Abstract
1. Background.
Tracking mosquitoes using current methods of mark-release-recapture are limited to small spatial and temporal scales exposing major gaps in understanding long-range movements and extended survival. Novel approaches to track mosquitoes may yield fresh insights into their biology which improves intervention activities to reduce disease transmission.
Stable isotope enrichment of natural mosquito breeding sites allows large-scale marking of wild mosquitoes absent human handling. Mosquito larvae that develop in 2H-enriched water are expected to be detectable for over four months using tissue mass-fraction 2H measurements, providing opportunities for long-term mark-capture studies on a large scale.
2. Approach.
A laboratory study followed by a field experiment of mosquito larval habitat 2H-enrichment was conducted in Mali, to evaluate potential labeling of wild mosquitoes. Twelve natural larval sites were enriched using [2H]-Deuterium-oxide (D2O, 99%). Enrichment level was maintained by supplementation following dilution by rains. Availability of 2H to mosquito larvae was enhanced by locally collected and cultured microorganisms (i.e. protozoa, algae and bacteria) reared in deuterated water, and provided as larval diet. Putative natural predators were removed from the larval sites and first instar larvae Anopheles gambiae s.l. larvae were added every other day. Emergence traps enabled collection of eclosing adults. Adult mosquitoes were kept at laboratory conditions for analysis of label attrition with age.
3. Results.
Deuterium enrichment of wild mosquitoes above background levels (maximum = 143.1 ppm) became apparent 5–6 days after initial exposure, after which 2H values increased steadily until ~24 days later (to a mean of approx. 220 ppm). Anopheles and Culex mosquitoes showed significantly different 2H values (211 and 194.2 ppm respectively). Both genera exhibited exponential label attrition (e(-x)) amounting to 21.6% by day 30 post emergence, after which attrition rate continuously decreased. Males of both taxa exhibited a higher mean 2H value compared to females.
4. Conclusions.
Deuterium-oxide proved useful in marking mosquitoes in their natural larval sites and although costly, may prove valuable for studies of mosquitoes and other aquatic insects. Based on our field study, we provide a protocol for marking mosquito larval sites using deuterium-oxide.
Keywords: deuterium, stable isotopes, Anopheles gambiae, mark-capture, vector dispersal
Introduction
Understanding disease transmission requires key insights into the biology and ecology of disease vectors. At a minimum, this involves understanding those factors influencing vector survival, biting behavior, and dispersal (Silver, Service, & Rogério dos Santos Alves; Alex Soares de Souza, 2008). Ecological studies of mosquito populations have therefore depended heavily on mark-release-recapture (MRR) techniques to estimate dispersal range, survival, population size, and even dietary resources (Gillies, 1961; Reisen & Aslamkhan, 1979; Lines, Lyimo, & Curtis, 1986; Costantini et al., 1996; Service, 1997; Midega et al., 2007; Cianci et al., 2013; Epopa et al., 2017). Marking has previously entailed the use of dyes, dusts, radio-isotopes, paints and stains which are applied in a wide variety of methods (Silver et al., 2008). Mosquito collection, preparation for marking, marking, release, and recapture are all time-consuming and tedious operations requiring skill and dedication, limiting the numbers of marked individuals released (generally to 10,000’s and only seldom above that). Often, marking alters mosquito behavior and reduces individual survival (Reisen, Lothrop, & Lothrop, 2003). Because of these limitations, and due to the large dilution factor of the marked individuals in the large unmarked population over distance from the release site (Taylor, 1978), experiments on mosquito dispersal had their most distant trapping points up to 3 km from the release point (Verdonschot & Besse-Lototskaya, 2014), thus limiting the maximal distance that could have been measured. Even at this distance and initiating recapture the day following release, recaptured rates were often very low (0.2–3%, but see below for exceptions) despite marking many thousands, limiting the statistical power of the inference (Service, 1997; Silver et al., 2008; Lehmann et al., 2010). Optimal marking must not be harmful to both insect and the environment, should be applied reliably on the targets, should persist through enough time to be informative, and should not be overly expensive to apply and detect (Hagler & Jackson, 2001).
Stable isotopes of carbon (δ 13C) and nitrogen (δ 15N) have previously been used to mark mosquitoes in both urban and peri-urban environments and were used to track dispersal of Culex pipiens, Aedes aegypti, C. quinquefasciatus and A. albopictus, finding maximal dispersal distances of under 3 km in Culex and under 1 km in Aedes (Hamer et al., 2014; Medeiros, Boothe, Roark, & Hamer, 2017). Stable isotope enrichment of natural mosquito larval sites potentially enables large-scale marking of wild mosquitoes by continuously and non-invasively marking newly hatched larvae in-situ. Unlike radioactive isotopes (Baldwin, James, & Welch, 1955; Rioux, Croset, Suquet, 1968; Papierok, Croset, & Rioux, 1973; Croset et al., 1976), stable isotopes do not emit radiation or decay, occur naturally in the environment including in our food, and are considered safe for all organisms (including humans) and the environment (Hood-Nowotny, 2009). Mosquito larvae that develop in water enriched with stable isotopes yield adults that are potentially marked for life with an elevated ratio of the enriched ‘heavy’ to lighter isotope (Hood-Nowotny & Knols, 2007; Hamer et al., 2012, 2014), providing opportunities to test hypotheses such as local persistence of Anopheles coluzzii through the dry season via aestivation (dormancy). Here, we describe a new method for marking Anopheles gambiae s.l. and co-habiting Culicine mosquitoes using 2H-deuterium-oxide in natural larval sites and its potential for mass marking in the wild. We also evaluated the persistence of the marking throughout the adult life. Deuterium oxide holds two major advantages over other stable isotopes: (a) hydrogen is not a limiting factor or a nutrient in the ecosystem (unlike N or C), thus distributed equally among organisms, and (b) has minimal residuals retained in the surroundings once 2H evaporates along with ambient water.
Materials and methods
A laboratory pilot experiment testing 2H-deuterium-oxide isotope (99.9% D2O, Cambridge Isotope Laboratories, Inc. Tewksbury, MA) (also: “Heavy water”, 2H2O or D2O; 2H in short) enrichment in Anopheles gambiae s.l larval stages preceded the field experiment. The laboratory protocol used was adapted with modifications from previous studies on stable isotope enrichment in mosquitoes (Hamer et al., 2012, 2014; Opiyo et al., 2016). The goal of the laboratory experiments was to test and optimize adult labeling through 2H isotopic enrichment of the rearing water. The goal of the field experiment was to evaluate and optimize our ability to enrich and mark mosquito populations in their natural sites, based on the laboratory findings.
Mosquito rearing - laboratory
Anopheles gambiae eggs (CDC G3 strain), and later larvae were reared following standard procedures (Benedict, 1997). For each respective experiment treatment, 200 eggs were placed in plastic trays (30 × 25 × 7 cm) with 1 L dechlorinated tap water. Trays of larvae were kept at constant conditions of 27°C, 85% RH (12:12 L:D, with 1 h of dusk and dawn). After hatching, control group trays were provided 2 ml yeast slurry (Slurry: 2 mg dry baker’s yeast in 50 ml Deionized-water) on days 1 and 2 and were then provided daily with 0.2–0.5 g finely-ground Koi fish food (38% protein, 13% ash) (Optimum Hi Pro, Perfect Companion Group Ltd., Samutprakarn, Thailand) until pupation, 7–9 days after hatching. Pupae were removed into dechlorinated water cups placed in cages by treatment and date of emergence. Adults were reared in standard conditions (see above) inside 1-gallon plastic cages and provided with 10% dark Karo® syrup (ACH Food Companies, Cordova, TN) on cotton wool balls replaced daily. Stable-isotope treatments were either supplemented with [2H]-deuterium-oxide 99% into the rearing water or reared in water supplemented with a deuterated microorganism culture (see in Supplementary Information). Treatments ‘spiked’ with deuterium-oxide 99% received normal fish food as described above. Microorganism-rich treatments did not receive any fish food supplementation, but the microorganisms provided (see ‘Laboratory experiments’ section).
