Abstract
This study was designed to compare systemic O2 utilization (V̇O2), and changes in tissue O2 extraction [deoxyhemoglobin (ΔHHb)] in the vastus lateralis (VL), gastrocnemius (GAST) and pre-frontal cortex (PFC) tissue; between aerobically short-term trained (STT) and long-term trained (LTT) older men (40 – 60 yr) who were matched for current training load. On separate occasions, 14 STT and 14 LTT participants completed ramp incremental (RI) and square-wave constant load (SWCL) tests on a cycle ergometer. In LTT compared to STT; (i) V̇O2 was higher during the RI (p > 0.001) and SWCL (p > 0.001) tests, (ii) ΔHHb in the GAST was greater in SWCL (p = 0.011); and (iii) ΔHHb in the PFC was greater at 90% GET during SWCL (p = 0.011). The additional years of training in LTT compared to STT (LTT 17.50yr ± 6.94yr vs STT 1.68yr ± 0.31yr) were associated with higher V̇O2peak, and sub-GET V̇O2, and ΔHHb in the GAST and PFC at sub-GET exercise, despite there being no difference in current training volume.
Keywords: Older men, aerobic training, V̇O2peak, oxygenation
INTRODUCTION
Regular aerobic exercise training increases peak oxygen uptake (V̇O2peak) in previously untrained adults irrespective of age and sex (6, 34). While the rate and magnitude of the increases in V̇O2peak depend largely on the training load (intensity and volume) of the intervention (18, 47), gains in V̇O2peak plateau within ~ 24 months (27, 46). The effect of many years of aerobic training on V̇O2peak is limited to longitudinal studies (7 – 20 yr) investigating how age-related declines in V̇O2peak are offset with regular aerobic training (17, 30); where the rate of decline is strongly related to changes in training volume over time (11, 23). If training volume is the primary mediator of increases in V̇O2peak, then the training length (years of training) should not affect V̇O2peak if the training volume is matched. Currently, differences in V̇O2peak between short and long-term trained individuals of the same age and current training volume is limited to one study from our laboratory on women aged 40 – 60 yr (8). However, as training volumes were significantly different between the women in that study and the men in the present study, and age-related declines in V̇O2peak differ between sexes (17, 53), a direct comparison could not be made. A unique finding in the study of older women (8) was that higher pulmonary ventilation (V̇E) in the LTT at peak exercise resulted from an increase in tidal volume (VT) not breathing frequency (BF) as previously reported (49). Whether the same applies to older men is unknown.
Increases in V̇O2peak from aerobic training result from enhanced O2 delivery (from increased peak cardiac output and vascular volume) and/or O2 extraction by active muscles (producing an increase in peak arterio-venous O2 difference). The relative contribution that each component has on increases in V̇O2peak differ between young and older men (35, 37). In healthy older men, based on variable responses (32, 33), the relative contribution that central and peripheral adaptations have on changes in V̇O2peak are less clear. Furthermore, while an increased arterio-venous O2 difference is strongly linked to muscle tissue O2 extraction, changes in arterio-venous O2 difference reflect O2 extraction of all tissues of the body, not isolated active muscles.
While peak or max values are commonly used to indicate an individual’s exercise capacity, longer bouts of submaximal exercise (below GET) are typically performed in training and competition. Moreover, endurance training can affect V̇O2 kinetics and the matching of O2 delivery to utilization during transitions from low to moderate intensity cycling in older men (2, 36), with greater improvements in long-term compared to short-term trained men (21). This adaptation could be due, it part, to a an increased affinity of O2 to hemoglobin and a decrease in blood lactate for a given intensity (51), which could improve O2 delivery and extraction at the muscle tissue during longer periods of moderate intensity exercise. However, while short term (4 wks) endurance training does not affect HHb amplitude or pattern during longer duration (15 min) constant load cycling (10), the effect of long-term training is not known.
Near-infrared spectroscopy (NIRS) provides a non-invasive indirect method of measuring real time changes in O2 extraction in isolated tissue via changes in deoxygenated hemoglobin (HHb) and oxyhemoglobin (O2Hb) (15, 22), with the HHb signal representing microvascular O2 extraction (balance between O2 delivery and utilization), and thus being indicative of arterio-venous O2 difference (12, 20). Previous studies have used the rate of response of HHb (normalised to peak HHb) during transitions from low to moderate exercise to report training induced improvements in active muscle tissue O2 extraction in men (21, 36). However, changes in HHb (ΔHHb) at peak exercise would provide an indication of the peak dynamic balance between O2 supply and demand, as total leg O2 extraction capacity is related to V̇O2peak (40, 44). Furthermore, some research indicates a link between cerebral (prefrontal cortex [PFC]) oxygenation and maximal exercise performance (4, 41), with greater oxygenation in trained and untrained individuals (45). While training length in years has no effect on the PFC oxygenation in women 40 – 60 yr (8) during low and high intensity exercise, the effect on men > 40 years is currently unclear.
The purpose of this study was to simultaneously investigate systemic O2 utilization (VO2), and tissue O2 extraction (ΔHHb) in two active muscles and the PFC between STT (6 – 24 months) and LTT (> 5 years) aerobically trained men aged 40 – 60 years matched for current training load, during ramp incremental (RI) and sub-gas exchange threshold (GET) square-wave constant load (SWCL) cycling. It was hypothesized that: 1) VO2 and ΔHHb in the vastus lateralis (VL) and gastrocnemius (GAST) would be greater in LTT compared to STT at 90% GET, GET and peak exercise during RI, and all measured relative intensities (25%, 80% and 90% GET) during SWCL cycling; and 2), that there would be no difference in the ΔHHb in the PFC between the groups at any exercise intensity.
