Abstract
A 67-year-old woman with a history of smoking and cardiovascular risk factors was admitted to the emergency room for uncontrolled diabetes, loss of appetite, nausea, significant weight loss and asthenia. The initial investigation, including cerebral and gastrointestinal explorations, were normal. One month later, she started presenting severe asymmetric proprioceptive ataxia of the lower extremities. She also reported paresthesia and neuropathic pain in both feet and ankles. A positron emission tomography (PET)-scanner showed a hypermetabolic nodule in the right lung. The neurological symptoms were attributed to paraneoplastic sensory and dysautonomic neuropathy, even though the bronchoscopic biopsies came back negative at first. Anti-Hu, anti-CV2/CRMP5 and anti-SOX1 antibodies were documented. Due to the severity and rapid progression of symptoms (from the lower to the upper limbs), corticosteroids, intravenous immunoglobulins and immunosuppressants were introduced prior to biopsies revealing a small-cell lung cancer. Despite these treatments and antineoplastic chemotherapy, her status deteriorated rapidly.
Keywords: neurology, immunology, respiratory cancer, malignant disease and immunosuppression
Background
Worldwide, broncho-pulmonary cancer is the first cause of cancer in men and the third cause in women.1 It is the first cause of cancer mortality and takes each year more lives than breast, prostate, pancreatic and colon cancers.2 Small-cell lung cancer (SCLC) accounts for about 15% of all lung cancers and is the most aggressive of these cancers. Its main cause (in 95% of cases) is tobacco.3 It is more frequently associated with paraneoplastic syndromes than other histological forms, with a prevalence of 10%–20%. There are a few paraneoplastic syndromes, and each has its own expression. These can be the first manifestation of cancer, often before the neoplasm’s discovery.4
Among paraneoplastic syndromes, subacute sensory neuropathy is a rare but typical expression of SCLC.5 It is important to investigate a neuropathy with no identified cause in patients at high risk of cancer. Indeed, an early discovery of cancer can lead to the prompt treatment of less advanced disease. The common presentation of paraneoplastic subacute sensory neuropathy is a development under 12 weeks, the severity of at least 3 on the Rankin score, numbness with pain and sensitive disturbance, non-length-dependent involvement of the arms and legs and often asymmetry at onset.4
Most antineuronal antibodies are secreted by the immune system in response to an autoantigen of the nervous system abnormally expressed by the SCLC.2 The autoimmune response targets the structures of the nervous system, which normally express this antigen, leading to the paraneoplastic syndrome. There are more than 20 known antigens associated with paraneoplastic syndromes in SCLC. A single patient can have several antineuronal antibodies, and therefore several paraneoplastic syndromes simultaneously.
Case presentation
A 67-year-old woman, with a history of cardiovascular risk factors including type 2 diabetes, hypertension and smoking was first oriented to the emergency room by her general practitioner for degradation of her general status and uncontrolled diabetes for a few months.
The patient had lost 17 pounds within a couple of weeks, was asthenic, had lost her appetite and presented with nausea and vomiting. Her glycated haemoglobin level was at 9.9%, whereas it had always been lower than 7%. The oral antidiabetic drugs (metformine and glicazide) had to be stopped because of the degradation of her renal function and insulin was initiated. Brain CT excluded any intracranial lesion which could have caused intracranial hypertension-related vomiting. The rest of her blood tests came back within normal ranges apart from hyponatraemia with a nadir of 121 mmol/L. A fluid restriction was initiated. The patient refused other explorations and was released on normalisation of natraemia. Gastroenterological explorations were scheduled on an outpatient basis.
One month later, the patient started complaining about a loss of coordination in her lower limbs and gait unsteadiness, leading to falls. She also reported tingling and pain in both feet and ankles. She visited several doctors, but no diagnosis was reached, and the symptoms were treated by pain medicines. Due to the worsening of her symptoms, she was finally admitted to the hospital 10 days later.