Laboratory experiments
Experiment 1.
The experiment consisted of two 2H-deuterium-oxide concentrations: a high dose (1 ml/L [0.1% v/v D2O]), low dose (0.25 ml/L [0.025% v/v D2O]), and control (no enrichment). Into rearing trays containing 1L of either enriched or non-enriched dechlorinated tap water, 200 L1-stage larvae were added per treatment and reared either 24, 72 or 120 hours in deuterated conditions. Larvae were then transferred to non-deuterated conditions until pupation, and emerging adults were caged by date and treatment. Standard mosquito rearing powdered fish food was provided daily to all larval stages.
Experiment 2.
Two treatments were set up: a deuterated microorganism culture enriched treatment, and a non-deuterated microorganism culture control treatment. Each treatment consisted of two rearing trays as described above. The two deuterated culture treatment larval rearing trays were concomitantly supplemented with 0.25 ml deuterium-oxide. L1-stage larvae were introduced into the treatment trays five days after 2H enrichment and microorganism culture inoculation to enable microorganism proliferation (determined by daily counts; see Supp. Info.) in deuterated conditions. No additional nutrition was provided in the treatment trays.
Experiment 3.
Setup of experiment 2 repeated; deuterated conditions treatment was additionally supplemented with 10% 2H ‘spike’ (25 μl/L v/v D2O) on days 3 and 6 of larval development.
Adult sampling
Adult mosquitoes were sampled in 5-day intervals for 2H analysis. Mosquitoes were aspirated from their cages and held at −20°C for 10 minutes, then transferred individually into 1.5 ml tubes containing Silica-gel granules (Cat. 10087. Sigma-Aldrich, St. Louis, MO) for desiccation. A layer of cotton wool separated the mosquito from direct contact with the silica-gel granules. Samples were desiccated 5–7 days in preparation for IRMS analysis to remove any residual moisture which may affect 2H values of the sample (see below). No sample weight loss was observed after three days of desiccation, meaning most residual moisture had been removed leaving the sample with minimal exchangeable hydrogen and suitable for IRMS analysis (Leonard I Wassenaar, Hobson, & Sisti, 2015) (data not shown).
2H-Enrichment quantification; Isotope Ratio Mass-Spectroscopy (IRMS)
Dry mosquito samples were prepared for IRMS as follows: mosquito samples from either the laboratory or the field experiments (and respective standards; see below) were exposed to the respective IRMS facility’s laboratory environment for at least 72 hours prior to analysis, to facilitate equilibration of exchangeable hydrogen with local atmospheric water vapor. Whole equilibrated mosquitos were weighed in 8×5 mm silver capsules (Cat. No. D2008. EA Consumables, Inc. Pennsauken, NJ, USA) in separate trials using single individuals. Individual mosquitoes were weighed to the nearest 0.01 mg (AD-6000 microscale. PerkinElmer, Inc. Waltham, MA). Crushed capsules were arranged into a 96-well plate with the appropriate standards and blanks (see below) and submitted for stable isotope analysis. Samples were analyzed independently at both the Smithsonian Institution (SI) Museum Support Center IRMS facility in Suitland, MD, and at the University of California, Davis (UCD) Stable Isotope Facility.
Stable hydrogen isotope analyses of laboratory reared mosquitoes were performed at the Smithsonian Institute, on a Thermo Delta V Advantage continuous-flow isotope-ratio mass spectrometer coupled to a Thermo Temperature Conversion Elemental Analyzer via a Conflo IV interface. Field samples were analyzed at UCD similarly, using an Elementar PyroCube (Elementar Analysensysteme GmbH, Hanau, Germany) interfaced to an Isoprime VisION (Isoprime Ltd., Stockport, UK, a unit of Elementar Analysensysteme GmbH, Hanau, Germany). Samples were thermally decomposed to H2 in a glassy carbon reactor filled with glassy carbon and graphite felt at 1450°C. Non-exchangeable δ2H values (i.e. δ2Hnon-ex) were determined via 3-point linear calibration as per (Wassenaar & Hobson, 2003) with three keratin reference materials dispersed every 10 samples: CBS (Caribou Hoof Standard, δ2Hnon-ex= −197.0 ‰), USGS42 (Tibetan Human Hair, δ2Hnon-ex= −78.5 ‰), and KHS (Kudu Horn Standard, δ2Hnon-ex= −54.1 ‰). Blanks (empty capsules, crushed) were inserted between each sample to minimize 2H carryover between samples. It should be noted that no chitin reference materials with known δ2Hnon-ex values were available commercially at the time of analysis. Keratin reference materials were used because the fraction of exchangeable hydrogen atoms in keratin (i.e. ~0.15–0.20) is similar to that of chitin (Schimmelmann, 1991; Chesson, Podlesak, Cerling, & Ehleringer, 2009). For natural abundance samples with values encompassed by the reference materials, analytical errors were ±2.0 ‰. Enriched samples with values outside the range of linear calibration encompassed by the reference materials may have higher errors estimated at ±5–10 ‰.
All data is reported in mass-fraction notation, in units of parts per-million (ppm), to accommodate for the gravimetric mass-fraction enrichment method used in the study. Conversion from δ2H‰ values (relative to Vienna Standard Mean Ocean Water (VSMOW)) to ppm followed the formula:
Ethics
Prior to the work, we have requested permission to carry out the experiments from the IRB-Safety Committee of the Faculty of Medicine and Odonto-Stomatology of Bamako university, Mali and from the community leaders and the village chiefs. It was explained that stable isotopes are non-radioactive, do not decay or emit radiation, and occur naturally in the environment. Stable isotopes pose no human health risks, are benign to the environment, and have been utilized in many studies investigating nutrition, resource allocation, dispersal, food-web structure and predation. The activities involved in the study were provided in writing and were followed up by meetings held in French and local language (Bambara). The meetings provided ample opportunities for everyone to ask questions and discuss all the concerns until satisfactory answers were provided.
Field study site
Mosquito larval sites were selected in the villages of Doneguebougou (12°48’19.37”N, 7°59’15.54”W) and Torodo (12°48’12.78”N, 7°56’59.99”W), Kati province, approximately 15 km north of Bamako, Mali. Both villages are farming communities of approximately 1500 people. The main crops are millet and maize and many households also own sheep, goats, and cattle. Larval sites selected were natural basins and catchments of run-off rain water at the vicinity of the villages. Annual precipitation averages 800–1000 mm concentrated mostly during the rainy season between June and October.
Twelve larval sites, both natural and man-made, were selected based on presence of A. gambiae s.l. larvae in the water. Larval sites varied in size, volume and structure (Table 1 and Fig. S1). To ensure long-term water availability of the larval sites we selected five of the sites within large (>5000 L) natural seasonal ponds. These sites were separated (termed: ‘Separated’) into smaller, more manageable larval sites along the edges of the ponds where larval activity was most pronounced, using common fiber sandbag (80×50×15 cm obtained in the local market) embankments. Larval site water volume was calculated by measuring maximum length and width and multiplying the area by the average depth (see Supp. Info.). To prevent complete drying of the sites, a 50–200L minimum (depending on the size of the larval habitat) was set as the lower threshold for water supplementation when rains lacked for several days. Sandbag embankments were erected to avert run-off around the edges of flooding-prone larval sites to prevent their flushing during heavy rains (Supp. Info. Fig. S1).