METHODS
Participants
The two participant groups of older (40 – 60 yr) Caucasian men consisted of one group of 14 short-term trained (STT; 6 – 24 months) men, and one group of 14 long-term trained (LTT; > 5 yr) men. Each participant had regularly completed > 230 min of moderate to vigorous aerobic exercise per week (all including cycling) missing no more than two weeks of training over any six-month period, as determined from self-reported physical activity training logs (refer Table 1). Current training load was determined by summing the product of each training session duration (min) and the intensity (1 = low, 2 = moderate, 3 = high) for the past week. All participants reported this training volume as a typical week of training completed within the previous six months. Participants’ physical characteristics and training history are provided in Table 1. Following medical screening (Physical Activity Readiness Questionnaire (7) and Medical Health Questionnaire), exclusion included any health related issues or medications that would affect participant safety and or exercise capacity or O2 utilization/extraction.
Table 1.
Participant characteristics of the short-term trained and long-term trained older men.
| Characteristic | STT | LTT |
|---|---|---|
| Age (yr) | 48.6 (5.5) | 46.1 (4.6) |
| Weight (kg) | 87.4 (10.6) | 79.9 (8.9) |
| Height (cm) | 180.9 (7.5) | 181.5 (6.2) |
| VL adipose (thickness) | 5.7 (1.3) | 4.9 (1.4) |
| GAST adipose (thickness) | 3.4 (1.6) | 3.3 (1.1) |
| Current training (yr) | 1.7 (0.3) | 17.5 (8.4) * |
| Lifetime training (yr) | 7.6 (5.5) | 17.5 (6.9) * |
| Current training load (AU) | 1184 (420) | 1242 (451) |
| GET V̇O2 (mL · kg−1 · min−1) | 26.6 (5.5) | 37.7 (5.4) * |
| GET % of Peak (mL · kg−1 · min−1) | 67.1 (8.0) | 72.9 (5.0) * |
Values are mean (SD).
Significant (p < 0.05) difference between groups.
Current training (yr) = current number of continuous years of training; Current training load (AU) = min × intensity (light = 1, moderate = 2 and high = 3).
STT: Short-term trained; LTT: Long-term trained; VL: Left vastus lateralis; GAST: Gastrocnemius; AU: Arbitrary units; GET: Gas exchange threshold.
Protocol
Session 1
The aim of session one was to determine participant characteristics, and V̇O2, ventilation (V̇E, VT, BF), HR, RPE (Table 2) and ΔHHb in the VL, GAST and PFC during a RI cycling (increments = 1 W per 2 s) test to volitional cessation, as previously described (8). To encourage peak results, participants received feedback and encouragement from the tester during the RI test. Expired gas was analyzed (Parvo Medics, Sandy, UT, USA) to determine V̇O2peak, and GET, with V̇O2peak determined as the highest 15-s average V̇O2 value within the last minute of the RI test, and GET determined using the V-slope method as described by Beaver, Wasserman and Whipp (3). The V-slope method has been reported to be more accurate and have smaller standard deviations compared to other methods (19). The GET in the present study was time matched with the power (Watts) of the cycle ergometer to determine the power outputs for the SWCL test. As systemic V̇O2 and muscle O2 extraction were not directly compared (i.e. VO2 to ΔHHb ratio) and the RI and SWCL were not compared, V̇O2 was not left shifted to accommodate for any potential phase 1-phase II V̇O2 lag time.
Table 2.
Systemic oxygen utilization, ventilation, heart rate and rating of perceived exertion of short-term trained and long-term trained older men at 90% Gas Exchange Threshold and peak exercise during Ramp Incremental (RI) cycling.
| Variable | 90% GET | Peak | ||
|---|---|---|---|---|
|
| ||||
| STT | LTT | STT | LTT | |
| V̇O2 (mL · kg−1 · min−1)a,b | 24.1 (3.6) | 34.4 (4.8) | 39.4 (5.8) | 51.7 (6.1) |
| VE (L · min−1)b | 52.6 (9.1) | 64.9 (13.0) | 144.3 (27.9) | 153.4 (25.3) |
| VT (L)b | 2.5 (0.7) | 2.9 (0.3) | 3.0 (0.6) | 3.1 (0.4) |
| BF (Breaths · min−1)b | 23.2 (5.4) | 24.6 (4.7) | 47.6 (9.8) | 53.2 (10.1) |
| HR (Beats · min−1) #b | 127.8 (13.7) | 134.0 (11.3) | 160.3 (10.8) | 168.0 (10.8) |
| RPEb | 3.6 (1.1) | 4.7 (1.2) | 9.9 (0.4) | 9.9 (0.4) |
Values are mean (SD).
significant group main effect;
significant intensity main effect;
significant group by intensity interaction;
significant difference between groups p < 0.05;
HR at 90% GET, STT n = 12, LTT n = 12. HR at Peak, STT n = 13, LTT n = 12.
GET: Gas exchange threshold; STT: Short-term trained; LTT: Long-term trained; V̇O2: Oxygen utilization; V̇E: Minute ventilation; VT: Tidal volume; BF: Breathing frequency; HR: Heart rate; RPE: Rating of perceived exertion.
Session 2
This session was conducted three to 14 days after session one. The aim of this session was to determine participants’ V̇O2, ventilation (V̇E, VT, and BF), HR, RPE (Table 4) and ΔHHb while each participant cycled at the same calculated relative intensity of 25%, 80% and 90% of GET power output obtained from the RI test. The timing for these intensities were as follows: 3 minutes at 25%, 80% and 25%; 20 minutes at 90%; and another 3 minutes at 25% of calculated GET.
Table 4.
