Abstract
Immune-checkpoint inhibitors (ICI), specifically inhibitors of programmed death ligand-1 (PD-L1) and receptor (PD-1) are the new standard of care for the treatment of patients with advanced non-small cell lung cancer (NSCLC) in front line setting as monotherapy or along with chemotherapy. Many of these agents are also approved for use in subsequent lines of treatment on progression on platinum doublet chemotherapy. Nivolumab, pembrolizumab and atezolizumab are currently approved ICI for advanced NSCLC. To date, no study has reported efficacy and safety of alternate PD-1/PD-L1 inhibitors in patients with NSCLC who have progressed on one ICI. Here, we report a case of a patient with advanced NSCLC who had a complete response to atezolizumab, following progression of disease on platinum doublet chemotherapy and then, nivolumab monotherapy.
Keywords: immunology, lung cancer (oncology)
Background
Immune-checkpoint inhibitors (ICIs) are the new standard of care for the treatment of patients with advanced non-small cell lung cancer (NSCLC) in front line setting as monotherapy or along with chemotherapy.1 Many of these agents are also approved for use in subsequent lines of treatment on progression on platinum doublet chemotherapy. Even though anti-programmed death-1 (PD-1) and anti-programmed death ligand-1 (PD-L1) inhibitors have similar mechanisms of anti-tumour activity which is essentially disrupting the interaction between the PD-1 and PD-L1, there are some differences.2 There is also some suggestion that there may be some differences in efficacy and incidence of immune-related toxicities between PD-1 and PD-L1 inhibitors.3 Limited data is available regarding the efficacy and safety of PD-L1 inhibitor use following the progression of disease (POD) on PD-1 inhibitor. Here, we report a case of a patient with advanced NSCLC who had a complete response to atezolizumab, following POD on platinum doublet chemotherapy and then, nivolumab monotherapy.
Case presentation
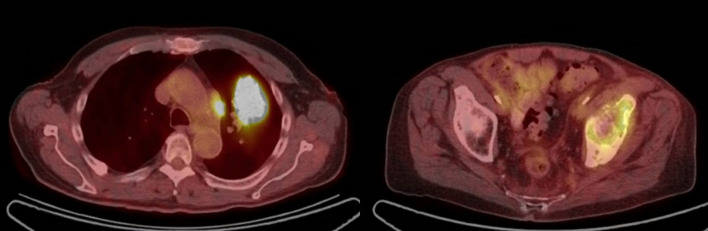
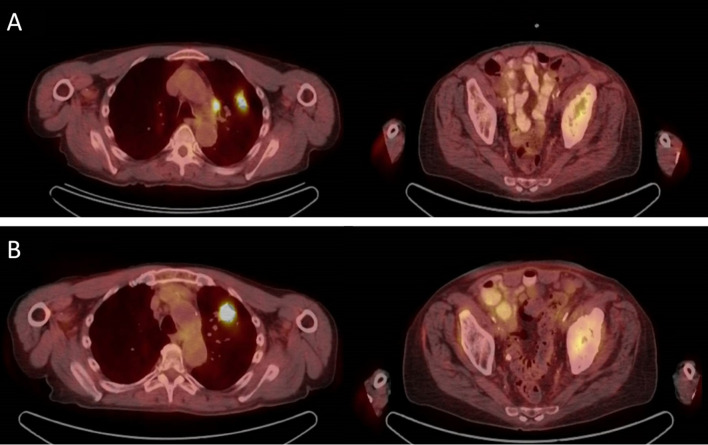
A 76-year-old white man with 50-pack-year smoking history presented in July 2016 with a progressive left hip pain that started 3 months prior. MRI of the left hip revealed a 6.1×5.6×7.5 cm soft tissue lesion in the left iliac bone involving the acetabulum. Biopsy of acetabular soft tissue mass revealed a poorly differentiated adenocarcinoma which was thyroid transcription factor-1 and napsin positive suggesting pulmonary origin. Next-generation sequencing showed no actionable mutations, tumour mutational burden was high (23.16 mutations/Mb) and PD-L1 by immunohistochemistry using the Dako 22C3 stain was 0%. Positron emission tomography (PET)-CT scan in July 2016 revealed an FDG-avid left lung mass measuring 4.3×3.5 cm with diffuse lymphadenopathy and multiple osseous lesions. Brain MRI showed no central nervous system involvement. On clinical evaluation, he was noted to have Eastern Cooperative Oncology Group (ECOG) performance status of 1. There was a marked decrease in the range of movement of left hip due to pain, but the examination was otherwise unremarkable. Our patient was treated with palliative radiation at a dose of 20 Gy in five fractions to T spine and left iliac bone metastases and initiated on carboplatin and pemetrexed in August 2016. PET-CT in October 2016 after fourth cycle of chemotherapy revealed progression of disease (figure 1) with development of new osseous and pulmonary metastases. He was started on nivolumab dosed 3 mg/kg every other week in November 2016. Subsequent PET-CT scans after 3 months of treatment showed partial response per RECIST 1.1 (figure 2A) but the scans done in July 2017 and September 2017 following 17 and 21 cycles, respectively, of nivolumab demonstrated progressive increase in the size of left upper lobe lung mass from 2.3×1.9 to 3.3×2.4 cm and then to 3.9×3.2 cm associated with increased standard uptake value in addition to persistent lymphadenopathy consistent with unequivocal progression of disease (figure 2B). Because of ECOG PS of 2 and patient’s wishes of not pursuing chemotherapy, he was switched to atezolizumab in August 2017 with the hypothesis that switching to a PD-L1 inhibitor from PD-1 inhibitor might overcome potential resistance through PD-L1 and CD80 interaction.