Her main symptom at admission was severe proprioceptive ataxia, which prevented her from standing or walking and was associated with an asymmetric pan-modality sensory loss (including significant vibration loss) up to the right knee, and below the left one. She also had paresthesia and complained about burning and dull neuropathic pain, poorly localised within the same areas (worse in the anterior part of the legs) and non-relieved by tramadol. At that time, she had a sensory loss in the lower abdominal regions (touching several dermatomes bilaterally, from T10 to T12) as well, and intermittent paresthesia in both hands, without additional neuropathic symptoms. Pregabaline and opioids were introduced and lowered the pain without ever making it completely disappear.
Investigations
Natraemia was normal at the time of the patient’s admission to the hospital for her neurological symptoms. Positron emission tomography (PET-scanner, however, showed a right pulmonary nodule and enlarged mediastinal lymph nodes with high metabolic activity (figure 1). She also had a costal lesion suspect of bone metastasis.
Figure 1.
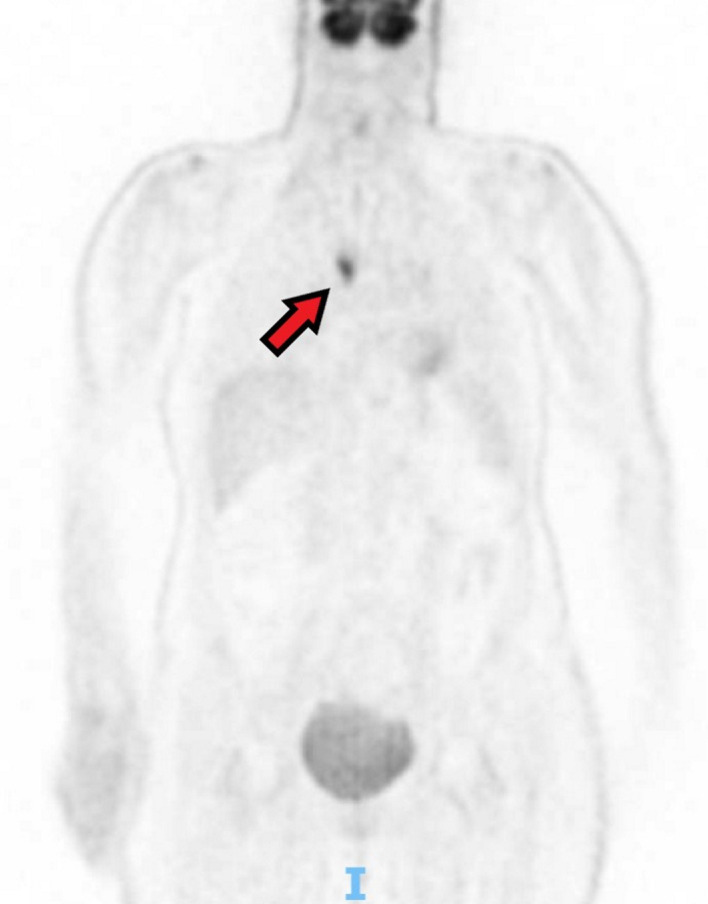
Positron emission tomography (PET)-scanner showing a highly metabolic pulmonary nodule.
Since the neuropathic symptoms were progressing up to mid-thigh, and she started experiencing numbness in both hands, with constant paresthesia, a spine MRI was conducted and showed no abnormality. The lumbar puncture was in favour of a lymphocytic meningitis: clear cerebrospinal fluid with augmented proteins (1.23 g/L) and 72×109/L white cells (97% of lymphocytes).
A body CT scan showed a thickened bronchus associated with homolateral hilar and mediastinal lymph nodes. There was also an anomaly in the posterior segment of the superior right lobe that the radiologist was not able to identify. No liver, bone or brain metastasis was seen.