Results of two-way Analysis of Covariance (ANCOVAs) for ventilatory parameters and ΔHHb during Ramp Incremental and Square-Wave Constant Load cycling.
| Variable | Test | Main effect for group | Group × intensity interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Df | F | p | ɳƤ2 | β | Df | F | p | ɳƤ2 | β | ||
| V̇O2 (mL · kg−1· min−1) | RI | 1,25 | 37.851 | < 0.001* | 0.602 | 1 | 1,25 | 2.669 | 0.115 | 0.096 | 0.35 |
| SWCL | 1,25 | 33.377 | < 0.001* | 0.572 | 1 | 2,50 | 36.74 | < 0.001* | 0.595 | 1 | |
| V̇E (L · min−1) | RI | 1,25 | 2.66 | 0.115 | 0.096 | 0.35 | 1,25 | 0.108 | 0.745 | 0.004 | 0.06 |
| SWCL | 1,25 | 5.091 | 0.033* | 0.169 | 1 | 2,50 | 4.746 | 0.013* | 0.16 | 0.77 | |
| VT (L) | RI | 1,25 | 2.19 | 0.151 | 0.081 | 0.3 | 1,25 | 4.052 | 0.055 | 0.139 | 0.49 |
| SWCL | 1,25 | 0.85 | 0.365 | 0.033 | 0.14 | 2,50 | 0.52 | 0.598 | 0.02 | 0.13 | |
| BF (Breaths · min−1) | RI | 1,25 | 0.051 | 0.823 | 0.002 | 0.01 | 1,25 | 0.372 | 0.547 | 0.015 | 0.09 |
| SWCL | 1,25 | 1.179 | 0.288 | 0.045 | 0.18 | 2,50 | 0.007 | 0.993 | < 0.001 | 0.05 | |
| HR (Beats · min−1) | RI | 1,21 | 0.108 | 0.745 | 0.005 | 0.06 | 1,21 | 3.369 | 0.081 | 0.138 | 0.42 |
| SWCL | 1,20 | 0.491 | 0.491 | 0.024 | 0.1 | 2,40 | 0.264 | 0.769 | 0.013 | 0.09 | |
| RPE | RI | 1,25 | 1.15 | 0.294 | 0.044 | 0.18 | 1,25 | 1.919 | 0.178 | 0.071 | 0.27 |
| SWCL | 1,25 | 0.201 | 0.657 | 0.008 | 0.07 | 2,50 | 1.325 | 0.275 | 0.05 | 0.27 | |
| VL ΔHHb | RI | 1,24 | < 0.001 | 0.993 | < 0.001 | 0.05 | 1,24 | 0.419 | 0.514 | 0.017 | 0.1 |
| SWCL | 1,24 | 0.932 | 0.344 | 0.037 | 0.15 | 2,48 | 1.464 | 0.242 | 0.057 | 0.3 | |
| GAST ΔHHb | RI | 1,22 | 3.213 | 0.087 | 0.127 | 0.4 | 1,22 | 0.016 | 0.899 | 0.001 | 0.05 |
| SWCL | 1,23 | 7.537 | 0.011* | 0.239 | 0.75 | 2,46 | 2.072 | 0.137 | 0.079 | 0.41 | |
| PFC ΔHHb | RI | 1,25 | 0.001 | 0.97 | 0.001 | 0.05 | 1,25 | 0.443 | 0.512 | 0.017 | 0.1 |
| SWCL | 1,25 | 7.599 | 0.011* | 0.233 | 0.76 | 2,50 | 5.135 | 0.009* | 0.17 | 0.8 | |
RI: Ramp incremental; SWCL: Square-wave constant load; V̇O2: Systemic oxygen utilization; V̇E : Minute ventilation; VT: Tidal volume; BF: Breathing frequency; HR: Heart rate; RPE: Rating of perceived exertion, VL: Vastus lateralis; GAST: Gastrocnemius; PFC: Pre-frontal cortex; HHb: deoxyhemoglobin.
Significant p < 0.05.
V̇O2, HR and ΔHHb in the VL, GAST and PFC were recorded continuously during exercise, while RPE was recorded within the last 10-s of the third minute of each of the three minute SWCL stages, and every fourth minute within the 20 minute stage using standard methods as previously described (8). To minimize cognitive stimulus, participants did not receive encouragement or feedback during this test.
Tissue Deoxyhemoglobin
Tissue oxygenation (HHb and O2Hb) data were measured continuously and simultaneously from the left VL, GAST and PFC during exercise with a single-channel NIRS system (PortaMon and Portalite, Artinis Medical Systems BV, Zetten, Netherlands). The muscle optodes were fixed to the skin at the mid-belly of the muscle using adhesive tape and wrapped with low compression black elastic bandage, and the PFC optode was fixed to the skin at the left PFC using adhesive tape, then covered with a black headband (8).
All NIRS primary data (HHb and O2Hb) were recorded at 10 Hz. The last 20-s of resting values were averaged to obtain baseline values. All changes were then expressed relative to these baseline values and then calculated and displayed as follows: 15-s averages for RI; 30-s averages for SWCL; total average data for the 15-s preceding 90% GET and peak exercise for RI; and total data for each exercise intensity for SWCL. In the present study, only HHb data have been presented, as compared to O2Hb, HHb is less affected by changes in blood hemodynamics (12, 16), thus a better indicator of oxygen extraction.
Statistical Analysis
All statistical analyses were performed using SPSS (version 22, SPSS Inc., Chicago, IL). Prior to statistical analysis, data were checked for normality using the Shapiro-Wilk test of normality and the Mauchly test for sphericity, and that the relevant assumptions for each test were met. To identify the presence of any significant group (STT vs LTT) differences in current training load, independent t-tests were conducted. To identify the presence of any significant exercise intensity and group (STT vs LTT) main effects or interactions, two-way Analysis of Covariance (ANCOVAs) were conducted on each dependent variable within each of the tests. For the RI, 2 (group: STT and LTT) × 2 (intensity: 90% of GET and peak) ANCOVAs were performed, and for the SWCL, 2 (group: STT and LTT) × 3 (intensity: 25% [first period of 25%] 80% and 90% of GET) ANCOVAs were performed. Due to the potential substantial effect of current training load on physiological responses to exercise, training load was included as a covariate in all analyses. For all analyses, the threshold for statistical significance was set to p < 0.05. Partial eta-squared was used to determine the effect size as small (ɳƤ2 > 0.01), medium (ɳƤ2 > 0.06) or large (ɳƤ2 > 0.14) as per Cohen (9).
