Abstract
We report a case of a hospitalised patient with COVID-19 who developed subacute thyroiditis in association with SARS-COV-2 infection. The patient presented with tachycardia, anterior neck pain and thyroid function tests revealing hyperthyroidism together with consistent ultrasonographic evidence suggesting subacute thyroiditis. Treatment with corticosteroids resulted in rapid clinical resolution. This case illustrates that subacute thyroiditis associated with viruses such as SARS-CoV-2 should be recognised as a complication of COVID-19 and considered as a differential diagnosis when infected patients present with tachycardia without evidence of progression of COVID-19 illness.
Keywords: thyroid disease, endocrinology, hyperthyroidism
Background
As of 1 May 2020, 17101 people with COVID-19 have been diagnosed in Singapore with migrant workers residing in dormitories forming the majority (n=14 776, 86.4%).1 We report a unique case of a migrant worker from Myanmar who developed subacute thyroiditis in association with COVID-19, which is the first case reported in Asia to our knowledge. On day 10 of illness, he developed anterior neck pain and sinus tachycardia with thyroid function tests revealing primary hyperthyroidism. Ultrasonography of his thyroid gland was consistent with subacute thyroiditis and he was administered oral corticosteroids, resulting in rapid clinical improvement. This case highlights the importance of good history taking and awareness of the diagnosis of thyroiditis in order to elicit the finding of anterior neck pain that can be conflated with upper respiratory tract symptoms in a persistently tachycardic patient with COVID-19.
Case presentation
History and clinical findings
A 34-year-old man from Myanmar with no medical history or known COVID-19 exposure presented to the emergency department at Singapore General Hospital with a 4-day history of fever, dry cough, headache and anosmia. On admission, he had a temperature of 37.7°C, blood pressure of 120/90 mm Hg, heart rate of 89 beats/min, respiratory rate of 19 breaths/min and an oxygen saturation (SpO2) of 96% on room air. Auscultation of the lungs was clear with no adventitious sounds. Given the clinical features and risk factors, an oropharyngeal swab and testing for COVID-19 was performed using reverse transcription real-time qualitative PCR on the Roche Cobas 6800 System using Roche Cobas SARS-CoV-2 test. The test for SARS-CoV-2 was positive. Initial laboratory tests on admission to the hospital showed a normal white cell count (9.8×109/L), haemoglobin level (14.3 g/dL) and platelet count (444 000/mm3). C reactive protein (CRP) level was mildly elevated at 11.3 mg/L, and lactate dehydrogenase (LDH) was within normal limits at 433 units/L. A chest X-ray (CXR) performed reported no pulmonary consolidation or pleural effusion. He was admitted to an isolation cohort ward for further management of COVID-19 upper respiratory tract infection (URTI) with consideration to transfer to a community isolation facility should he continue to remain stable without any progression of COVID-19.
Clinical course and management
On the third day of admission, the patient complained of ongoing dry cough associated with sore throat. Vital signs remained stable and he remained afebrile. Symptomatic treatment with paracetamol and dequalinium lozenges were given.
However, from the fifth day of the hospital stay (day 9 of illness), he had new complaints of an anterior neck pain with a score 5/10 that was refractory to symptomatic treatment and a new onset of tachycardia ranging from 90 to 120 beats/min. He remained afebrile with SpO2>96% on room air. His oropharynx was not injected nor had any exudates. Auscultation of the lungs revealed no adventitious sounds. On examination of his neck, a diffuse asymmetric goitre was found with regions on both lobes that were hard and tender to palpation. There was no retrosternal extension or palpable bruit. Few cervical lymph nodes were palpable bilaterally. He did not have any eye signs of thyrotoxicosis or thyroid eye disease, pretibial myxoedema or hand tremors. Additionally, examination of his skin revealed no exanthem.