The bronchoscopy confirmed the presence of a thickened bronchus, but the biopsies came back negative. Even though it was not proven, lung cancer with paraneoplastic neurological syndrome remained a highly probable diagnosis, and antineuronal antibodies were dosed. Anti-Hu, anti-CV2/CRMP5 and anti-SOX1 were positive while anti-N-methyl-D-aspartate receptor (NMDAR) antibodies came back negative. Electroneuromyography was in favour of a non-length-dependent sensory axonal neuropathy (ganglionopathy). Due to these findings (including anti-Hu positivity), the initial non-specific gastrointestinal symptoms were suspected to be secondary to autonomic dysfunction, causing gastroparesis. In line with this, the patient was diagnosed with mild orthostatic hypotension without an appropriate increase in heart rate.
Because of its severity, an immunomodulatory treatment was initiated for this sensory and dysautonomic neuropathy, associated with triple positive antineuronal antibodies, with steroids, intravenous immunoglobulins and cyclophosphamide, while cancer remained to be proven at this stage.
The patient had a second bronchoscopy with biopsies which could not show any cancer cells. Finally, a thoracoscopy was scheduled. Pathological analyses eventually confirmed the diagnosis of SCLC.
Differential diagnosis
Diabetes is one of the most common cause of neuropathy, and its classical type is sensory neuropathy, possibly associated with dysautonomic neuropathy.6 Diabetes can impact nerves through several mechanisms, mainly vascular and metabolic. While diabetic neuropathy usually begins gradually, it can also appear as an acute form, with a motor deficit at the forefront (the so-called Bruns-Garland syndrome or diabetic amyotrophy) in case of physical stress.7 At first, our patient was oriented to the emergency room for uncontrolled diabetes, so it was a main diagnosis to rule out. It was, however, later found that the lack of balance of her diabetes was rather of a consequence than a cause of her condition.
The second cause of sensory neuropathy in developed countries is alcohol abuse.6 Our patient reported abstaining from alcoholic beverages. Alcohol misuse/abuse can directly affect nerves by bonding to the membrane phospholipids or indirectly by inducing group B vitamin deficiency (B1, B6, B12) or traumatic lesions.8 In case of profound vitamin B1 deficiency, the patient may present with ataxia, delirium, oculomotor disorders and memory loss.9 Our patient did not present all of these symptoms; however, toxic neuropathies have previously been described and can be at least partially related to alcoholism.10
In a case with neurological signs in both upper and lower limbs to the clinical presentation of our patient, it is important to exclude a spinal cord lesion. Proprioceptive ataxia being the first and the most invalidating symptom in our patient, vitamin B12 deficiency-related subacute combined degeneration had to be considered. It is important to know that patients suffering from pernicious anaemia do not systematically present low haemoglobin levels or macrocytosis and the laboratory work does not always report on low serum levels of B12.11 In this hypothesis, the Electromyography would rather have shown a sensory-motor neuropathy.
Since our patient did not have spinal pain or symptoms compatible with an upper motor neuron syndrome, spinal cord compression (as a result of a vertebral metastasis or cervico-arthrosic myelopathy) was not expected, and the spine MRI ruled out this hypothesis.
Other chronic causes of sensory ganglionopathy should be excluded, as well.12 In our case, the acute onset and the extra-neurological symptoms were not consistent with an autoimmune disease such as Sjögren’s syndrome, a genetic disorder or a viral infection, and the corresponding laboratory tests were negative.
Treatment
The patient was treated monthly with intravenous corticosteroids 1 g/day 3 days in a row, intravenous immunoglobulins 0.4 g/kg/day for 5 days and 1 g of cyclophosphamide on the first day. Overall, our patient received four cures. Cyclophosphamide was stopped after the first cure to limit the risk of the adverse event because the patient was about to start the antineoplastic chemotherapy. The cancer was treated with carboplatin associated with etoposide every 3 weeks for three cures at first. A further three cures were decided on the basis of a good CT response.