RESULTS
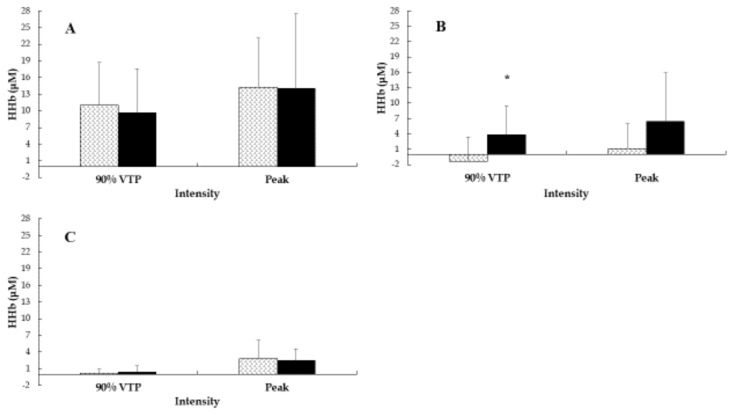
Current training loads were not significantly different between STT and LTT (Table 1). For all dependent variables in both tests, there was a significant (p < 0.05) main effect of exercise intensity with a large effect size. These variables increased with increased exercise intensity, as physiologically expected (descriptive statistics are shown in Tables 2 and 3). Results for group main effects and group by intensity interactions are given in Table 4. There were significant (p < 0.05) group main effects for V̇O2 (mL· min−1; mL · kg−1 . min−1) during both the RI and SWCL tests and V̇E during SWCL. There were significant (p < 0.05) group by intensity interactions for V̇O2 (mL· kg−1 . min−1) and V̇E during the SWCL test. The difference in V̇O2 and VE between groups increased with increased intensity. The group mean ΔHHb in the RI and SWCL tests are presented in Figures 1 and 2. There were significant (p < 0.05) group main effects for ΔHHb in the GAST and PFC during the SWCL test. There were significant (p < 0.05) group by intensity interactions for ΔHHb in the PFC during the SWCL test (Figures 3 and 4).
Table 3.
Systemic oxygen utilization, ventilation, heart rate and rating of perceived exertion of short-term trained and long-term trained older men at 25%, 80% and 90% Gas Exchange Threshold during Square-Wave Constant Load (SWCL) cycling.
| Variable | 25% VT | 80% VT | 90% VT | |||
|---|---|---|---|---|---|---|
|
| ||||||
| STT | LTT | STT | LTT | STT | LTT | |
| V̇O2 (mL · kg−1 · min−1)a,b,ab | 10.6 (1.6) | 13.5 (2.3) * | 21.3 (3.2) | 29.5 (4.4) * | 29.3 (4.4) | 39.7 (4.9) * |
| VE (L · min−1)a,b,ab | 24.1 (6.3) | 27.0 (8.6) | 46.9 (6.2) | 55.8 (10.2) * | 73.9 (7.1) | 85.7 (16.9) * |
| VT (L)b | 1.5 (0.4) | 1.6 (0.3) | 2.3 (0.5) | 2.5 (0.4) | 2.8 (0.5) | 2.9 (0.3) |
| BF (Breaths · min−1)b | 16.6 (2.5) | 18.9 (8.4) | 21.0 (4.4) | 23.5 (7.4) | 27.5 (4.0) | 30.0 (6.6) |
| HR (Beats · min−1) #b | 89.2 (5.1) | 84.7 (7.4) | 116.3 (7.7) | 117.2 (6.9) | 140.5 (10.0) | 145.4 (7.3) |
| RPEb | 1.2 (0.6) | 1.0 (0.0) | 3.1 (0.7) | 3.4 (0.6) | 6.5 (1.2) | 5.7 (1.0) |
Values are mean (SD).
significant group main effect;
significant intensity main effect;
significant group by intensity interaction;
significant difference between groups p < 0.05;
HR at 25% VT; STT n = 9, LTT n = 13. HR at 80% and 90% VT; STT n = 10, LTT n = 13.
GET: Gas exchange threshold; STT: Short-term trained; LTT: Long-term trained; V̇O2: Oxygen utilization; V̇E : Minute ventilation; VT: Tidal volume; BF: Breathing frequency; HR: Heart rate; RPE: Rating of perceived exertion.
Figure 1.
Mean ΔHHb in the vastus lateralis, gastrocnemius and pre-frontal cortex within Ramp Incremental cycling. Panel (A) ΔHHb in the VL. Panel (B) ΔHHb in the GAST. Panel (C) ΔHHb in the PFC. STT 0 – 8 min (n = 14); 8 – 11 min (n = 9 – 13); 11 – 13.5 min (n = 2 – 8). LTT 0 – 10.5 min (n = 14); 10.5 – 13 (n = 8 – 13); 13 – 15.5 (n = 2 – 8). Square trace LTT, diamond trace STT.
Figure 2.
Mean ΔHHb in the vastus lateralis, gastrocnemius and pre-frontal cortex during Square-Wave Constant load cycling. Panel (A) ΔHHb in the VL. Panel (B) ΔHHb in the GAST. Panel (C) ΔHHb in the PFC. Square trace LTT, diamond trace STT.
Figure 3.
Group mean ΔHHb in the VL, GAST and PFC during Ramp Incremental cycling. Panel (A) ΔHHb in the VL. Panel (B) ΔHHb in the GAST. Panel (C) ΔHHb in the PFC at 90% of GET and peak exercise. Pattern fill STT, solid fill LTT. * Significant (p < 0.05) differences between groups.