Further relevant history to his thyroid status revealed no palpitations, diarrhoea, increased appetite, unintentional weight loss or heat intolerance on presentation or preceding this admission. He did not have history of thyroid disease in the past but had a positive family history for thyroid disease.
Investigations
In view of tachycardia and a tender goitre found, a thyroid function test was done revealing primary hyperthyroidism with elevated free thyroxine 3 (13.4 pmol/L), free thyroxine 4 (41.8 pmol/L) and suppressed thyroid-stimulating hormone (TSH) (<0.01 mU/L). Thyrotropin receptor antibody (TRAb) and thyroperoxidase antibody (TPOAb) were negative. Notably, CRP was also markedly elevated to 122 mg/L, while procalcitonin remained unremarkable (0.13 µg/L). Alkaline phosphatase was also mildly elevated (218 U/L) without hyperbilirubinaemia. The ECG corresponding to the tachycardia episodes revealed a sinus tachycardia rhythm with no evidence of atrial fibrillation. A CXR that was repeated did not reveal any new pulmonary consolidation. A summary of investigations on admission and during the onset of tachycardia can be seen in table 1.
Table 1.
Summary of investigation findings on admission and after the onset of tachycardia
| Investigation (units) | Normal range | On admission | Onset of tachycardia |
| LDH (U/L) | 222–454 | 433 | 440 |
| CK (U/L) | 56–336 | – | 45 |
| CKMB (μg/L) | 1.0–5.0 | – | <1 |
| Trop (ng/L) | <30 | <13 | <13 |
| Ur (mmol/L) | 2.7–6.9 | 3.7 | 3.6 |
| Na (mmol/L) | 136–146 | 133 | 138 |
| K (mmol/L) | 3.6–5.0 | 4.4 | 4.7 |
| Cl (mmol/L) | 100–107 | 100 | 100 |
| HCO3 (mmol/L) | 19.0–29.0 | 22.6 | 27.3 |
| Cr (μmol/L) | 54–101 | 73 | 72 |
| Alb (g/L) | 40–51 | –— | 38 |
| Bili (μmol/L) | 7–32 | – | 19 |
| ALP (U/L) | 39–99 | – | 218 |
| ALT (U/L) | 6–66 | – | 77 |
| AST (U/L) | 12–42 | – | 44 |
| GGT (U/L) | 19–94 | – | 234 |
| CRP (mg/L) | 0.2–9.1 | 11.3 | 122 |
| Procal (UG/L) | <0.50 | – | 0.13 |
| Hb (G/DL) | 14.0–18.0 | 14.3 | 13.7 |
| WCC (×109/L) | 4.0–10.0 | 9.6 | 11.56 |
| Plt (×109/L) | 140–440 | 444 | 592 |
| Free T4 (pmol/L) | 8.8–14.4 | – | 41.8 |
| Free T3 (pmol/L) | 3.2–5.3 | – | 13.4 |
| TSH (μ/L) | 0.65–3.70 | – | <0.01 |
| TRAb (IU/L) | <1.76 | – | <1.10 (negative) |
| TPOAb (IU/ML) | <9.0 | – | 2.0 (negative) |
| COVID-19 | – | Detected | – |
Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Bili, bilirubin; CK, creatinine kinase; CKMB, creatinine kinase myocardial band; Cr, creatinine; CRP, C reactive protein; GGT, gamma-glutamyl transferase; Hb, haemoglobin; HCO3, bicarbonate; K, potassium; LDH, lactate dehydrogenase; Na, sodium; Plt, platelet; Procal, procalcitonin; T3, thyroxine 3; T4, thyroxine 4; TPOAb, thyroid peroxidase antibody; TRAb, thyrotropin receptor antibody; Trop, troponin; TSH, thyroid-stimulating hormone; Ur, urea; WCC, white cell count.
Further investigations done upon diagnosis of thyroiditis
Urine dipstick : negative for bilirubin, nitrite, leucocyte, glucose, ketone, blood and protein. Urobilinogen: 0.2, specific gravity: 1.01, urine: pH 6.5.
