed] [Google Scholar]
- 7. Leape LL, Cullen DJ, Clapp MD, et al. . Pharmacist participation of physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267-270. [DOI] [PubMed] [Google Scholar]
- 8. Bond CA, Raehl CL, Franke T. Clinical pharmacy services and hospital mortality rates. Pharmacotherapy. 1999;19:556-564. [DOI] [PubMed] [Google Scholar]
- 9. Rivkin A, Yin H. Evaluation of the role of the critical care pharmacist in identifying and avoiding or minimizing significant drug-drug interactions in medical intensive care patients. J Crit Care. 2011;26:104, e1-e6. [DOI] [PubMed] [Google Scholar]
- 10. Lee H, Ryu K, Sohn Y, et al. . Impact on patient outcomes of pharmacist participation in multidisciplinary critical care teams: a systematic review and meta-analysis. Crit Care Med. 2019;47:1243-1250. [DOI] [PubMed] [Google Scholar]
- 11. Stringer KA, Puskarich MA, Kenes MT, Dickson RP. COVID-19: The uninvited guest in the intensive care unit (ICU): implications for pharmacotherapy. Pharmacotherapy. 2020. doi: 10.1002/phar.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fox ER, Birt A, James KB, et al. . ASHP guidelines on managing drug product shortages in hospitals and health systems. Am J Health-Syst Pharm. 2009;66:1399-1406. [DOI] [PubMed] [Google Scholar]
- 13. Paskovaty A, Lucarelli CD, Patel P, et al. . Antimicrobial stewardship efforts to manage a pentamadine shortage. Am J Health-Syst Pharm. 2014;71:2014-2018. [DOI] [PubMed] [Google Scholar]
- 14. Gross AE, MacDougall C. Roles of the clinical pharmacist during the COVID-19 pandemic. J Am Coll Clin Pharm. 2020. doi: 10.1002/jac5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song Z, Hu Y, Zheng S, et al. . Hospital pharmacists’ pharmaceutical care for hospitalized patients with COVID-19: recommendations and guidance from clinical experience. Res Social Adm Pharm. 2020. doi: 10.1016/j.sapharm.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Society of Health-System Pharmacists. ASHP statement on the role of health-system pharmacists in emergency preparedness. Am J Health-Syst Pharm. 2003;60:1993-1995. [DOI] [PubMed] [Google Scholar]
- 17. Ijo I, Feyerharm J. Pharmacy intervention on antimicrobial management of critically ill patients. Pharm Pract (Granada). 2011;9:106-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alkhalili M, Ma J, Grenier S. Defining roles for pharmacy personnel in disaster response and emergency preparedness. Disaster Med Public Health Prep. 2017;11:496-504. [DOI] [PubMed] [Google Scholar]
- 19. Barlam TF, Cosgrove SE, Abbo LM, et al. . Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51-e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collins CD, Kabara JJ, Michienzi SM, Malani AN. Impact of an antimicrobial stewardship care bundle to improve the management of patients with suspected or confirmed urinary tract infection. Infect Control Hosp Epidemiol. 2016;37:1499-1501. [DOI] [PubMed] [Google Scholar]
- 21. Brumley PE, Malani AN, Kabara JJ, et al. . Effect of an antimicrobial stewardship bundle for patients with Clostridium difficile infection. J Antimicrob Chemother. 2016;71:836-840. [DOI] [PubMed] [Google Scholar]
- 22. Antworth A, Collins CD, Kunapuli A, et al. . Impact of an antimicrobial stewardship program comprehensive care bundle on management of candidemia. Pharmacotherapy. 2013;33:137-143 [DOI] [PubMed] [Google Scholar]
- 23. State of Michigan. Coronavirus.Michigan.gov; Web site. https://www.michigan.gov/coronavirus. Accessed April 21, 2020. [Google Scholar]
- 24. US National Library of Medicine. Expanded access treatment protocol: remdesivir (RDV; GS-5734) for the treatment of SARS-CoV2 (CoV) infection.ClinicalTrials.gov; Web site. https://clinicaltrials.gov/ct2/show/NCT04323761. Accessed April 30, 2020. [Google Scholar]
- 25. US National Library of Medicine. Expanded access to convalescent plasma for the treatment of patients with COVID-19.ClinicalTrials.gov; Web site. https://clinicaltrials.gov/ct2/show/NCT04338360. Accessed April 30, 2020. [Google Scholar]
- 26. Luo M, Cao S, Wei L, et al. . Precautions for intubating patients with COVID-19. Anesthesiology. 2020. doi: 10.1097/ALN.0000000000003288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh PM, Rewari V, Chandralekha , et al. A retrospective analysis of determinants of self-extubation in a tertiary care intensive care unit. J Emerg Trauma Shock. 2013;6:241-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leguelinel-Blache G, Nguyen TL, Louart B, et al. . Impact of quality bundle enforcement by a critical care pharmacist on patient outcomes and costs. Crit Care Med. 2018;46:199-207. [DOI] [PubMed] [Google Scholar]
- 29. Hammond DA, Gurnani PK, Flannery AH, et al. . Scoping review of interventions associated with cost avoidance able to be performed in the intensive care unit and emergency department. Pharmacotherapy. 2019;39:215-231. [DOI] [PubMed] [Google Scholar]
- 30. Miller S, Patel N, Vadala T, et al. . Defining the pharmacist role in the pandemic outbreak of novel H1N1 influenza. J Am Pharm Assoc. 2012;52:763-767. [DOI] [PubMed] [Google Scholar]
- 31. Einav S, Hick JL, Hanfling D, et al. . Surge capacity logistics: care of the critically Ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146:e17S-e43S. [DOI] [PubMed] [Google Scholar]
- 32. American Association of Colleges of Pharmacy House of Delegates. Oath of a pharmacist.American Pharmacists Association; Web site. https://www.pharmacist.com/oath-pharmacist. Accessed April 21, 2020. [Google Scholar]