Abstract
A middle-aged woman with end-stage renal disease (ESRD) due to obstructive nephropathy presented to the hospital for an episode of unresponsiveness and hypoglycaemia. Initially, she was diagnosed with hypoglycaemia associated with ESRD and was discharged. However, she returned to the hospital after experiencing tonic–clonic seizures and recurrent hypoglycaemia. Her hypoglycaemia workup revealed an elevated insulin-like growth factor 2 (IGF2) to IGF1 ratio consistent with paraneoplastic IGF2 secretion. Subsequently, a CT abdomen revealed a retroperitoneal mass, found to be a retroperitoneal sarcoma. Her hypoglycaemia was treated with glucocorticoids and growth hormone. Surgical debulking of her tumour was attempted, but she expired due to postoperative haemorrhagic shock. Doege-Potter syndrome is a rare cause of hypoglycaemia which should be suspected in any new-onset, worsening, inexplicable or refractory hypoglycaemia, particularly in non-diabetic ESRD. Here we present a report of retroperitoneal sarcoma presenting with hypoglycaemia in a patient with ESRD without diabetes.
Keywords: endocrinology, metabolic disorders, chronic renal failure, endocrine cancer
Background
Doege-Potter syndrome (DPS) is a small subset of non-islet cell tumour hypoglycaemia (NICTH), and is defined as a rare paraneoplastic form of hypoglycaemia characterised by low insulin levels and elevated insulin-like growth factor 2 (IGF2) levels typically secreted from a pleural or abdominal solitary fibrous tumour. The most common presentation of DPS is as a secondary manifestation of an already diagnosed malignancy; however, the primary presentation of the tumour as hypoglycaemia has also been described.1 2 On the other hand, many types of tumours have been associated with NICTH, with the most common tumours being of mesenchymal or hepatic origin.3
Hypoglycaemia in end-stage renal disease (ESRD) is a common occurrence, although seen less frequently in patients without diabetes. Hypoglycaemia from ESRD is often underdiagnosed and is independently associated with poorer outcomes.4 In patients with ESRD, hypoglycaemia should be evaluated using an algorithm-based approach.
A diagnosis of DPS should be considered after more common aetiologies of hypoglycaemia have been ruled out.5 Here, we describe a case of DPS from a retroperitoneal sarcoma that was initially thought to be hypoglycaemia secondary to ESRD. Due to the patient’s comorbid conditions, the diagnosis of the sarcoma was delayed, stressing the importance of a systemic approach to the evaluation of hypoglycaemia, especially in patients with ESRD.
Case presentation
A woman in her mid-50s presented to the hospital for an episode of unresponsiveness and new-onset of hypoglycaemia. Her previous medical history was significant for ESRD secondary to bilateral obstructive nephropathy from nephrocalcinosis several years prior to the presentation but was otherwise unremarkable. She did not previously have symptoms related to hypoglycaemia. There was no history of alcohol or tobacco consumption, and she resided with her family. None of her parents or siblings reported any medical history of cancer.
Her physical examination at the initial presentation revealed a body mass index of 29 kg/m2 with normal vital signs and no evidence of palpable masses or other systemic abnormalities with the exception of an arteriovenous fistula in her right arm.
On initial presentation, her hypoglycaemia was attributed to her ESRD. Subsequently, she was discharged from the hospital with instructions to follow-up with her primary care doctor. One week after her hospitalisation, she returned to the hospital and was noted to be hypoglycaemic in the emergency room with blood glucose of 38 mg/dL. Her glucose improved with injections of dextrose 50% but due to persistent hypoglycaemia, she was started on a continuous infusion of 10% dextrose.
Endocrinology was consulted for recurrent hypoglycaemia. The patient was placed on a 72 hours fast in order to obtain labs for completing a hypoglycaemia workup. The patient’s glucose fell to 42 mg/dL within 2 hours of stopping dextrose infusion.
Investigations
An MRI head and electroencephalogram (EEG) were performed for the evaluation of seizures. The MRI showed no focal lesions. The EEG was notable for diffuse encephalopathy consistent with a metabolic cause, such as hypoglycaemia.
Hypoglycaemia labs were drawn when the patient became hypoglycaemic off of continuous dextrose fluid. The labs showed low-normal serum insulin and normal C-peptide levels. The normal C-peptide level was attributed to impaired renal clearance due to her ESRD. Most notably, her labs showed a suppressed IGF1 and elevated IGF2 levels (table 1). An IGF2/IGF1 ratio of 12:1 was consistent with a diagnosis of paraneoplastic IGF2 secretion.
Table 1.
Laboratory evaluation of the patient
| Biochemical marker | Measured value | Normal range |
| Serum glucose | Arrival: 42 mg/dL Postglucagon: 103 mg/dL |
70–110 mg/dL (fasting) |
| Insulin | <2 µIU/mL | <17 µIU/mL |
| Proinsulin | 9.7 pmol/L | <18.8 pmol/L |
| Beta-hydroxybutyrate | 0.18 mmol/L | <0.28 mmol/L |
| C-peptide | 2.3 ng/mL | 0.8–3.1 ng/mL |
| IGF1 | 39 ng/mL | 80–225 ng/mL |
| IGF2 | 498 ng/mL | 267–616 ng/mL |
IGF, insulin-like growth factor.