Figure 1.
Progression of disease after fourth cycle of carboplatin and pemetrexed.
Figure 2.
(A) Interim PET-CT scans showed partial response on nivolumab. (B) PET-CT progression of disease/unequivocal after 17 cycles of nivolumab. PET, positron emission tomography.
Outcome and follow up
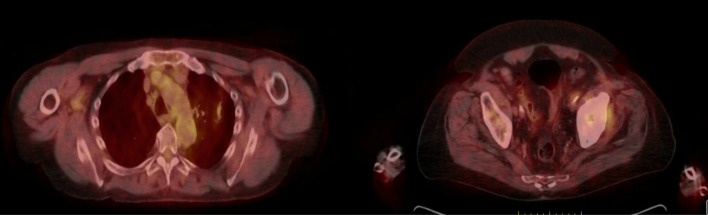
Our patient experienced a complete response to atezolizumab based on the 6-month scan (figure 3). The patient has received 35 cycles of atezolizumab thus far with no immune-related adverse events and a sustained complete response. Patient is alive and well.
Figure 3.
Positron emission tomography-CT showing a complete response to atezolizumab: done after 6 months of initiation atezolizumab.
Discussion
Lung cancer is a significant health burden globally as it is the leading cause of cancer death in men worldwide and the leading cause of cancer death in both men and women in the USA.4
NSCLC is the most common subtype accounting for about 85% of all lung cancer cases. Cancer immunotherapy is a paradigm shift in the treatment of NSCLC. Due to the improved survival benefit shown in multiple clinical trials several anti-PD-1 and anti-PD-L1 drugs have been approved for the treatment of NSCLC. These ICI are approved as first line and second or later line as monotherapy and/or combination therapy.5
PD-1 is an inhibitory receptor and is expressed on regulatory and activated T cells (CD4 andCD8), activated B cells and natural killer cells. PD-1 has two receptor ligands, PD-L1 and PD-L2. While PD-L2 is expressed mainly on antigen-presenting cells, PD-L1 is also expressed in a wide variety of tumour cells. The function of the PD-1 receptor is to prevent the destruction of the body’s own cells by activated T cells. However, PD-1 is non-selective in working against T cells and hence inhibits T cells from attacking pathogens and cancer cells as well.6
PD-1 gets upregulated when T cells get activated and this happens through a process called PD-1 signaling. This process occurs when PD-1 receptor ligands bind to PD-1. Due to the PD-1 signaling the tumour-infiltrating lymphocytes, which frequently express PD-1, get inhibited and are unable to attack the tumour cells. On the basis of this mechanism, anti-PD-1 and anti-PD-L1 antibodies were developed and are approved for their use as the first line or second line, either alone or in combination. Even though PD-1 and PD-L1 inhibitors have similar mechanism of action there are certain differences in their efficacy and mechanism of action. The efficacy of PD-1 and PD-L1 antibodies were compared using half-maximal effective concentration (EC50) values which were obtained using functional assays. The EC50 values for the PD-L1 antibodies were significantly lower compared with the EC50 values of the PD-1 antibodies suggesting that PD-L1 antibodies are perhaps more effective blockers than PD-1 antibodies.3
From a practical standpoint, PD-1 and PD-L1 inhibitors are considered to be equally efficacious even though they have not been compared head-to-head. Mechanistically, PD-1 inhibitors block interaction between PD-1 and its ligands PD-L1 and PD-L2 both of which are negative regulators of T cell activation. PD-L1 inhibitors, on the other hand block the engagement of PD-L1 with its receptors PD-1 and CD80.3 While CD80 is generally considered as a co-stimulatory receptor, and it may also function as a negative regulator of effector and memory T cells.7 Over expression of CD-80 has been surmised to be one of the mechanisms for resistance to PD-1 inhibitors.8
There have been no prospective studies and only a handful of small retrospective chart review studies and case series reported in literature exploring the concept of switching from anti-PD-1 antibody therapy to anti-PD-L1 therapy or vice versa.9 These reports have suggested that this intervention may have very limited benefit. There is one case report in the literature where nivolumab was discontinued in a patient with stable NSCLC due to the immune-related side effects and the patient was successfully administered atezolizumab resulting in a stable disease without any side effects.10 In the case of our patient, atezolizumab therapy led to complete remission following progression on nivolumab. The patient completely tolerated both the agents and did not experience any side effects from either nivolumab or atezolizumab therapy. There are now many ongoing clinical trials that are evaluating the use of atezolizumab either alone or in combination with other agents in patients with advanced NSCLC who had POD on prior treatment with either chemotherapy and/or anti-PD-1 therapy (table 1).