Outcome and follow-up
Apart from ataxia, the main symptom that was invalidating for the patient was neuropathic pain. It decreased after the second cure of immunoglobulins, associated with opioids and clonazepam. However, the patient described a progression of her proprioceptive ataxia and paresthesia up to the thighs, abdomen and upper limbs. A decrease of her anal tonus was noted in the context of saddle anaesthesia. Between the third and the fourth cycle of chemotherapy, the patient had a febrile urinary tract infection related to treatment-induced neutropenia. Our patient ultimately had a brain haemorrhage due to a lack of platelets and became haemiplegic. Following this episode, palliative care was provided, and our patient died in the following weeks.
Discussion
The most frequent antibody in subacute sensory neuropathy is anti-Hu, while other antibodies such as anti-CV2/CRMP5, antiamphiphysin or anti-Yo are less frequently evidenced.4 Oncological treatments seem to provide symptomatic stabilisation, while the additional benefit provided by immunomodulatory treatments (eg, corticosteroids, intravenous immunoglobulin, plasma exchange, rituximab and cyclophosphamide for the most common) appears to be minimal.13 14 However, improvements have been reported in a few cases, especially when immunotherapies were administered timely and all experts agree about the fact that it should be considered when the neuropathy is severe and/or before a diagnosis of cancer has been reached, as in the present case.15 16
Anti-SOX1 antibodies were discovered in patients with paraneoplastic disorders associated with SCLC, particularly in Lambert-Eaton myasthenic syndrome and, to a lesser extent, in patients with anti-Hu positive paraneoplastic syndromes.17 One study (n=22) reported that anti-SOX1 antibodies were positive in four patients with a neuropathy of unknown origin (without any tumour after a 4-year follow-up, but no details regarding the investigations were provided); however, it has not been confirmed in larger studies.18 19 Hence, anti-SOX1 antibodies are mostly considered as biomarkers of SCLC, whose frequency is the highest (around 60%) in patients with associated paraneoplastic neurological syndrome, but which can occur in up to 15% of patients with this type of cancer alone.19
In our case, we found three antineuronal antibodies, anti-Hu, anti-CV2/CRMP5 and anti-SOX1 associated with this subacute paraneoplastic neuropathy. In most cases, there is only one antibody found, and to the best of our knowledge, there is, to this day, only a single case report available in the literature pertaining to triple positive antineuronal antibodies.20 A 56-year-old man was brought to the emergency room for cognitive and behavioural changes with motor disturbances for a couple of months. An MRI showed limbic encephalitis and a chest CT showed hilar lymphadenopathy with a necrotic centre, whose high metabolic activity was confirmed via PET-scanner, leading to the diagnosis of SCLC. In this case, antineuronal antibodies were positive with anti-Hu, anti-SOX1 and antiamphiphysin.
Similarly to our case, it was the paraneoplastic neurological symptoms that revealed the cancer and brought the patient into care. Unfortunately, the very limited number of cases does not allow us to generalise any conclusions; however, both cases appeared to be very severe and eventually led to the death of the patients, despite the association of intensive immunotherapy and chemotherapy.
Learning points.
A rapidly progressing neuropathy can be paraneoplastic, even without any typical signs of cancer
Antineuronal antibodies are quite rare, but reliable markers in the diagnosis of unexplained acute or subacute neurological signs.
Patients with paraneoplastic dysautonomic neuropathy (especially with anti-Hu antibodies) can present with unspecific gastrointestinal symptoms due to gastroparesis.
Multiple antineuronal antibodies positivity is extremely rare but seems to be associated with a very severe outcome.
Acknowledgments
We thank Dr Olivier Cerles for his precious help in improving the manuscript.