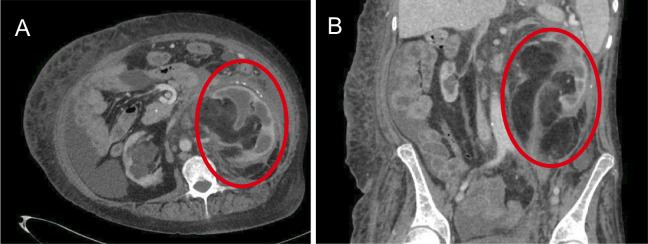
After a biochemical diagnosis of NICTH was established, a CT scan of the abdomen and pelvis (based on clinical suspicion) was performed to identify the source of ectopic IGF2 secretion that revealed an ill-defined 17 cm calcified retroperitoneal mass with an invasion of the bilateral pararenal spaces and infiltration of the left kidney and ureter (figure 1). Due to the size of the mass, there was also mass effect on the right kidney, pancreas and adrenal gland with encasement of the aorta. A core biopsy was inconclusive and showed an admixture of dense fibrous tissue and unremarkable mature adipose tissue. A follow-up CT scan performed 4 weeks after the first one, which did not reveal changes in the size of the tumour.
Figure 1.
Axial (A) and coronal (B) sections of the CT abdomen demonstrating the retroperitoneal sarcoma (red circles).
Differential diagnosis
Initially, when the patient presented with hypoglycaemia, a comprehensive hypoglycaemia workup was not completed as she was thought to have hypoglycaemia secondary to her ESRD. Subsequently, when she presented with hypoglycaemia provoked seizures, poor response to dextrose boluses and significant dextrose requirements, more extensive differential diagnosis was considered, and a hypoglycaemia workup was completed. Insulin-mediated causes such as exogenous (insulin injection) or endogenous (insulinoma) were excluded based on appropriately low insulin and C-peptide levels. She was noted to have elevated IGF2 level compared with low IGF1 level, which indicated a paraneoplastic aetiology. This was confirmed by visualisation of a retroperitoneal tumour. Other clinical features that supported a paraneoplastic aetiology included malnutrition and changes in body weight (unspecified).
Treatment
The patient was initially started on dextrose 10% infusion for acute treatment of her hypoglycaemia. However, after NICTH was suspected, she was started on hydrocortisone 20 mg two times per day, which was increased to 40 mg two times per day. Due to continued hypoglycaemia, she was then switched over to dexamethasone 4 mg two times per day. Unfortunately, she remained dependent on the dextrose infusion despite high dose dexamethasone. Recombinant growth hormone (rhGH) was started at 3 mg subcutaneous daily with immediate stabilisation of her glucose levels. The dextrose fluid was gradually discontinued and the rhGH was then tapered to 1 mg daily. The patient was able to maintain euglycaemia on a combination of dexamethasone 4 mg two times per day and rhGH 1 mg daily, so she was discharged from the hospital.
The patient was then referred to a tertiary care centre for surgical resection, given the size of her retroperitoneal mass and the extent of her disease. Notably, an attempt at the curative resection of the tumour had failed at an outside hospital after the surgeons were unable to dissect the tumour from the aorta and decided to abort the procedure and refer her to a tertiary centre for further management. Growth hormone was restarted and increased from 1 mg daily to 2 mg daily. Her steroids had been held initially but were restarted at dexamethasone 3 mg daily. However, she now required continuous dextrose infusion, which may have been indicative of worsening disease. Due to concerns that a complete resection would not be possible, other potential options, including radiation therapy, were explored but she was determined to not be a candidate. Ultimately the decision was made to debulk the tumour in the hopes that this would improve her hypoglycaemia and quality of life. Intraoperatively, the tumour was found to be significantly adherent to local vascular structures, including the aorta, the superior mesenteric artery and the coeliac artery. Attempts to mobilise the tumour led to significant bleeding requiring massive transfusions. Given that the tumour resection could not be safely resected or debulked, the surgery was aborted.
Surgical pathology showed fragments of unremarkable skeletal muscle and adipose tissue with extensive stromal fibrosis, necrosis and haemorrhage, mature adipose tissue mixed with lipoblasts and mild mixed inflammation. Collectively, these features were suggestive with an adjacent liposarcoma. In light of the patients’ deceased status, additional ancillary studies were not be performed.
Outcome and follow-up
Her postoperative course was complicated by haemorrhagic shock and multiorgan failure, resulting in her death at the postoperative day 1 and after spending 11 days in the hospital.