Figure 4.
Group mean ΔHHb in the VL, GAST and PFC during Square-Wave Constant Load cycling. Panel (A) ΔHHb in the VL. Panel (B) ΔHHb in the GAST. Panel (C) ΔHHb in the PFC at 25%, 80% and 90% of GET. Pattern fill STT, solid fill LTT. * Significant (p < 0.05) differences between groups.
DISCUSSION
This study examined systemic V̇O2, and O2 extraction (ΔHHb) in the VL, GAST and PFC at peak and sub-GET intensities between short-term and long-term aerobically trained men aged 40 – 60 yr, who were matched for current training load. The primary findings were that VO2 was significantly higher in LTT compared to STT, and O2 extraction (ΔHHb) was greater in GAST but not the VL or PFC in LTT compared to STT. These data suggest that without decreasing training volume per year, additional years of training can significantly improve V̇O2peak for which increased muscle O2 extraction may be a major contributing factor. While this result supports the suggestion that peripheral adaptations are likely to be responsible for increases in V̇O2peak from aerobic training (33), as peak cardiac output was not measured in the present study it is not possible to quantify the effect the greater O2 extraction had on the higher V̇O2peak in LTT.
The V̇O2peak values in the LTT (2.6 ± 0.4 L·min−1 / 39.4 ± 5.8) and STT (2.1 ± 0.4 L·min−1 / 51.7 ± 6.1) are consistent with normative values of well-trained men aged 40 – 60 yr (50). These data support the hypothesis in that the additional training years of the LTT resulted in significantly higher V̇O2peak than the STT. The present results support the findings of a similar study of women aged 40 – 60 yr (8), and results of a longitudinal study; where over a 10-year period starting from the age of 42 – 45 yr, men who were already somewhat trained increased their V̇O2peak without changing their training volume (29). Collectively, these studies suggest that while gains in V̇O2peak significantly plateau within ~ 24 months of starting aerobic exercise training (27, 46), with regular training and maintained training volume, V̇O2peak can continue to increase for 5 – 10 yr between the ages of 40 – 60 yr.
In the present study, V̇O2 at GET was, as expected, significantly higher in LLT compared to the STT. However, an unexpected finding was that this difference still existed when expressed as a percentage of V̇O2peak. This result suggests a higher GET as a percentage of V̇O2peak may be associated with higher V̇O2peak. While previous studies support the possible influence of GET on V̇O2peak and performance, V̇O2peak, not GET is regarded as the major determinant of aerobic exercise performance in adults.
For V̇O2 during SWCL, the present results supported our hypothesis in that V̇O2 was significantly higher in LTT compared to STT while cycling at a power calculated to be 25%, 80% and 90% of GET. Furthermore, the difference between groups increased with increases in intensity (as indicated by the ANCOVA interactions) from 0.2 L · min−1 at 25%, to 0.4 L · min−1 at 80% and 0.5 L · min−1 at 90% of GET. Similar results have been reported as a function of aerobic training in older men (21) and women (8, 13). Taken together these data suggest that aerobic exercise increases systemic O2 utilization during sub-GET constant load exercise.
Differences in pulmonary ventilation (V̇E, VT, BF) have been previously reported between STT and LTT older women (8). Concurring with the findings of that study, there was a significant interaction for VE in SWCL in the present study. However, there were no significant differences between groups for VT and BF in either the RI or SWCL in the present study. This provides further support for the suggestion that the effects of aerobic exercise on ventilation may be different between sexes (1, 8).
For ΔHHb in the VL during RI, the present data were contrary to the hypothesis with ΔHHb not being different between STT and LTT. However, in support of the hypothesis ΔHHb in the VL was not different between groups during SWCL. This finding suggests that in men aged 40 – 60 yr, additional training years beyond 24 months may not improve O2 extraction (ΔHHb) in the VL at any intensity.
Previously, only two studies have investigated the effect of aerobic exercise training on muscle tissue O2 extraction (ΔHHb) in exercising adults between 40 and 60 yr (8, 21). Within the first study (21) the matching of O2 distribution (V̇O2) and extraction (ΔHHb) in the VL was compared in untrained and long-term (≥ 7 yr) trained men aged 40 – 59 yr during transitions from light to moderate exercise below GET. The faster V̇O2 but not ΔHHb kinetics (thus a lower ΔHHb: V̇O2 ratio) in the trained compared to the untrained indicated a better matching of local O2 delivery to extraction. Furthermore, while peak exercise tests were conducted in the previous study to calculate the work load (as a percentage of GET) for subsequent sub-GET tests, no peak ΔHHb values were presented. The results of the second study (8) on short and long-term trained women aged 40 – 60 yr do not support those of the present study with the LTT compared to the STT having greater peak ΔHHb in the RI, and at the same relative intensity during the SWCL test (8). Collectively, these results suggest that sex differences exist in the relative contribution that peripheral and central components have on increases in, or maintenance of V̇O2peak in adults aged 40 – 60 yr.
For the ΔHHb in the GAST, the present results were contrary to the hypothesis with the ΔHHb in the GAST significantly greater in LTT compared to STT during SWCL but not during RI. This new finding suggests that in men aged 40 – 60 years, long-term (> 5 years) aerobic exercise provides adaptations to and/or maintains the ability of the GAST muscle to utilize O2 during sub-GET exercise, which was not observed in the VL. Previously, only three studies have reported oxygenation (HHb or O2Hb) changes in the GAST during exercise (8, 25, 31), with only one of these investigating the effect of aerobic training (8). In that study on STT and LTT women (40 – 60 yr), the ΔHHb in the GAST was not significantly different between groups during RI and SWCL exercise (8). Possible explanations for the disparity in the results between these two studies include sex difference in muscle fiber distribution (28), and reduced leg blood flow and perfusion pressure reported in older women (43). However, the opposing HHb patterns of the GAST (which decreased substantially) and the VL (which progressively increased) at the onset of exercise in the present study were also observed in women (8). This could potentially be due to a greater increase in mechanical vasoconstriction at the GAST compared to the VL at the onset of cycling, with the initial increase in blood flow during exercise (in the lower limbs) being driven by muscle pump activation (54).
