Abstract
Thalamic deep brain stimulation (DBS) for chronic pain is performed in selected patients with a variable success rate. We report the use of recently developed directional DBS in a patient with hemibody central poststroke pain (CPSP) and its added value in the induction of pleasant, pain-distracting paresthesia’s throughout the contralateral body side. A 68-year-old man suffered from multiple strokes in the left hemisphere 11 years before presentation, resulting in medically refractory right-sided hemibody CPSP. He was implanted with a directional DBS electrode in the left ventrocaudal nucleus of the thalamus. A directional single-segment contact configuration produced a better improvement throughout the contralateral body side than ring-mode and other directional configurations. Treatment led to a reduction of almost 50% in pain. This case demonstrates the value of directional DBS in the treatment of chronic pain, as steering increases selectivity and reduces side effects in a small target area surrounded by structures with high functional diversity.
Keywords: stroke, pain (neurology), neurosurgery
Background
Central poststroke pain (CPSP), first described in 1906 by Déjerine and Roussy as Le Syndrome Thalamique, is a chronic central neuropathic pain disorder.1 2 Its occurrence and symptomatology are linked to the location of the lesion, with particularly high occurrence after infarction of the lateral medulla and the ventrocaudal nucleus (Vc) of the thalamus.2 The Vc serves as the somatosensory relay nucleus of the thalamus. The medial part, also known as ventral posteromedial nucleus, is involved in the sensation of the contralateral head and upper extremity, while the lateral part, also known as ventral posterolateral nucleus, is involved with the sensation of the contralateral lower extremity.
CPSP can be a great burden for the patient, as the response to medication is usually low and limited by the side effects.2 3 Thalamic deep brain stimulation (DBS), a neurosurgical treatment involving the implantation of electrodes that send electrical impulses to specific locations in the brain (figure 1A), may be a useful tool in the treatment of CPSP when all other treatment modalities have failed.4 5 The goal of DBS in CPSP is to induce pleasant, pain-distracting paresthesias in the affected body part. The characteristic anatomical topology of Vc led to recommendations on where to treat patients with DBS for thalamic pain syndromes.6 However, DBS treatment of hemibody CPSP can be very challenging as a result of the inherent complex and small functional Vc target area; it may not be possible to induce pleasant, pain-distracting paresthesias in the leg, when the DBS electrode is located preferentially in the face or arm area of Vc and vice versa.
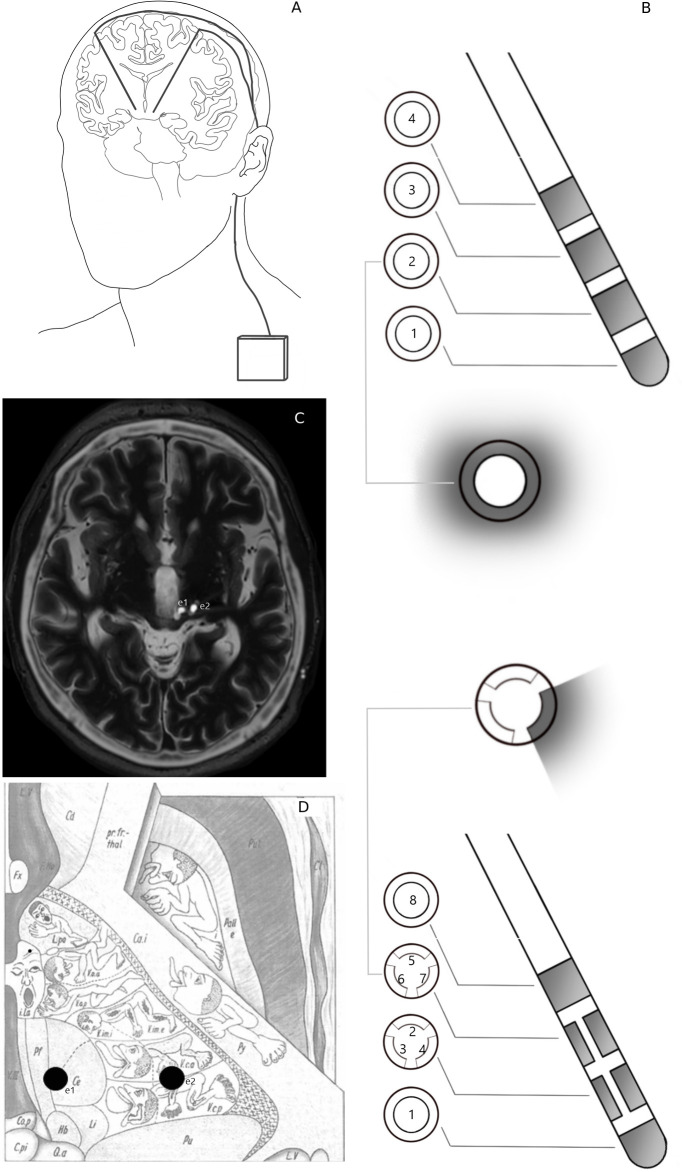
Figure 1.