Discussion
Hypoglycaemia in patients with ESRD without diabetes is relatively uncommon, with a reported incidence of 1%–3%.4 6 7 The causes of hypoglycaemia in patients with ESRD without diabetes include reduced clearance of endogenous insulin, reduced renal gluconeogenesis, malnutrition, glucose-containing dialysate fluid causing glycaemic fluctuations and proteinuria and uremia-related hypoglycaemia.4 Additionally, metabolic acidosis leads to diminished ability to maintain normoglycaemia due to impaired hepatic gluconeogenesis. Further, episodes of hypoglycaemic unawareness also occur frequently, causing the under-recognition of such episodes.4 It is essential to fully evaluate the cause of the hypoglycaemia as rare conditions other than the ESRD, can be contributing to the hypoglycaemia and diagnosis can be delayed.
DPS is a rare condition reported mainly as case reports in the literature. A review of 45 case reports reported a mean age of diagnosis of 60.8±10.0 with a range of 38–79 years. Most of the tumours were larger than 10 cm at the time of diagnosis, including in our patient.8 A review of 288 published cases, reported that the most common site of the primary neoplasm was pleura, followed by retroperitoneum, abdomen and pelvis, mandible and thigh with greater than two-thirds (67%) of the tumours being malignant.5 The authors also described a female preponderance for this entity.5 In a more recent review of 71 cases by Han et al the most common cause of DPS were solitary fibrous tumours of the pleura and pelvis followed by liver and peritoneum, and a malignant aetiology of DPS was more common than a benign aetiology.2 DPS-related tumours were grossly described to be large and well-circumscribed, revealing fascicles with interspersed hypercellularity on histopathological examination.2 Furthermore, they reported a younger mean age (59 years) and male predominance in DPS, unlike prior reviews.2 5 DPS causes hypoglycaemia by secretion of IGF2 that has insulin-like activity and is a rare paraneoplastic cause of hypoglycaemia generally seen in the setting of an already established malignancy.
The Endocrine Society recommends confirmation of Whipple’s triad and lists NICTH on the differential diagnosis in patients without diabetes. Further, measurement of plasma glucose, proinsulin, C-peptide, beta-hydroxybutyrate, IGF1 and IGF2 is recommended in workup, as was done in this patient.9
This case is novel since it presents the first report of a very rare form of hypoglycaemia as a primary presentation of malignancy, in the setting of ESRD, making the diagnosis more challenging.
Learning points.
Hypoglycaemia is a life-threatening condition which usually affects patients with insulin-dependent diabetes and should be excluded in patients presenting with unresponsiveness or seizures.
Doege-Potter syndrome (DPS) represents a rare cause of hypoglycaemia.
Patients with (large) malignancies may have paraneoplastic secretion of IGF2 resulting in clinical conditions, including DPS.
DPS should be suspected in any new-onset, worsening or refractory hypoglycaemia.
In patients with end-stage renal disease without diabetes, a comprehensive guideline-based approach to the diagnosis of hypoglycaemia should be adopted.
Footnotes
Twitter: @skandshekharMD
Contributors: SS drafted portions of the manuscript, was involved in patient care, obtained images, made editorial changes and formatted the text. JC assisted in drafting the manuscript, was involved in patient care as a fellow physician, reviewing the literature and assembling tables. KD conceptualised the manuscript, was involved in patient care as attending physician, drafted portions of the manuscript and made revisions. All the authors approved the final version of the manuscript.
Funding: SS receives funding from the Intramural Research Program (IRP) of the National Institutes of Health (NIH).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor-II producing non-islet-cell tumor hypoglycemia. Growth Horm IGF Res 2006;16:211–6. 10.1016/j.ghir.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 2.Han G, Zhang Z, Shen X, et al. Doege-Potter syndrome: a review of the literature including a new case report. Medicine 2017;96:e7417-e. 10.1097/MD.0000000000007417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips LS, Robertson DG. Insulin-Like growth factors and non-islet cell tumor hypoglycemia. Metabolism 1993;42:1093–101. 10.1016/0026-0495(93)90265-P [DOI] [PubMed] [Google Scholar]
- 4.Arem R. Hypoglycemia associated with renal failure. Endocrinol Metab Clin North Am 1989;18:103–21. 10.1016/S0889-8529(18)30391-8 [DOI] [PubMed] [Google Scholar]
- 5.Bodnar TW, Acevedo MJ, Pietropaolo M. Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab 2014;99:713–22. 10.1210/jc.2013-3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsahli M, Gerich JE. Hypoglycemia in patients with diabetes and renal disease. J Clin Med 2015;4:948–64. 10.3390/jcm4050948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avram MM, Wolf RE, Gan A, et al. Uremic hypoglycemia. A preventable life-threatening complication. N Y State J Med 1984;84:593–6. [PubMed] [Google Scholar]
- 8.Meng W, Zhu H-H, Li H, et al. Solitary fibrous tumors of the pleura with Doege-Potter syndrome: a case report and three-decade review of the literature. BMC Res Notes 2014;7:515. 10.1186/1756-0500-7-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2009;94:709–28. 10.1210/jc.2008-1410 [DOI] [PubMed] [Google Scholar]