Table 1.
Larval site descriptions.
| Larval site | Location | Site type | Area+ | Mean | Volume range | Dose Start | Dose End1 | 2H (ppm)b | IRMS | |
|---|---|---|---|---|---|---|---|---|---|---|
| (m2) | volume (L) | (L) | Min. | Max. | (n)a | |||||
| 1 | Doneg | Mud pit | 5 | 250 | 100–750 | Low | Low | 161.9 | 270.7 | 23 |
| 2 | Torodo | Mud pit | 4 | 220 | 80–660 | Low | Low | 135.0 | 573.3 | 104 |
| 3 | Doneg | Mud pit | 4 | 520 | 100–1300 | Low | Low | 128.5 | 362.4 | 29 |
| 4* | Torodo | Pond | 5 | >1000 | None | 126.8 | 143.1 | 19 | ||
| 5^ | Doneg | Separated | 3 | 750 | 430–1650 | High | Low | n/a | n/a | 0 |
| 6 | Doneg | Separated | 3 | 1200 | 480–1900 | High | Low | 131.8 | 216.5 | 42 |
| 7 | Doneg | Separated | 7 | 930 | 530–1300 | High | Low | 136.6 | 299.6 | 87 |
| 9 | Doneg | Mud pit | 3 | 750 | 520–1300 | High | Low | 146.7 | 273.9 | 6 |
| 10 | Doneg | Mud pit | 1 | 160 | 50–450 | High | Low | 244.4 | 244.3 | 1 |
| 11 | Doneg | Separated | 3 | 1200 | 570–2200 | High | Low | 129.4 | 247.4 | 10 |
| 12 | Doneg | Separated | 3 | 420 | 200–1050 | Low | Low | 156.7 | 449.3 | 21 |
Site No. 4 was a control larval site receiving no enrichment and thus was not measured for volume
No A. gambiae emerged from site no. 5, thus no mosquitoes were tested
Mean water surface area
Larval site enrichment dose after dose change
Number of mosquitoes analyzed by IRMS
Values obtained from mosquitoes
To ensure adult productivity in our selected larval sites, 200 L1–2-stage larvae were added daily. Larvae were obtained by collecting either gravid or blood-fed A. gambiae s.l females within the villages and transporting them to the lab for controlled oviposition. We additionally conducted daily predator removal from the larval sites, sieving out possible mosquito larvae predators (e.g. notonectids, dragonfly nymphs, tadpoles, aquatic bugs and beetles, etc.), transferring them to distant puddles (see Fig. 1S panel d).
Figure 1.
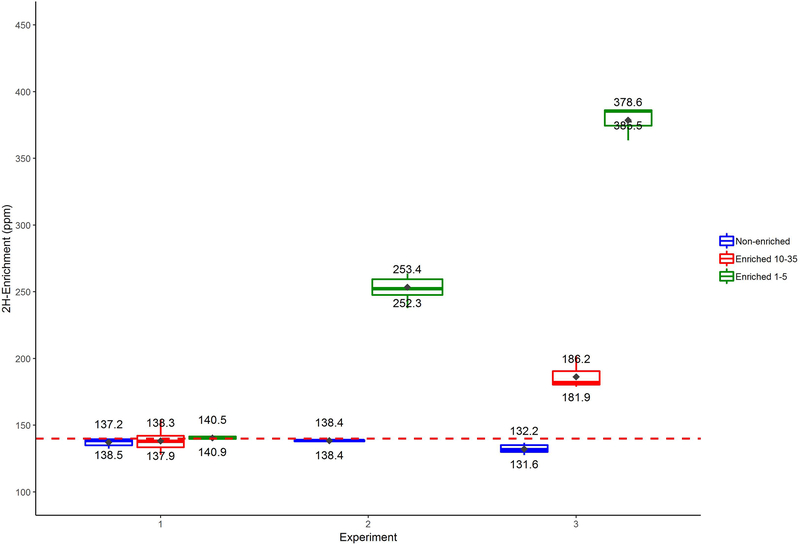
Laboratory 2H-enrichment Box plot. Non-enriched control adult mosquitoes (blue), 10-day-old enriched (red), and 1 day-old enriched (green). Experiment 1; Direct supplementation of 2H-deuterium-oxide into mosquito larvae rearing water. Only values of longest exposure to deuterated conditions (5 days) shown, 1-day-old adults are presented (Controls; n=6, 10-day old; n=6 and 1-day old; n=16). Experiment 2; Deuterated microbiota culture fed to larvae reared in 0.1 v/v% deuterated rearing water. Results from 1-day-old adults (Controls; n=4 and 1-day old; n=13). Experiment 3; Deuterated microbiota culture fed to larvae in 0.1 v/v% deuterated water with additional 2H-spiking at days 3 and 6 post hatch (Controls; n=6, 10-day old; n=3 and 1-day old; n=6). Enrichment threshold (dashed red line, 140 ppm) was set at the level of the highest control value (rounded up to next integer). Diamonds inside boxes denote the means, horizontal line denote medians (corresponding value shown). Whiskers are 25 (bottom) and 75 (top) percentiles.
[2H]-deuterium-oxide enrichment of larval sites
Enrichment of eleven field larval sites (one additional site was left untreated) commenced between July 5–15, 2017, depending on completion of separations and rain storms flooding the sites.
Based on the laboratory experiment, showing that mosquito enrichment was reached only after a 5–6 day ‘ripening’ period of the water, i.e. proliferation of deuterated microorganisms, mosquitoes collected from the larval sites were assigned to 6-day cohorts, based on the time that had elapsed between initial enrichment of the habitat and the subsequent collection date. Mosquitoes collected within the first 6 days after initial enrichment were termed ‘Cohort 0’. Similarly, mosquitoes collected on days 7–12 post-enrichment were termed ‘Cohort 1’, and so forth every 6 days.
Seven larval sites were predetermined to be 2H-enriched with a 2H dose of 0.02% (v/v = 0.2 ml/L), and four others with a dose of 0.05% (v/v = 0.5 ml/L). For example, if a low-dose (0.02%) site volume was measured to hold 500L, 100 ml [2H]-deuterium-oxide was added to it. A long-handle, 1L-ladle was filled with 900 ml larval site water and mixed with 100 ml [2H]-deuterium-oxide, then dispensed uniformly 20 cm inward, along the edges of the larval site where most larval activity takes place (Fig. S1, panel a, Supp. Info.).
Larval sites were additionally supplemented with a 10% v/v deuterium-oxide ‘pulse’, such that low-dose sites (0.02%) were pulsed every 6 days, and high-dose sites (0.05%) every 4 days. Based on the volume of the example above, a 10-ml deuterium-oxide pulse was provided. Following rains, deuterium-oxide supplementation was conducted as soon as possible to address dilution due to volume increases in the larval sites. Supplemental deuterium-oxide volume was calculated based on the volume difference including, losses to ground by infiltration and evaporation prior to the rain event (See Supp. Info.).
Microbiota culture enrichment
Deuterated microbiotic culture (1% [10 ml/L v/v]) (see Supp. Info.), originally collected from the village larval sites, was added to larval sites on alternating days to facilitate larval feeding on deuterated material from as early a stage possible. Either 100 or 200mL microbiotic culture was supplemented into larval sites below or exceeding 500L respectively. The culture was dispersed using the long-handle, 1L-ladle as described above.