Footnotes
Contributors: VH and MK wrote the manuscript. J-PC and PC critically reviewed the manuscript. MK and PC managed the case. MK accepts full responsibility for the work, had access to the data and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Kazarian M, Laird-Offringa IA. Small-Cell lung cancer-associated autoantibodies: potential applications to cancer diagnosis, early detection, and therapy. Mol Cancer 2011;10:33. 10.1186/1476-4598-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015;121:664–72. 10.1002/cncr.29098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanaji N, Watanabe N, Kita N, et al. Paraneoplastic syndromes associated with lung cancer. World J Clin Oncol 2014;5:197–223. 10.5306/wjco.v5.i3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135–40. 10.1136/jnnp.2003.034447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra UK, Kalita J, Nair PP. Diagnostic approach to peripheral neuropathy. Ann Indian Acad Neurol 2008;11:89–97. 10.4103/0972-2327.41875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaghan BC, Cheng HT, Stables CL, et al. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2012;11:521–34. 10.1016/S1474-4422(12)70065-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammoud N, Jimenez-Shahed J. Chronic neurologic effects of alcohol. Clin Liver Dis 2019;23:141–55. 10.1016/j.cld.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 9.Dhir S, Tarasenko M, Napoli E, et al. Neurological, psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Front Psychiatry 2019;10:207. 10.3389/fpsyt.2019.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamel J, Logigian EL. Acute nutritional axonal neuropathy. Muscle Nerve 2018;57:33–9. 10.1002/mus.25702 [DOI] [PubMed] [Google Scholar]
- 11.Wolffenbuttel BHR, Wouters HJCM, Heiner-Fokkema MR, et al. The Many Faces of Cobalamin (Vitamin B12) Deficiency. Mayo Clin Proc Innov Qual Outcomes 2019;3:200–14. 10.1016/j.mayocpiqo.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowell A, Gwathmey KG. Sensory neuronopathies. Curr Neurol Neurosci Rep 2017;17:79. 10.1007/s11910-017-0784-4 [DOI] [PubMed] [Google Scholar]
- 13.Keime-Guibert F, Graus F, Broët P, et al. Clinical outcome of patients with anti-Hu-associated encephalomyelitis after treatment of the tumor. Neurology 1999;53:1719–23. 10.1212/WNL.53.8.1719 [DOI] [PubMed] [Google Scholar]
- 14.Graus F, Keime-Guibert F, Reñe R, et al. Anti-Hu-Associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain 2001;124:1138–48. 10.1093/brain/124.6.1138 [DOI] [PubMed] [Google Scholar]
- 15.Widdess-Walsh P, Tavee JO, Schuele S, et al. Response to intravenous immunoglobulin in anti-Yo associated paraneoplastic cerebellar degeneration: case report and review of the literature. J Neurooncol 2003;63:187–90. 10.1023/A:1023931501503 [DOI] [PubMed] [Google Scholar]
- 16.Berzero G, Karantoni E, Dehais C, et al. Early intravenous immunoglobulin treatment in paraneoplastic neurological syndromes with onconeural antibodies. J Neurol Neurosurg Psychiatry 2018;89:789–92. 10.1136/jnnp-2017-316904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tschernatsch M, Singh P, Gross O, et al. Anti-SOX1 antibodies in patients with paraneoplastic and non-paraneoplastic neuropathy. J Neuroimmunol 2010;226:177–80. 10.1016/j.jneuroim.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 18.Berger B, Dersch R, Ruthardt E, et al. Prevalence of anti-SOX1 reactivity in various neurological disorders. J Neurol Sci 2016;369:342–6. 10.1016/j.jns.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-García R, Martínez-Hernández E, García-Ormaechea M, et al. Caveats and pitfalls of Sox1 autoantibody testing with a commercial line blot assay in paraneoplastic neurological investigations. Front Immunol 2019;10:769. 10.3389/fimmu.2019.00769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda TG, do Rosário MS, Branco RCC, et al. Multiple paraneoplastic antibodies (anti-SOX1, anti-Hu, and anti-Amphiphysin) detected in a patient with limbic encephalitis and small cell lung cancer. Neurol India 2017;65:1127. 10.4103/neuroindia.NI_1256_15 [DOI] [PubMed] [Google Scholar]