Compared to the GAST, the VL has a low percentage of Type I fibers and a higher percentage of Type IIa and IIx fibers that contribute to a faster onset of O2 extraction (14, 24). Furthermore, age-related reductions in citrate synthase activity and thus oxidative capacity have been reported in the GAST but not VL in older men, with V̇O2peak positively related to citrate synthase in the GAST but not the VL (26). The higher O2 extraction (ΔHHb) in the GAST but not the VL in LTT compared to STT in the present study suggests that long-term (> 24 months) aerobic exercise might reduce the age-related reductions in citrate synthase activity and muscle oxidative capacity in the male GAST.
For the ΔHHb in the PFC during RI, the present results support the hypothesis in that the ΔHHb was not different between STT and LTT at 90% of GET or at peak exercise. However, an unexpected finding was the greater ΔHHb in the PFC in LTT compared to STT at 90% of GET during SWCL exercise. This finding suggests that the additional training years of the LTT may not affect O2 extraction in the PFC at peak exercise. However, during moderate intensity constant load exercise for a longer duration (20 min), the present data suggest LTT older men utilize more O2 in the PFC compared to STT older men.
In attempting to link PFC oxygenation and fatigue, there are multiple studies and reviews on oxygenation patterns in the PFC during exhaustive exercise (4, 45, 52), and following aerobic training in men (42, 48). Despite this, there are currently no data on sub-maximal constant load exercise, thus the present findings are unique. It is plausible that the greater ΔHHb in the PFC in LTT compared to STT at 90% of GET is related to the higher V̇O2peak in the LTT. However, in contrast, a systematic review reported that a positive relationship exists between ΔHHb in the PFC and V̇O2peak at hard to very hard intensity exercise but not at light to moderate exercise (45). Furthermore, changes in cerebral blood flow, partial pressure of arterial carbon dioxide and cerebral autoregulation at different intensities during exercise may explain the variations in ΔHHb in the PFC between LTT and STT in the present study (39).
A potential limitation of this present study was that while regular cycling was a requirement for recruitment, cycling was not necessarily each participants’ major mode of aerobic exercise and the amount of cycling completed by the participants in each group may have changed during the respective training (years). This could have influenced the cycling technique, and thus muscle activation. Another potential limitation is that the ramp data was not left shifted to account for the mean response time of V̇O2, thus potentially overestimating the power output at GET. Furthermore, while differences were found between groups it is acknowledged that these were within a cross sectional design.
The results of this study have implications for aerobic exercise in men aged 40 – 60 yr. Specifically, the present data suggest that, in addition to the typical adaptive improvements in systemic O2 utilization, and peripheral O2 extraction following starting of regular aerobic exercise, additional years of training without necessarily increasing training volume, may provide significant further improvements. Future research could include training studies extending beyond 12 months and monitor changes in central delivery to, and the extraction of O2 in multiple peripheral tissues.
It is concluded that in men aged 40 – 60 yr, regular aerobic exercise beyond 24 months significantly increases V̇O2 at peak and sub-GET exercise intensities, without increased current training volume. Concomitant with these adaptations there is increased O2 extraction (ΔHHb) in the GAST and PFC at moderate intensity (sub-GET) exercise, but not at peak exercise.
ACKNOWLEDGEMENTS
Hugo Kerhervé and Yuri Kriel provided technical assistance.
REFERENCES
- 1.Anaya SA, Church TS, Blair SN, Myers JN, Earnest CP. Exercise dose-response of the v(e)/vco(2) slope in postmenopausal women in the drew study. Med Sci Sports Exerc. 2009;41(5):971–976. doi: 10.1249/MSS.0b013e3181930009. [DOI] [PubMed] [Google Scholar]
- 2.Babcock MA, Paterson DH, Cunningham DA. Effects of aerobic endurance training on gas exchange kinetics of older men. Med Sci Sports Exerc. 1994;26(4):447–452. [PubMed] [Google Scholar]
- 3.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60(6):2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 4.Bhambhani Y, Malik R, Mookerjee S. Cerebral oxygenation declines at exercise intensities above the respiratory compensation threshold. Respir Physiol Neurobiol. 2007;156(2):196–202. doi: 10.1016/j.resp.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Borg GA. Perceived exertion. Exerc Sport Sci Rev. 1974;2:131–153. [PubMed] [Google Scholar]
- 6.Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, Rao DC, Rankinen T. Genomic predictors of the maximal o(2) uptake response to standardized exercise training programs. J Appl Physiol. 2011;110(5):1160–1170. doi: 10.1152/japplphysiol.00973.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredin SSD, Gledhill N, Jamnik VK, Warburton DER. New risk stratification and physical activity clearance strategy for physicians and patients alike. Can Fam Physician. 2013;59(3):273–277. [PMC free article] [PubMed] [Google Scholar]