Blood cultures : no bacterial growth in both aerobic and anaerobic.
Oropharyngeal respiratory swab PCR : negative for respiratory syncytial viruses A and B, influenza A and B, parainfluenza viruses 1–4, metapneumovirus, rhinoviruses A/B/C, human coronavirus OC43, human coronavirus 2229E, human coronavirus NL63, adenovirus, human enterovirus and human bocavirus 1/2/3/4.
Hepatitis B core antibody : negative, hepatitis B surface antigen : negative, hepatitis B surface antibody : negative.
HIV : negative.
Mumps, measles and rubella IgG : positive.
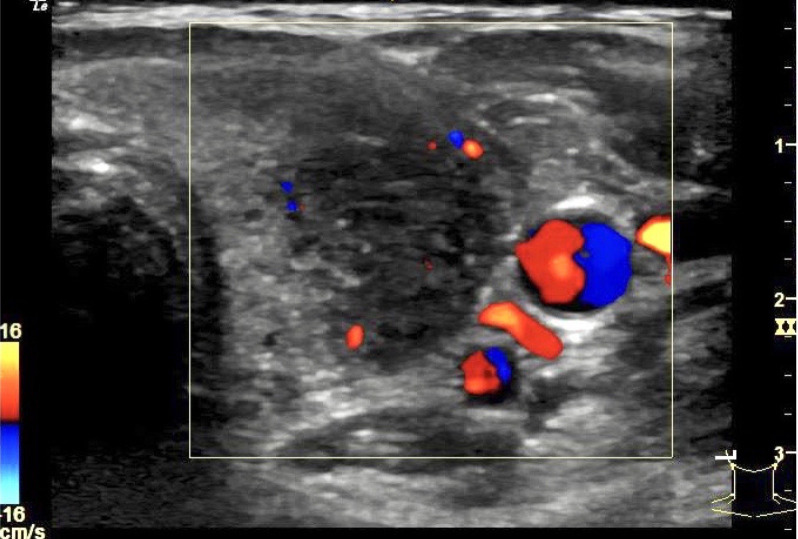
Ultrasound of the neck (figure 1) showed an enlarged thyroid gland with heterogenous echotexture. Both lobes had hypoechoic areas with ill-defined margins corresponding to the hard regions palpable. Colour flow Doppler showed reduced blood flow in both lobes. There were no definite nodules seen in the thyroid gland. A few cervical lymph nodes with normal morphology were seen.
Figure 1.
Ultrasound of the left lobe of the thyroid gland showing an enlarged lobe with reduced blood flow on Doppler study. A hypoechoic heterogenous area with ill-defined margins corresponded to the palpable hard region on the left lobe of the thyroid.
Differential diagnosis
Subacute thyroiditis is fundamentally a clinical diagnosis, and our patient presented with clinical manifestations that were sufficient to establish this as a leading differential diagnosis, given the new-onset neck pain, thyroid tenderness and a diffuse asymmetric goitre in the context of a preceding upper respiratory tract viral illness and hyperthyroidism. The classical ultrasound finding of hypoechoic and heterogenous enlarged thyroid gland with reduced blood flow was also supportive of the diagnosis. Furthermore, TRAb and TPOAb were negative, rendering the diagnoses of Grave’s disease and autoimmune thyroiditis unlikely. Investigations also did not support an acute infectious thyroiditis of a ‘suppurative’ type with normal procalcitonin levels and blood cultures that did not grow any bacteria after 48 hours in incubation. Further results of tests performed to exclude other common viral infective causes of thyroiditis were normal. Notably, a throat swab sent for a respiratory virus multiplex PCR was negative for influenza A and B, adenovirus and human enterovirus. Measles, mumps and rubella virus serologies suggested immunity. Hence, SARS-CoV-2 was the likely viral trigger of the subacute thyroiditis in this particular case.