Adult mosquito collection
Visual scanning and recording of A. gambiae larvae numbers was conducted daily to assess mosquito productivity and development (Fig. S1, panel e, Supp. Info.). Once L4-stage larvae or pupae were observed, the larval sites were covered with a tight-woven plastic net stretched tent-like over 2–4 poles encompassing the larval site and secured to the ground from all sides (Fig. S1, panel c, Supp. Info.). Adult mosquitoes, found under the net cover were collected each morning, transported to the laboratory and maintained under insectary conditions for a time-series analysis of isotopic enrichment by age (27°C, 80% RH, 12:12 L:D photoperiod). For the isotopic analysis, mosquitoes were either killed by their age or collected dead during the daily routine assessing viability. Dead mosquitoes were transferred to 0.6 ml micro-centrifuge tubes containing Silica-gel granules (Silica-gel orange, Cat. No. 10087. Sigma-Aldrich, St. Louis, MO) desiccant plugged by a layer of cotton wool and preserved desiccated.
Statistical analysis
To optimize mosquito 2H-enrichment in natural larval sites, we evaluated the effects of enrichment dose, type of larval site (“whole” vs. “separated” by embankment as described above), and time since initiation of larval site enrichment (cohort) on adult mosquito enrichment level. We consider a cohort to last 6 days as past work showed development time of first instar to pupa in natural larval sites of this region takes 6 days (Diabaté et al., 2005), and due to the 5–6 day ‘ripening’ period (i.e. proliferation of deuterated microorganisms) of the larval site after initial enrichment. Cohorts were used to reflect temporal dynamics of 2H availability to mosquito larvae in the larval sites. Availability may vary based on 2H flow between aquatic organisms, only some of which are consumed by larvae, even when overall 2H concentration has been stable in the total volume.
The 2H level in an adult mosquito is expected to decline with age due to biological elimination (e.g. excretion, metabolism, etc.), and the normal exchange of the artificially enriched 2H (from larval sites) with the more common 1H, which occurs throughout the tissues except in the core of the chitin (Arndt Schimmelmann & DeNiro, 1986; Ogawa, Kimura, Saito, & Wada, 2012). Thus, isotopic attrition is expected over time after the mosquito has left the enriched larval sites, which we modeled by a standard ‘half-life’ exponential decay function (although this process is not related to atomic decay):
| 1) |
with 2Hppm [0] and 2Hppm [t] representing 2H at adult eclosion and at age ‘t’, representing the adult age in days post eclosion, respectively (Silver et al., 2008). After logarithmic transformation, the model becomes linear with raw values transformed as Ln (2H ), and the models appears as follows:
| 2) |
with an intercept (constant) reflecting the enrichment upon adult eclosion and a slope measuring the rate of decay over time (days).
We used Proc GLIMMIX on SAS (Schabenberger, 2005) to analyze the log-transformed decay model, initially incorporating all fixed effects of type of larval sites (above), enrichment dose (excluding the non-enriched control mosquitoes), cohort, separation barriers, mosquito species and sex. To account for possible decomposition between mosquito time of death and time of preservation the model included the variable ‘killed’ (killed and preserved immediately) vs. ‘live’ (preservation 1–24 h after uninduced death). The variation between larval sites was estimated as a random variable in this analysis. We evaluated several models starting with simple effects without interaction of all variables. Only the effect of larval site separation was not statistically significant (P>0.32) and was removed before second order interactions were introduced sequentially and kept if they were significant. All second order interactions (e.g. sex*species, age*sex, and age*species) were not significant (P>0.2) and accordingly were removed from the final model.
Results
Laboratory experiments
Adding [2H]-deuterium-oxide directly into the rearing water to reach concentrations of 0.1- and 0.025% (Exp. 1), yielded no evidence of significant enrichment levels (above background) in adult A. gambiae G3 mosquitoes (in 1-day old adults) in any of the exposure treatments (Fig. 1, Exp.1). In ‘Experiment 2’, a deuterated larval diet composed of outdoor puddle water microorganism culture (protozoa, algae and bacteria; See in Supp. Info.) was added to 0.1% v/v deuterium-oxide in the rearing water. Mean adult marking in 1-day-old enriched treatment mosquitoes significantly increased over the non-enriched controls (253.4 ppm and 138.4 ppm respectively) (Fig. 1, Exp. 2).
To evaluate the effect of 2H (‘dose’) on marking of the adult mosquitoes, and the change in 2H over the age of the mosquito, adults emerging from the deuterated diet conditions were sampled 1- and 10-days post emergence. Mean adult 2H values were significantly above background (=138.4 ppm) in both 1- and 10-day-old mosquitoes (378.6 and 253.4 ppm respectively; Exp. 2 and 3), whereas mean 2H values dropped 80.1% between 1-day old adults and 10-day-old adults (378.6 and 186.2 ppm respectively, Exp. 3) yet even the older age group was significantly above the mean background 2H level (Fig. 1). Percent decrease in 2H values between 1- and 10-day old mosquitoes was calculated only on the values above background (i.e. after subtracting 138.4 ppm from both values).
Field experiments
Natural 2H values in mosquitoes and the effect of enrichment dose
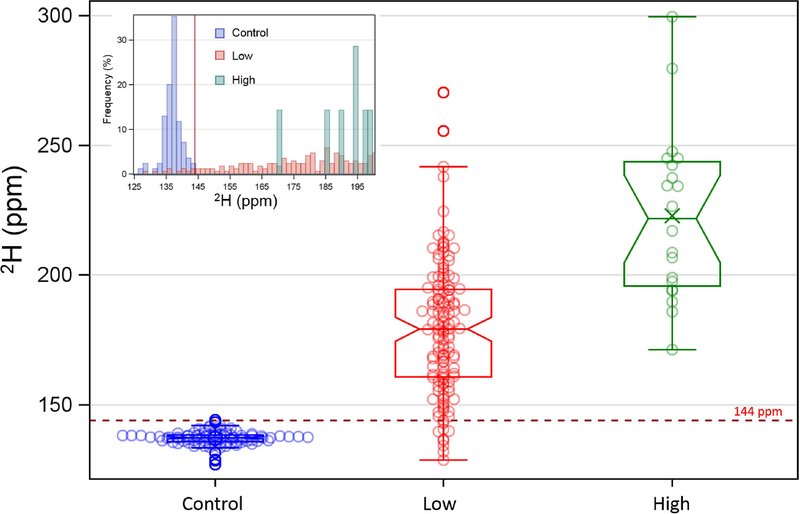
The non-enriched control mosquitoes, all collected before enrichment began (n=85), in the non-enriched larval site (n=18) or 20–100 km from the villages where enrichment took place (n=67), showed 2H values ranging between 127.7 and 143.1 ppm, with a mean of 136.7 ppm, which was close to expectations based on our previous laboratory assessment of background enrichment levels. No significant difference in mean 2H values was found between the mosquitoes from the villages up to 100 km away from Doneguebougou (P>0.278; data not shown), thus ‘Control’ villages were pooled. Based on the highest background level of enrichment rounded up to the next integer we selected 144 ppm as our 2H enrichment threshold (see Fig. 2 inset).
Figure 2.
Differences in mean and median 2H values between enrichment doses: non-enriched, natural abundance (Control, Blue), Low dose (2H=0.02% (v/v = 0.2 ml/L), Red) and High dose (2H=0.05% (v/v = 0.5 ml/L), Green) used in the study. Inset: the distribution of 2H values encompassing the natural abundance (blue) and lowest range of experimentally enriched (High dose range truncated). Threshold used to separate the natural 2H abundance and the experimentally enriched is 144 ppm (dashed horizontal red line [Inset: vertical red line]).