- 8.Buzza G, Lovell GP, Askew CD, Kerherve H, Solomon C. The effect of short and long term endurance training on systemic, and muscle and prefrontal cortex tissue oxygen utilisation in 40–60 year old women. PLoS One. 2016;11(11):e0165433. doi: 10.1371/journal.pone.0165433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: L. Erlbaum Associates; 1988. [Google Scholar]
- 10.Costes F, Prieur F, Feasson L, Geyssant A, Barthelemy JC, Denis C. Influence of training on nirs muscle oxygen saturation during submaximal exercise. Med Sci Sports Exerc. 2001;33(9):1484–1489. doi: 10.1097/00005768-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Dehn MM, Bruce RA. Longitudinal variations in maximal oxygen intake with age and activity. J Appl Physiol. 1972;33(6):805–807. doi: 10.1152/jappl.1972.33.6.805. [DOI] [PubMed] [Google Scholar]
- 12.DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary o2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol. 2003;95(1):113–120. doi: 10.1152/japplphysiol.00956.2002. [DOI] [PubMed] [Google Scholar]
- 13.Dogra S, Spencer MD, Murias JM, Paterson DH. Oxygen uptake kinetics in endurance-trained and untrained postmenopausal women. Appl Physiol Nutr Metab. 2013;38(2):154–160. doi: 10.1139/apnm-2012-0173. [DOI] [PubMed] [Google Scholar]
- 14.Edgerton VR, Smith JL, Simpson DR. Muscle fibre type populations of human leg muscles. Histochem. 1975;7(3):259–266. doi: 10.1007/BF01003594. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fnirs) development and fields of application. NeuroImage. 2012;63(2):921–935. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira LF, Townsend DK, Lutjemeier BJ, Barstow TJ. Muscle capillary blood flow kinetics estimated from pulmonary o2 uptake and near-infrared spectroscopy. J Appl Physiol. 2005;98(5):1820–1828. doi: 10.1152/japplphysiol.00907.2004. [DOI] [PubMed] [Google Scholar]
- 17.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 18.Foulds HJA, Bredin SSD, Charlesworth SA, Ivey AC, Warburton DER. Exercise volume and intensity: A dose-response relationship with health benefits. Eur J Appl Physiol. 2014;114(8):1563–1571. doi: 10.1007/s00421-014-2887-9. [DOI] [PubMed] [Google Scholar]
- 19.Gaskill SE, Ruby BC, Walker AJ, Sanchez OA, Serfass RC, Leon AS. Validity and reliability of combining three methods to determine ventilatory threshold. Med Sci Sports Exerc. 2001;33(11):1841–1848. doi: 10.1097/00005768-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol. 2003;95(1):149–158. doi: 10.1152/japplphysiol.00695.2002. [DOI] [PubMed] [Google Scholar]
- 21.Grey TM, Spencer MD, Belfry GR, Kowalchuk JM, Paterson DH, Murias JM. Effects of age and long-term endurance training on vo2 kinetics. Med Sci Sports Exerc. 2015;47(2):289–298. doi: 10.1249/MSS.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 22.Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt. 2007;12(6):062105. doi: 10.1117/1.2805437. [DOI] [PubMed] [Google Scholar]
- 23.Heath GW, Hagberg JM, Ehsani AA, Holloszy JO. A physiological comparison of young and older endurance athletes. J Appl Physiol. 1981;51(3):634–640. doi: 10.1152/jappl.1981.51.3.634. [DOI] [PubMed] [Google Scholar]
- 24.Hickey MS, Weidner MD, Gavigan KE, Zheng D, Tyndall GL, Houmard JA. The insulin action-fiber type relationship in humans is muscle group specific. Am J Physiol-Endocrinol Metab. 1995;269(1 Pt 1):E150–154. doi: 10.1152/ajpendo.1995.269.1.E150. [DOI] [PubMed] [Google Scholar]
- 25.Hiroyuki H, Hamaoka T, Sako T, Nishio S, Kime R, Murakami M, Katsumura T. Oxygenation in vastus lateralis and lateral head of gastrocnemius during treadmill walking and running in humans. Eur J Appl Physiol. 2002;87(4–5):343–349. doi: 10.1007/s00421-002-0644-y. [DOI] [PubMed] [Google Scholar]
- 26.Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol. 1998;85(4):1337–1341. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- 27.Huang G, Wang R, Chen P, Huang SC, Donnelly JE, Mehlferber JP. Dose-response relationship of cardiorespiratory fitness adaptation to controlled endurance training in sedentary older adults. Eur J Prev Cardiol. 2016;23(5):518–529. doi: 10.1177/2047487315582322. [DOI] [PubMed] [Google Scholar]
- 28.Hunter GR, Bamman MM, Larson-Meyer DE, Joanisse DR, McCarthy JP, Blaudeau TE, Newcomer BR. Inverse relationship between exercise economy and oxidative capacity in muscle. Eur J Appl Physiol. 2005;94(5–6):558–568. doi: 10.1007/s00421-005-1370-z. [DOI] [PubMed] [Google Scholar]
- 29.Kasch FW, Boyer JL, Schmidt PK, Wells RH, Wallace JP, Verity LS, Guy H, Schneider D. Ageing of the cardiovascular system during 33 years of aerobic exercise. Age and ageing. 1999;28(6):531–536. doi: 10.1093/ageing/28.6.531. [DOI] [PubMed] [Google Scholar]
- 30.Katzel LI, Sorkin JD, Fleg JL. A comparison of longitudinal changes in aerobic fitness in older endurance athletes and sedentary men. J Am Geriatr Soc. 2001;49(12):1657–1664. doi: 10.1046/j.1532-5415.2001.t01-1-49276.x. [DOI] [PubMed] [Google Scholar]
- 31.Kriel Y, Kerherve HA, Askew CD, Solomon C. The effect of active versus passive recovery periods during high intensity intermittent exercise on local tissue oxygenation in 18–30 year old sedentary men. PLoS One. 2016;11(9):e0163733. doi: 10.1371/journal.pone.0163733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall P, Al-Timman J, Riley R, Wright J, Williams S, Hainsworth R, Tan LB. Randomized controlled trial of home-based exercise training to evaluate cardiac functional gains. Clin Sci. 2001;101(5):477–483. [PubMed] [Google Scholar]