Treatment
In view of the significant pain and discomfort and hyperthyroidism with tachycardia, he was commenced on prednisolone at a dose of 20 mg. A steroid-tapering regimen was planned for him to decrease the dosage of prednisone to the minimum required for symptomatic relief with periodic monitoring of thyroid function. Beta-blocker treatment was also initiated with atenolol at a dosage of 25 mg every morning.
Outcome and follow-up
Two days after starting oral steroid treatment, the anterior neck pain had substantially reduced to a pain score of 2/10 from 5/10. The palpable hard swellings on either lobe of the thyroid gland that were found at diagnosis had rapidly disappeared after treatment, which supports the diagnosis of subacute thyroiditis.
On day 5 of treatment, the CRP level was noted to be downtrending markedly from 122 mg/L, which was documented on the diagnosis of thyroiditis to a much lower level of 16.5 mg/L. There was clinical commensuration with the downtrending inflammatory markers as the tachycardia had resolved with the neck pain.
He subsequently continued to remain well with supportive management for URTI symptoms and was discharged for follow-up with an endocrinologist after recovery from COVID-19.
He was reviewed after 10 weeks in the outpatient clinic. He had completed his tapering course of steroids and was clinically well with no symptoms. His thyroid function tests normalised with an FT4 level of 11.0 pmol/L and a TSH level of 2.10 mU/L. The thyroid gland was normal to palpation with no palpable cervical lymph nodes. His steroid therapy was stopped in view of complete resolution of his symptoms with a scheduled early follow-up.
Discussion
We report the first case of subacute thyroiditis in an Asian population where the temporal sequence and the exclusion of other viral infections suggest COVID-19 as the causal factor. The importance of detailed history taking and physical examination of the neck is illustrated by our case. Capturing the distinctive history of neck pain which can often be conflated with symptoms of pharyngitis, a very common complaint in COVID-19 URTI, was key to the spearhead the clinical reasoning process that led to the diagnosis.
Subacute thyroiditis is presumed to be caused by a viral infection or a postviral inflammatory process with clusters of disease reported during outbreaks of viral infections drawing much parallel to that of the current COVID-19 pandemic situation. This further highlights the importance for physicians to be vigilant of the diagnosis while treating patients with COVID-19 who may have multiple upper respiratory symptoms. Reviews of literature have shown that evidence for viral infection in subacute thyroiditis was linked to mumps virus, coxsackievirus, adenovirus, Epstein-Barr virus, rubella and cytomegalovirus, though a specific viral cause is not always found.2 In our case of a mild COVID-19 URTI, there was sufficient clinical and virological evidence within reasonable limits that failed to yield any other alternative viral trigger other than SARS-CoV-2.
From a pathophysiological standpoint, previous studies examining the pathology of the thyroid in Severe Acute Respiratory Syndrome proposed several mechanisms of thyroid organ damage that include host immune overreaction, immune deficiency related to infection, destruction of lymphocytes, inhibition of the innate immune response and direct cellular destruction with apoptosis playing a key role.3 Much as been discussed about ACE2, which is key to the mechanism of SARS-CoV-2 infection with the virus using it as a host cell receptor to invade human cells. More recent studies based on SARS-CoV-2 in 2020 have shown that ACE2 expression levels were highest in thyroid among other organs, such as the small intestine, kidneys, heart and adipose tissue, which does give insight into a plausible mechanism for pathophysiology of thyroiditis in COVID-19.4 5 We acknowledge similar cases reported in an 18-year-old woman in Italy and also in a middle-aged Caucasian woman in Turkey.6 7 Given prior described literature by Ohsako et al on genetic aspects of subacute thyroiditis and certain human leucocyte antigens that predispose to subacute thyroiditis in a Japanese study,8 our case further sheds light onto possible research directions into gender, ethnic and therefore genetic predilection of viral subacute thyroiditis in the different populations demonstrated in reports so far.