The effect of dose was tested twice: once on the entire data set, and once only on mosquitoes from the same cohorts and ages (that matched between dose), because high dose samples were fewer and only available from earlier cohorts. In both analyses, higher dose yielded significantly higher enrichment in adult mosquitoes (P<0.001, Fig. 2 and Table 1) which generally corresponded with their respective concentrations. Enriched mosquitoes at the low dose showed a mean 2H=~197.8 ppm indicating that adequate marking was achieved in the enriched larval sites compared to background levels. However, among the enriched mosquitoes were few individuals with 2H as low as the control (e.g. 2H=128.5 ppm), suggesting that some individuals were not enriched (Table 1).
Closer examination (Fig. 2) showed that 8 of 308 enriched mosquitoes (2.6%) had 2H values similar to control (i.e. below 144 ppm). Three of these 8 came from cohort 1 which was the largest (n=114) and five from cohort 0 (n=29). Notably 6 of the 8 were female Culex spp., despite Culex being under represented compared with A. gambiae (n=121 vs 230). Culex mosquitoes from the first two cohorts tended to be indistinguishable from controls although they originated from enriched sites. Whether this was due to minimal exposure to 2H before adult emergence or due to un-enriched females that rested near the site when emergence traps were set remains unclear.
Effect of larval site separation, cohort, and variation among larval sites
Eclosing mosquitoes were assigned to cohorts based on the number of days elapsing since the day of enrichment. Since enrichment in adult mosquitoes became clear 5–6 days after larval site enrichment we regarded a cohort as a 6-day period. This time-period allowed us to follow enrichment dynamics in the larval sites on groups of mosquitoes (see Materials and Methods). Some differences between the cohorts were apparent, suggesting a buildup trend in the first three cohorts, followed by stabilization around a 220 ppm mean (Fig. S2).
Although site no. 9 was higher than our high dose site no. 11, the variation among sites (a random factor in the analysis) was non-significant (P>0.09) (Table 1). The effect of the sandbag separation barriers was not significant (GLIMMIX procedure, P>0.214; data not shown), however, larval-site no. 12 which was separated showed an above-average level of enrichment (Table 1). For reasons of sub-optimal mosquito productivity and due to budgetary constraints, sites 6 and 7 were reverted to low dose sites. However, there was a slight trend showing separated sites (no. 6–7 and 11–12) were prone to lower enrichment, possibly due to unaccounted for water exchange/loss through the sandbag separation barriers or the bottom mud underneath.
Effect of species, sex and age
Values of 2H were significantly higher in A. gambiae s.l than in Culex spp. (P<0.01) (Fig. 3), and males of both genera showed significantly higher 2H values than females (Culex: 204.3 and 184.0 ppm, A. gambiae s.l.: 214.4 and 207.2 ppm; P<0.0001).
Figure 3.
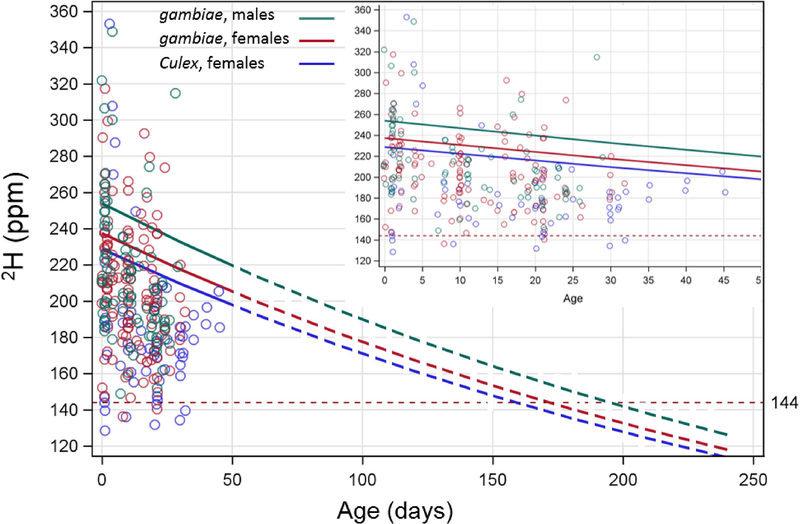
Estimated 2H signal attrition functions with mosquito age (computed using Proc GLIMMIX, see Materials and Methods). Note that the variance among individual mosquitoes (circles) also reflects their cohort, larval-site, and dose. Extrapolation (dashed colored curves) of the effect of age on 2H values up to 240 days (~8 months) post-emergence from enriched sites based on experimental data from field-collected mosquitoes at the range of 1–50 days (see inset). According to this extrapolation, initial 2H enrichment levels between 240–255 ppm in A. gambiae s.l. may potentially enable detection of 50% of enriched individuals up to 180 days (6 months) after eclosion. Dashed horizontal red line denotes the threshold (144 ppm) we used to separate the natural 2H abundance and the experimentally enriched.
As expected, adult age had a significant effect on 2H values (P<0.001), showing a decay curve (Fig. 3). In A. gambiae s.l. females, the effect of age on mean enrichment showed a decrease of 21.6% by 30 days of age, after which measured decay rate decreased (in Culex sp. only; due to longer survival in the laboratory). Similarly, male A. gambiae s.l. exhibited an overall elevated mean enrichment level at day 0, decreasing 18.7% by day 30. Signal decrease between mosquito ages accounted for enrichment levels above the 144 ppm threshold only (see Laboratory experiments section above). As expected, based on the species effect, the mean of Culex females at 30 days old was lower (171.3 ppm) than that of female A. gambiae (183.8) (Fig. 3), consistent with no significant interaction between age and species (not shown, see Materials and Methods).
Surprisingly, despite the short time after death, mosquitoes which died ‘naturally’ and were preserved 1–24 h after death had significantly lower 2H values than freshly killed mosquitoes preserved in desiccant immediately upon being killed, indicating changes in 2H values possibly due to decomposition.
Discussion
We demonstrate for the first time, to the best of our knowledge, that using 2H in natural mosquito larval sites enabled lifelong marking of emerging adults (both A. gambiae s.l. and Culex sp.) with elevated 2H values that can be readily detected by IRMS analysis, and without human handling of the mosquitoes at the marking stage. By experimenting under field conditions, we gained key insights to key factors facilitating successful marking and subsequent retention in wild, adult mosquitoes.
Our protocol development of larval site enrichment was based on the following key elements:
1. Selection of productive larval sites showing moderate or high density of the larvae of the target species. In our case with A. gambiae, s.l., when the size of the larval site to be enriched is beyond the enrichment budget capacity (due to large volume; see Conclusions), separation of a suitable sub-section maximizing productive edges by a solid embankment is a viable option. The embankment is constructed as described in the Supp. Info. Testing of embankment permeability using a water tracer dye helps to determine if major leaks occur (Supp. Info.) prior to enrichment. Whether separation by embankment is required or not, the larval site should not dry up in less than 14 days to enable a full developmental cycle when rain is absent. Supplementing water and 2H for a site which is near desiccation is another option for certain conditions (see below).
2. Enrichment with 2H is especially advised where and when long term environmental persistence (e.g., the following year) of the marker should be minimized. Other stable isotopes might be used if it is clear that traces remaining in the area for years are of no concern.
3. To maximize survival success of mosquito larvae, removal of putative predators, including back swimmers (Notonecta spp.) dragon fly nymphs, tadpoles, etc. is advisable (Supp. Info.). To further maximize adult production, addition of early larval instars (L1–2) to enriched sites is recommended.
4. If an estimate of the number of adults produced is required, emergence traps should be employed and their area out of the total productive area computed (Supp. Info.).