Table 1.
Ongoing clinical trials investigating the use of atezolizumab in NSCLC patients
| Identifier, phase | Disease | Intervention | Primary outcome | Completion date |
| NCT03014648, II | Advanced NSCLC previously treated with anti-PD-1 therapy | Atez | Best overall response | September 2023 |
| NCT03977467, II | Advanced NSCLC previously treated with anti-PD-1 therapy | Chemo+Atez vs Chemo alone | ORR of Atez+Chemo in NSCLC patients who progressed after anti-PD-1 monotherapy | July 2021 |
| NCT03689855, II | Advanced NSCLC previously treated with any ICIs | Ramucirumab+Atez | ORR—percentage of participants with complete or partial response | August 2023 |
| NCT03559647 | Locally advanced or metastatic NSCLC previously treated with chemo | Atez | Time to loss of clinical benefit and duration of response | May 2023 |
| NCT03922997, III | Advanced or metastatic NSCLC progressed after systemic chemo (either given after anti-PD-1 therapy or in combination with anti-PD-1 therapy) | Atez | Serious Adverse Events Incidence Rates related to Atez | July 2022 |
Atez, atezolizumab; Chemo, chemotherapy; ICIs, Immune-checkpoint inhibitors; NSCLC, non-small cell lung cancer; ORR, overall response rate; PD-1, programmed death-1; PD-L1, programmed death ligand-1.
While most cases in the literature are of patients who had primary refractoriness to ICIs, our patient had an initial partial response to nivolumab, which was lost after many cycles of treatment. To the best of our knowledge, this is the first reported case of complete response with the use of anti-PD-L1 immunotherapy following progression on anti-PD-1 therapy without any immune-related toxicity to either agent.
Conclusion
To summarise, this case report shows the potential role of switching to anti-PD-L1 treatment in selected patients with NSCLC who acquire resistance to PD-1 inhibition after an initial response. Clinical trials investigating this strategy would be highly beneficial for this increasing cohort of patients.
Learning points.
Cancer immunotherapy has resulted in a shift in treatment paradigm due to the improved survival outcomes in non-small cell lung cancers.
Programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) inhibitors have slight differences in their mechanisms of action.
Anti-PD-L1 antibodies may have the ability to revert resistance to PD-1 inhibition.
Our case is an important addition to the literature as it suggests that PD-L1 inhibitors may be an effective treatment for patients who have progressed on prior PD-1 therapies.
Footnotes
Contributors: NSeetharamu and NSingh, under the guidance and immense support of NSeetharamu did the entire work for the case.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sui H, Ma N, Wang Y, et al. Anti-Pd-1/Pd-L1 therapy for non-small-cell lung cancer: toward personalized medicine and combination strategies. J Immunol Res 2018;2018:1–17. 10.1155/2018/6984948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spagnuolo A, Gridelli C. "Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer": is there a substantial difference or not? J Thorac Dis 2018;10:S4065–8. 10.21037/jtd.2018.09.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Sousa Linhares A, Battin C, Jutz S, et al. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci Rep 2019;9:11472. 10.1038/s41598-019-47910-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016;893:1–19. 10.1007/978-3-319-24223-1_1 [DOI] [PubMed] [Google Scholar]
- 5.Reck M, Shankar G, Lee A, et al. Atezolizumab in combination with bevacizumab, paclitaxel and carboplatin for the first-line treatment of patients with metastatic non-squamous non-small cell lung cancer, including patients with EGFR mutations. Expert Rev Respir Med 2020;14:125–36. 10.1080/17476348.2020.1701439 [DOI] [PubMed] [Google Scholar]
- 6.Tartarone A, Roviello G, Lerose R, et al. Anti-Pd-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: a meta-analysis. Future Oncol 2019;15:2423–33. 10.2217/fon-2018-0868 [DOI] [PubMed] [Google Scholar]
- 7.Tatari-Calderone Z, Semnani RT, Nutman TB, et al. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J Immunol 2002;169:6162–9. 10.4049/jimmunol.169.11.6162 [DOI] [PubMed] [Google Scholar]
- 8.Rollins MR, Gibbons Johnson RM. CD80 Expressed by CD8+ T Cells Contributes to PD-L1-Induced Apoptosis of Activated CD8+ T Cells. J Immunol Res 2017;2017:1–6. 10.1155/2017/7659462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita K, Uchida N, Yamamoto Y, et al. Retreatment with anti-PD-L1 antibody in advanced non-small cell lung cancer previously treated with anti-PD-1 antibodies. Anticancer Res 2019;39:3917–21. 10.21873/anticanres.13543 [DOI] [PubMed] [Google Scholar]
- 10.Swami U, Lenert P, Furqan M, et al. Atezolizumab after Nivolumab-Induced inflammatory polyarthritis: can anti-PD-L1 immunotherapy be administered after Anti-PD-1-Related immune toxicities? J Thorac Oncol 2018;13:e102–3. 10.1016/j.jtho.2018.01.027 [DOI] [PubMed] [Google Scholar]