- 33.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas bedrest and training study: II. Effect of age on cardiovascular adaptation to exercise training. Circulation. 2001;104(12):1358–1366. [PubMed] [Google Scholar]
- 34.Montero D, Diaz-Canestro C. Endurance training and maximal oxygen consumption with ageing: Role of maximal cardiac output and oxygen extraction. Eur J Prev Cardiol. 2016;23(7):733–743. doi: 10.1177/2047487315617118. [DOI] [PubMed] [Google Scholar]
- 35.Montero D, Diaz-Cañestro C, Lundby C. Endurance training and vo2max: Role of maximal cardiac output and oxygen extraction. Med Sci Sports Exerc. 2015;47(10):2024–2033. doi: 10.1249/MSS.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 36.Murias JM, Kowalchuk JM, Paterson DH. Speeding of vo2 kinetics with endurance training in old and young men is associated with improved matching of local o2 delivery to muscle o2 utilization. J Appl Physiol. 2010;108(4):913–922. doi: 10.1152/japplphysiol.01355.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murias JM, Kowalchuk JM, Paterson DH. Time course and mechanisms of adaptations in cardiorespiratory fitness with endurance training in older and young men. J Appl Physiol. 2010;108(3):621–627. doi: 10.1152/japplphysiol.01152.2009. [DOI] [PubMed] [Google Scholar]
- 38.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: Mechanisms of regulation. J Appl Physiol. 2009;107(5):1370–1380. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- 40.Okushima D, Poole DC, Barstow TJ, Rossiter HB, Kondo N, Bowen TS, Amano T, Koga S. Greater vo2peak is correlated with greater skeletal muscle deoxygenation amplitude and hemoglobin concentration within individual muscles during ramp-incremental cycle exercise. Physiological Reports. 2016;4(23) doi: 10.14814/phy2.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oussaidene K, Prieur F, Bougault V, Borel B, Matran R, Mucci P. Cerebral oxygenation during hyperoxia-induced increase in exercise tolerance for untrained men. Eur J Appl Physiol. 2013;113(8):2047–2056. doi: 10.1007/s00421-013-2637-4. [DOI] [PubMed] [Google Scholar]
- 42.Oussaidene K, Tagougui S, Abaidia A, Matran R, Mucci P, Prieur F. Aerobic fitness influences cerebral oxygenation response to maximal exercise in healthy subjects. Respir Physiol Neurobiol. 2015;205:53–60. doi: 10.1016/j.resp.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Proctor DN, Koch DW, Newcomer SC, Le KU, Smithmyer SL, Leuenberger UA. Leg blood flow and vo2 during peak cycle exercise in younger and older women. Med Sci Sports Exerc. 2004;36(4):623–631. doi: 10.1249/01.mss.0000121951.10417.b5. [DOI] [PubMed] [Google Scholar]
- 44.Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle o2 transport at vo2max. J Appl Physiol. 1992;73(3):1067–1076. doi: 10.1152/jappl.1992.73.3.1067. [DOI] [PubMed] [Google Scholar]
- 45.Rooks CR, Thom NJ, McCully KK, Dishman RK. Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: A systematic review. Prog Neurobiol. 2010;92(2):134–150. doi: 10.1016/j.pneurobio.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Scharhag-Rosenberger F, Meyer T, Walitzek S, Kindermann W. Time course of changes in endurance capacity: A 1-yr training study. Med Sci Sports Exerc. 2009;41(5):1130–1137. doi: 10.1249/MSS.0b013e3181935a11. [DOI] [PubMed] [Google Scholar]
- 47.Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Endurance training in older men and women. I. Cardiovascular-responses to exercise. J Appl Physiol. 1984;57(4):1024–1029. doi: 10.1152/jappl.1984.57.4.1024. [DOI] [PubMed] [Google Scholar]
- 48.Seifert T, Rasmussen P, Brassard P, Homann PH, Wissenberg M, Secher NH, Nielsen HB, Nordby P, Stallknecht B. Cerebral oxygenation and metabolism during exercise following three months of endurance training in healthy overweight males. Am J Physio Regul Integr Comp Physiol. 2009;297(3):R867–R876. doi: 10.1152/ajpregu.00277.2009. [DOI] [PubMed] [Google Scholar]
- 49.Sheel AW, Romer LM. Ventilation and respiratory mechanics. Compr Physiol. 2012;2(2):1093–1142. doi: 10.1002/cphy.c100046. [DOI] [PubMed] [Google Scholar]
- 50.Shvartz E, Reibold RC. Aerobic fitness norms for males and females aged 6 to 75 years: A review. Aviat Space Environ Med. 1990;61(1):3–11. [PubMed] [Google Scholar]
- 51.Stringer W, Wasserman K, Casaburi R, Porszasz J, Maehara K, French W. Lactic acidosis as a facilitator of oxyhemoglobin dissociation during exercise. J Appl Physiol. 1985;76(4):1462–1467. doi: 10.1152/jappl.1994.76.4.1462. 1994. [DOI] [PubMed] [Google Scholar]
- 52.Subudhi AW, Olin JT, Dimmen AC, Polaner DM, Kayser B, Roach RC. Does cerebral oxygen delivery limit incremental exercise performance? J Appl Physiol. 2011;111(6):1727–1734. doi: 10.1152/japplphysiol.00569.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka H, Seals DR. Endurance exercise performance in masters athletes: Age-associated changes and underlying physiological mechanisms. J Physiol. 2008;586(1):55–63. doi: 10.1113/jphysiol.2007.141879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tschakovsky ME, Saunders NR, Webb KA, O’Donnell DE. Muscle blood-flow dynamics at exercise onset: Do the limbs differ? Med Sci Sports Exerc. 2006;38(10):1811–1818. doi: 10.1249/01.mss.0000230341.86870.4f. [DOI] [PubMed] [Google Scholar]