Prior to enrichment, protection of the larval site from runoff flow of water during rain events is important. Such surface flow results in flushing and isotope dilution of the enriched water. A combination of well-maintained ditches, protective mud walls and embankments may be necessary to minimize such flooding. Accurate rain gages in the study area should be available to determine the exact rainfall amount.
Enrichment maintenance requires daily estimates of the volume of water in each larval site (Supp. Info.). It is especially important to have accurate measurements before, and shortly after a rain event, (i.e. <24 hours) to follow up the changes in the volume and estimate the dilution. In most soils, the difference may not account for the full dilution and additional “invisible volume” (i.e. water loss due to absorption or evaporation between the end of the rain and the actual time of measurement) can be estimated (Supp. Info.).
5. After initiation of enrichment, supplementation of v/v ml/ 500L larval site of enriched microbiota (Supp. Info.) every other day is recommended. Dilution by rains must be quickly met by augmentation of stable isotope. Finally, a weekly pulse of 10% (of the initial 2H dose) in addition to the above compensates for slow dilution by absorption into the ground.
Once enrichment is ongoing, adults caught in emergence traps should be preserved promptly in desiccant and their age post emergence recorded to ascertain mosquito enrichment.
6. Finally, sampling of adults indoors, in resting sites, or in bait stations can be used to determine the proportion of marked adults in the population.
As our laboratory experiments have shown, robust, detectable mosquito 2H-enrichment requires nutrients consumed by the larvae eating microorganisms rather than by exposure to ambient 2H-rich rearing water; None of our mosquitoes exposed as larvae to 2H-enriched water directly exhibited 2H values significantly above the background level (Fig. 1, Exp. 1; showing results from longest exposure [120 hours] only). It is likely that mosquito tissue 2H enrichment by water passage through the larval body cavity is minimal, and that enrichment is dependent on metabolism of ingested, 2H-enriched fodder. Although enriched microorganisms (e.g. yeasts, protozoa, bacteria and algae) are expected to build up naturally in the rearing water, the period required for this buildup may be longer than a single mosquito cohort in the laboratory due to larval consumption and low microorganism counts to begin with. Microorganism populations have previously been shown to thrive in larval rearing water, utilizing nutrients from the food provided to the larvae (e.g. fish food, etc.) and other organic materials (e.g. larval excrement, larvae exuviae, larvae carcasses, etc.) (Merritt, Dadd, & Walker, 1992; Gimnig et al., 2002; Coon, Vogel, Brown, & Strand, 2014; Opiyo et al., 2016). Feeding mosquito larvae with deuterated microorganisms cultured from a rain puddle, allowing them to grow and amplify for five days before introduction of mosquito larvae into the water necessitated no additional larval food for normal developmental completion.
The variation among cohorts showed a steady increase in 2H-enrichment levels during the first three cohorts, followed by a stabilization in enrichment levels which remained relatively constant until the end of the trial (Fig. S2). The increase seen over the first three cohorts may be explained by the period necessary for the natural microorganisms in the larval habitat to metabolize the 2H and become available fodder within the larval habitat as the 2H becomes incorporated in the larval site food-web. Additionally, during these early days, we sorted out how to prepare the enriched-microbiota in the lab and revised how to compensate for the rainfall dilution, which possibly contributed to the pattern of increase. Additional studies are required to elucidate the variation among cohorts and explain the roles of microbiota buildup vs. other processes.
The natural 2H values in individual mosquitoes varied between 126.9 and 143.1 2 ppm, with a mean and median values of 135.9 and 135.7 ppm respectively. This wide distribution indicates a degree of heterogeneity in field conditions or measurement variation (i.e. error). For this study we determined 144 ppm to be a useful threshold, although it is conceivable that higher values among natural wild mosquitoes in this area will be detected with increasing sample size. Within the low dose treatment, eight mosquitoes collected from enriched sites resulted with enrichment levels similar to background levels (i.e. below 144 ppm). Some of these mosquitoes had been late L4 instar larvae or pupae at the time of 2H-enrichment, thus exposed to early deuterated conditions for 1–4 days only. This result is consistent with the effect of cohort on enrichment (Table 1 and Supp. Info. Fig. S2). This highlights that the added 2H becomes available to mosquito larvae after 5–6 days, and continues to increase during at least 15 days (Supp. Info. Fig. S2).
Although, the effect of dose was only crudely assessed given the limited number of mosquitoes emerging from our two high dose sites (n=20, Fig. 2), the median 2H value was higher in the higher dose (235.1 vs 176.3 ppm), it was roughly proportional to the 2.5x concentration of the deuterium (Table 1) but suggested diminishing mosquito enrichment with dose.
In the interest of reducing costs, we expanded treatment with lower dose over higher dose larval sites. Importantly, low dose larval sites also produced individuals with sufficiently high enrichment levels (Fig. 2). High dose sites were costly to maintain during the rainy season when large dilutions (>10% volume increase) by rains occurred frequently.
Both the variation among larval sites and the effect of the separation embankments were not statistically significant. A slight trend of lower enrichment was found in the separated sites, suggesting that infiltration either under the embankment or through it allowed for some dilution of added 2H. Future studies in such habitats may require supplemental addition on a weekly basis in the order of the difference (approx. 10–15% v/v).
Effect of age
As expected, age was found to have a major influence on 2H-enrichment of adults; A. gambiae s.l. exhibited a 10.1% median reduction by day 10 and 21.6% by day 30, (Fig. 3, inset). Although based on available age-series data due to laboratory mosquito life-span, overall, both genera exhibited a reduction of enrichment attrition rate over age. This attrition rate is crucial for long-term studies and may prove vital for any field observations substantially longer than 60 days. Future long-term field experimentation is needed to shed light on this matter. Extrapolation of our age data beyond 50 days to assess long-term marking retention suggested a marking mean signal significantly above the enrichment threshold up to 150 days given our low dose enrichment, but this may not be the case after 200 days (Fig. 3). Increasing initial enrichment levels by using higher doses appears to be required for this marking technique to test the hypothesis that A. coluzzii persist locally through the 7-month long Sahelian dry season.
Difference between taxa and sex
Mean enrichment differences between A. gambiae s.l and Culex sp. were significant, as were the differences between males and females (Fig. 3). Because chitin is the primary long-term storage tissue of hydrogen in insects, these differences probably reflect lower fraction of cuticle (chitin) to soft tissues in Culex and females as opposed to A. gambiae and males. Alternatively, the different foraging behavior of larvae of these two genera (or sexes) might have resulted in differential consumption of the isotopically enriched diet. Given that at least four identified and four unidentified culicine species were included under our designation of Culex spp. (not shown), additional work may be necessary to elucidate if differences in enrichment potential are found among them. Some support for the first explanation, regarding the ratio of the chitinized portion of the body to the rest was obtained by comparing different body parts (not shown), the thorax appears to show elevated levels over the abdomen, whereas the head seems intermediate among the body sections.
Conclusions
We describe a method to mark adult mosquitoes with stable 2H isotopes by enrichment of natural larval sites, typical of A. gambiae s.l. in Africa, including puddles and small seasonal ponds. This marking method offers a number of advantages, most notably the lack of handling of mosquitoes for marking, its neutral effect on their biology, prolonged persistence of the marking, and the potential for scaling up numbers of marked individuals. Thus, it can be used to address important ecological questions that have eluded rigorous research, such as tracking mosquitoes through the 5–8 months-long dry season, or once large numbers or marked adults are attained – tracking their movement over tens of kilometers. The use of enriched microbiota early on and dosage compensation after rains are key. Optimal mosquito enrichment levels are attained after 10–15 days post enrichment initiation, although marked adults can be detected within 5–7 days.