In a hospitalised patient for mild to moderate COVID-19, a presentation of sinus tachycardia may lead to a differential diagnosis of worsening sepsis or progression of COVID-19 illness, which we agree should be dutifully ruled out as illustrated in our case report. Our case highlights the importance of being cognisant of the wide differentials in a patient with COVID-19 who develops tachycardia, such as cardiac, pulmonary, haematological and also thyroid dysfunctions, which eventually surfaced in our case. In an outbreak setting, we also note to consider physical deconditioning and anxiety as other causes of persistent unexplained tachycardia in the daytime, which has been described in prior cohort studies of SARS patients in 2004.9
In summary, thyroiditis and resultant thyrotoxicosis should always be considered as a differential in patients with COVID-19. Though we acknowledge the wide array of differentials of tachycardia in a hospitalised patient with COVID-19, the eventual recognition of clinical, biochemical and radiological findings led to the diagnosis of a viral thyroiditis that was amenable to inexpensive treatment and rewarding outcomes of rapid symptom relief. Furthermore, the importance of clinically recognising thyroiditis transcends all levels of medical care from tertiary care providers in hospitals to primary care physicians holding the fort in the community for well and stable cases for which thyroiditis may present as a delayed complication of COVID-19.
Learning points.
COVID-19 is a novel disease for which its clinical presentation, potential complications and organ involvement are still being elucidated in literature.
Anterior neck pain, which can be conflated with upper respiratory tract symptoms especially in the setting of COVID-19, should not be dismissed and warrants further examination and investigation as required.
Subacute thyroiditis is a rare complication of COVID-19 that should be considered especially in the setting of persistent tachycardia without any suggestion of progression of COVID-19 and other common cardiorespiratory causes.
Acknowledgments
We thank Dr Juliana Kan and Dr Tan Yuyang from the Department of Internal Medicine in Singapore General Hospital for their assistance in acquiring the ultrasound images of the thyroid gland.
Footnotes
Contributors: SAMM, SJQK, SRC and BPZC all contributed to the planning, conduct and reporting of the work described in the article. SAMM was the primary physician managing the patient with SJQK. SRC was the endocrinologist consulted and assisted in acquiring the ultrasound images. BPZC was the infectious diseases consultant with an overview of the patients with COVID-19 admitted to Singapore General Hospital.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ministry of Health Singapore Updates on COVID-19 (coronavirus disease 2019) local situation. Ministry of health, Singapore. Available: www.moh.gov.sg/covid-19 [Accessed 2 May 2020].
- 2.Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J 2009;6:5. 10.1186/1743-422X-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei L, Sun S, Xu C-H, et al. Pathology of the thyroid in severe acute respiratory syndrome. Hum Pathol 2007;38:95–102. 10.1016/j.humpath.2006.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M-Y, Li L, Zhang Y, et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020;9:45. 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Human Protein Atlas - Tissue expression of ACE2. Available: https://www.proteinatlas.org/ENSG00000130234-ACE2/tissue [Accessed 2 May 2020].
- 6.Brancatella A, Ricci D, Viola N, et al. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocr Metab 2020;105:2367–70. 10.1210/clinem/dgaa276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asfuroglu Kalkan E, Ates I. A case of subacute thyroiditis associated with Covid-19 infection. J Endocrinol Invest 2020;43:1173–4. 10.1007/s40618-020-01316-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohsako N, Tamai H, Mukuta T, et al. Clinical characteristics of subacute thyroiditis classified according to human leukocyte antigen typing 1995;80:3653–6. [DOI] [PubMed] [Google Scholar]
- 9.Lau S-T, Yu W-C, Mok N-S, et al. Tachycardia amongst subjects recovering from severe acute respiratory syndrome (SARS). Int J Cardiol 2005;100:167–9. 10.1016/j.ijcard.2004.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]