The use of deuterium-oxide is effective across mosquito species, and probably for other insects that develop in the same habitats and may facilitate in ecological studies involving movement (e.g. notonectid dispersal between aquatic habitats), predation and trophic level interactions. Compared with other stable isotopes, the 2H spike leaves no lasting traces in the seasonal environment (i.e. after the larval sites dried up), so experiments can be repeated in the same sites repeatedly, over several seasons. Carbon (δ13C) and nitrogen (δ15N) stable isotopes on the other hand, are likely to persist for longer in the environment which could compromise multi-year studies. That said, these isotopes theoretically do not evaporate like 2H, but rather are recycled within the food-web as they are consumed as nutrients by bacteria, fungi and plants. Baring total flushing of enriched larval sites, this suggests possible usage of significantly smaller doses of enriched material over the trial period, and with IRMS analysis costs half that of 2H, thus significantly lower expenditure.
Like other stable isotopes, the shortcomings of working with 2H spiking include no accurate estimate of the number of enriched adults that are released into the environment (true for any isotope), it is labor intensive requiring routine compensation for lost enriched material, especially during frequent rains, and finally, its cost. Over the entire trial we had used a total of 16.6 liters of deuterium-oxide amounting to $6400. We estimate that our cost of the 2H spiking alone for enrichment of a 500L larval site over a period of 20 days was approximately $250 (US), and the cost of IRMS (see Materials and Methods) analysis per sample was approximately $14 at the time of writing this paper. The proportion of enriched adults can be readily monitored after standard collections in resting sites, host-baited traps etc., but emergence traps sampling enriched habitats may be needed to estimate the absolute number of marked adults produced. However, tracking insects over long temporal and spatial scales may well justify these costs and efforts, enabling new insights into the ecology of insects affecting public health and wellbeing.
Supplementary Material
Table 2.
Mixed model main effects on the log of enrichment level (ANOVA using GLIMMIX procedure). Bold face denotes statistical significance (P<0.001)
| Factor | FdfN/dfD+ | Estimate | P |
|---|---|---|---|
| Larval site#* | 1.41/296 | 0.002 | 0.0986 |
| Residual# | 12.21/296 | 0.016 | 0.0001 |
| Age (days) | 16.151/296 | −0.003 | 0.0001 |
| Killeda | 19.911/296 | 0.074 | 0.001 |
| Species (g-Cx)b | 6.631/296 | −0.045 | 0.0105 |
| Sex (F-M)c | 23.781/296 | −0.079 | 0.0001 |
| Dose | 21.581/296 | 0.276d | 0.0001 |
| Cohort | 14.298/296 | −0.178e | 0.0001 |
z value instead of F value for random variables test and estimate of variance instead of the effect of unit change between levels
model random effects (covariate parameter)
dfN is the number of degrees of freedom used in the estimate of the variance. dfD is the degrees of freedom error
Factor determining whether mosquitoes had been deliberately killed by experimenter or found dead before preservation
Species: A. gambiae s.l. (g) was overall more enriched than Culex sp. (Cx); (negative estimate)
Sex: Males (M) were more enriched than females (F) in both species; (negative estimate)
Dose estimate; High over Low, derived from model matched by Cohorts, after removing all non- significant factors
Cohort estimate derived by subtraction of smallest from largest cohort estimates
Acknowledgements
We thank the people of the villages of Doneguebougou and Torodo in Mali for their support of the field work. We thank Drs. James Hagler, Peter Holder, Rebecca Nowotny-Hood and Carolina Barillas-Mury for valuable discussions and comments on previous versions of this manuscript. We wish to thank Mr. André Laughinghouse and Kevin Lee for logistical support. We thank the three anonymous reviewers for their helpful suggestions for improvement of our manuscript. This study was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda MD.
Footnotes
Data Accessibility
Data used in this paper was deposited in the Dryad repository:
Provisional DOI: doi:10.5061/dryad.b1866n0
Data files: Faiman_etal_MarkingMosquitoesDeuteriumData
References
- Baldwin WF, James HG, & Welch HE (1955). A Study of Predators of Mosquito Larvae and Pupae with a Radio-active Tracer. The Canadian Entomologist, 87(8), 350–356. doi: 10.4039/Ent87350-8 [DOI] [Google Scholar]
- Benedict MQ (1997). Care and maintenance of anopheline mosquito colonies In The Molecular Biology of Insect Disease Vectors (pp. 3–12). Dordrecht: Springer Netherlands. doi: 10.1007/978-94-009-1535-0_1 [DOI] [Google Scholar]
- Chesson LA, Podlesak DW, Cerling TE, & Ehleringer JR (2009). Evaluating uncertainty in the calculation of non-exchangeable hydrogen fractions within organic materials. Rapid Communications in Mass Spectromentry, 23, 1275–1280. doi: 10.1002/rcm.4000 [DOI] [PubMed] [Google Scholar]
- Cianci D, Van Den Broek J, Caputo B, Marini F, Torre A Della, Heesterbeek H, & Hartemink N (2013). Estimating Mosquito Population Size From Mark–Release–Recapture Data. Journal of Medical Entomology, 50(3), 533–542. doi: 10.1603/ME12126 [DOI] [PubMed] [Google Scholar]
- Coon KL, Vogel KJ, Brown MR, & Strand MR (2014). Mosquitoes rely on their gut microbiota for development. Molecular Ecology, 23(11), 2727–2739. doi: 10.1111/mec.12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini C, Li S-G, Della-Torre A, Sagnon N, Coluzzi M, & Taylor EC (1996). Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a West African Sudan savanna village. Medical and Veterinary Entomology, 10(3), 203–219. Retrieved from https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1365-2915.1996.tb00733.x [DOI] [PubMed] [Google Scholar]
- Croset H, Papierok B, Rioux JA, Gabinoud A, Cousserans J, & Arnaud D (1976). Absolute estimates of larval populations of culicid mosquitoes: comparison of ‘capture-recapture’, ‘removal’ and ‘dipping’ methods. Ecological Entomology, 1(4), 251–256. doi: 10.1111/j.1365-2311.1976.tb01229.x [DOI] [Google Scholar]
- Diabaté A, Dabire RK, Kim EH, Dalton R, Millogo N, Baldet T, … Med Entomol J (2005). Larval Development of the Molecular Forms of Anopheles gambiae (Diptera: Culicidae) in Different Habitats: A Transplantation Experiment. J Med Entomol, 42(4), 548–553. Retrieved from https://academic.oup.com/jme/article-abstract/42/4/548/911125 [DOI] [PubMed] [Google Scholar]
- Epopa PS, Millogo AA, Collins CM, North A, Tripet F, Benedict MQ, & Diabate A (2017). The use of sequential mark-release-recapture experiments to estimate population size, survival and dispersal of male mosquitoes of the Anopheles gambiae complex in Bana, a west African humid savannah village. Parasites & Vectors, 10(1), 376. doi: 10.1186/s13071-017-2310-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiman R et al. (2019) Data from: Marking mosquitoes in their natural larval sites using 2H-enriched water: a promising approach for tracking over extended temporal and spatial scales. Methods in Ecology and Evolution doi: 10.5061/dryad.b1866n0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies MT (1961). Studies on the dispersion and survival of Anopheles gambiae Giles in East Africa, by means of marking and release experiments. Bulletin of Entomological Research, 52(1), 99–127. doi: 10.1017/S0007485300055309 [DOI] [Google Scholar]
- Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, & Walker ED (2002). Density-Dependent Development of Anopheles gambiae (Diptera: Culicidae) Larvae in Artificial Habitats. J. Med. Entomol, 39(1), 162–172. Retrieved from https://academic.oup.com/jme/article-abstract/39/1/162/879576 [DOI] [PubMed] [Google Scholar]
- Hagler JR, & Jackson CG (2001). Methods for Marking Insects : Current Techniques and Future Prospects. Annual Review of Entomology, 46(1), 511–543. doi: 10.1146/annurev.ento.46.1.511 [DOI] [PubMed] [Google Scholar]
- Hamer GL, Anderson TK, Donovan DJ, Brawn JD, Krebs BL, Gardner AM, … Walker ED (2014). Dispersal of Adult Culex Mosquitoes in an Urban West Nile Virus Hotspot: A Mark-Capture Study Incorporating Stable Isotope Enrichment of Natural Larval Habitats. PLoS Neglected Tropical Diseases, 8(3), e2768. doi: 10.1371/journal.pntd.0002768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer GL, Donovan DJ, Hood-nowotny R, Kaufman MG, Goldberg TL, & Walker ED (2012). Evaluation of a Stable Isotope Method to Mark Naturally-Breeding Larval Mosquitoes for Adult Dispersal Studies. J. Med. Entomol, 49(1), 61–70. doi: 10.1603/ME11076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood-Nowotny R (2009). Manual for the Use of Stable Isotopes in Entomology. Retrieved from http://www-naweb.iaea.org/nafa/ipc/public/IAEA_SI_Hi-Res_final.pdf [Google Scholar]
- Hood-Nowotny R, & Knols BGJ (2007). Stable isotope methods in biological and ecological studies of arthropods. Entomologia Experimentalis et Applicata, 124(1), 3–16. doi: 10.1111/j.1570-7458.2007.00572.x [DOI] [Google Scholar]
- Lehmann T, Dao A, Yaro AS, Adamou A, Kassogue Y, Diallo M, … Coscaron-Arias C (2010). Aestivation of the African malaria mosquito, Anopheles gambiae in the Sahel. The American Journal of Tropical Medicine and Hygiene, 83(3), 601–6. doi: 10.4269/ajtmh.2010.09-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines JD, Lyimo EO, & Curtis CF (1986). Mixing of indoor- and outdoor-resting adults of Anopheles gambiae Giles s.l. and A. funestus Giles (Diptera: Culicidae) in coastal Tanzania. Bulletin of Entomological Research, 76(1), 171. doi: 10.1017/S0007485300015388 [DOI] [Google Scholar]
- Medeiros MCI, Boothe EC, Roark EB, & Hamer GL (2017). Dispersal of male and female Culex quinquefasciatus and Aedes albopictus mosquitoes using stable isotope enrichment. PLOS Neglected Tropical Diseases, 11(1), e0005347. doi: 10.1371/journal.pntd.0005347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt RW, Dadd RH, & Walker ED (1992). Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annual Review of Entomology, 37(1), 349–376. Retrieved from www.annualreviews.org [DOI] [PubMed] [Google Scholar]
- Midega JT, Mbogo CM, Mwambi H, Wilson MD, Ojwang G, Mwangangi JM, … Beier JC (2007). Estimating Dispersal and Survival of Anopheles gambiae and Anopheles funestus Along the Kenyan Coast by Using Mark–Release–Recapture Methods. Journal of Medical Entomology, 44(6), 923–929. doi: 10.1093/jmedent/44.6.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kimura S, Saito Y, & Wada M (2012). Infrared study on deuteration of highly-crystalline chitin. Carbohydrate Polymers, 90(1), 650–657. doi: 10.1016/J.CARBPOL.2012.05.092 [DOI] [PubMed] [Google Scholar]
- Opiyo MA, Hamer GL, Lwetoijera DW, Auckland LD, Majambere S, & Okumu FO (2016). Using Stable Isotopes of Carbon and Nitrogen to Mark Wild Populations of Anopheles and Aedes Mosquitoes in South-Eastern Tanzania. PLOS ONE, 11(7), e0159067. doi: 10.1371/journal.pone.0159067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papierok B, Croset H, & Rioux JA (1973). Estimation of the effective larval population of Aedes (O.) cataphylla Dyar, 1916 (Diptera- Culicidae). I. Capture-marking-recapture method. Cahiers ORSTOM, Serie Entomologie Medicale et Parasitologie, 11(4), 243–9. Retrieved from https://www.cabdirect.org/cabdirect/abstract/19752900427 [Google Scholar]
- Reisen WK, & Aslamkhan M (1979). A release-recapture experiment with the malaria vector, Anopheles stephensi Liston, with observations on dispersal, survivorship, population size, gonotrophic rhythm and mating behaviour. Annals of Tropical Medicine & Parasitology, 73(3), 251–269. doi: 10.1080/00034983.1979.11687255 [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, & Lothrop B (2003). Factors Influencing the Outcome of Mark-Release-Recapture Studies with Culex tarsalis (Diptera: Culicidae). POPULATION AND COMMUNITY ECOLOGY J. Med. Entomol (Vol. 40). Retrieved from https://academic.oup.com/jme/article-abstract/40/6/820/835185 [DOI] [PubMed]
- Rioux JA, Croset H, Suquet P, and T. S (1968). ‘Essais de marquage par le phosphore radioactif P32 pour l’estimation absolue des populations larvaires de culicides (Diptera-Culicidae).”. Vie et Milieu, C(19), 55–62. [Google Scholar]
- Schabenberger O (2005). Introducing the GLIMMIX Procedure for Generalized Linear Mixed Models. Retrieved from http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.463.6093&rep=rep1&type=pdf
- Schimmelmann A (1991). Determination of the Concentration and Stable Isotopic Composition of Nonexchangeable Hydrogen in Organic Matter. Int. J. Mass Spectrom. Ion Phys, 63(21), 91–97. Retrieved from https://pubs.acs.org/sharingguidelines [Google Scholar]
- Schimmelmann A, & DeNiro MJ (1986). Stable isotopic studies on chitin. III. The D/H and 18O/16O ratios in arthropod chitin. Geochimica et Cosmochimica Acta, 50(7), 1485–1496. doi: 10.1016/0016-7037(86)90322-4 [DOI] [Google Scholar]
- Service MW (1997). Mosquito (Diptera: Culicidae) Dispersal-The Long and Short of It. J. Med. Entomol, 34(6), 579–588. Retrieved from https://academic.oup.com/jme/article-abstract/34/6/579/2221644 [DOI] [PubMed] [Google Scholar]
- Silver JB, Service MW, & dos Santos Alves Rogério; Soares de Souza Alex, et al. (2008). Mosquito Ecology: Field Sampling Methods (3rd edition). Igarss 2014. doi: 10.1007/978-1-4020-6666-5 [DOI]
- Taylor RAJ (1978). The relationship between density and distance of dispersing insects. Ecological Entomology, 3(1), 63–70. doi: 10.1111/j.1365-2311.1978.tb00903.x [DOI] [Google Scholar]
- Verdonschot PFM, & Besse-Lototskaya AA (2014). Flight distance of mosquitoes (Culicidae): A metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica, 45, 69–79. Retrieved from file:///C:/Users/faimanr/Downloads/2013VerdonschotBesse_LototskayaFlightdistanceofmosquitoes.pdf [Google Scholar]
- Wassenaar LI, & Hobson KA (2003). Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes in Environmental and Health Studies, 39(3), 211–217. doi: 10.1080/1025601031000096781 [DOI] [PubMed] [Google Scholar]
- Wassenaar LI, Hobson KA, & Sisti L (2015). An online temperature-controlled vacuum-equilibration preparation system for the measurement of δ 2 H values of non-exchangeable-H and of δ 18 O values in organic materials by isotope-ratio mass spectrometry. Rapid Commun. Mass Spectrom, 29, 397–407. doi: 10.1002/rcm.7118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.