(A) Schematic of implanted deep brain stimulation (DBS) system, consisting of brain electrodes which are connected to an implantable pulse generator by subcutaneous extension cables. (B) Schematic comparison of traditional ring-shaped four-contact DBS electrode and directional eight-segment DBS electrode (Cartesia, Boston Scientific, Marlborough, Massachusetts, USA). (C) Fused axial images of stereotactic T2-weighted MRI and coregistered postoperative CT showing implantation of DBS electrodes in the left periventricular grey matter (PVG (e1) and left ventrocaudal nucleus of the thalamus (Vc (e2)). (D) Schematic axial representation of PVG and Vc DBS electrodes into Fig. 29 (this was adapted from Hassler et al,15 p315 copyright 1979).
In the past decennia, only traditional ring-shaped electrodes were available for DBS, applying spherical stimulation of surrounding brain tissue. With recent technological advancement, ‘directional’ DBS electrodes were shown the ability to steer current in a certain direction (figure 1B). Here, we report the first case of a patient with hemibody CPSPs in whom directional DBS allowed for selective induction of pleasant, pain-distracting paresthesias throughout the contralateral body side at very low amplitudes.
Case presentation
A 68-year-old man with a history of hypertension and diabetes suffered multiple strokes 11 years before presentation. He reported slowly progressive neuropathic pain on the right side of the body since 10 years, with the pain being most severe in the right hand and the right leg. On a scale from 0 to 10 (with 10 being the most severe pain the patient had ever felt), he reported variable levels of pain intensity over the day, with a maximum value of 9. In the past, he tried multiple pain medications including paracetamol, gabapentin, pregabalin, codeine, morphine, lidocaine and ketamine. No medication regimen significantly reduced the pain. Transcutaneous electrical nerve stimulation was applied without success. Physical examination revealed a right arm motor drift and a symmetrical decreased sensitivity of the distal part of the legs and feet, attributed to the patient’s history of diabetes. MRI showed generalised cerebral atrophy, multiple vascular white matter lesions in left- and right-sided corona radiata and a small infarction of the right ventral intermediate nucleus of the thalamus.
The patient was implanted with two DBS electrodes (Cartesia; Boston Scientific, Marlborough, Massachusetts, USA): one in the left Vc, and one in the left periventricular grey matter (PVG), another well-established anatomical target for DBS in the treatment of intractable pain syndromes.7 8 For the PVG electrode (targeted at 8 mm lateral, 3 mm anterior and 0 mm relative to the posterior commissure (PC)), intraoperative test stimulation was set to a frequency of 30 Hz and a pulse width of 450 µs, which elicited a cold feeling in the right arm (and to a lesser extent in the right side of the face and the right leg) at an amplitude of 2 mA. The tip of the PVG electrode was implanted at 2.5 mm lateral, 4.5 mm posterior and 6 mm inferior relative to PC. For the Vc electrode (targeted at 17 mm lateral, 3 mm anterior and 0 mm relative to PC, with a third ventricle width of 11 mm), test stimulation was set to a frequency of 100 Hz and a pulse width of 100 µs, which elicited a cramping contraction of the right hand at an amplitude of 1.5 mA. It was concluded that the Vc electrode was positioned too closely to the motor pathways in the nearby internal capsule. Subsequently, a 3 mm more medial trajectory was explored. Test stimulation at target depth elicited pleasant paresthesias in right arm and hand. At deeper levels, the patient also reported paresthesias in his right leg and foot. The tip of the Vc electrode was implanted at 11 mm lateral, 0 mm anterior and 5 mm inferior relative to PC. The leads were fixed and connected to an implantable pulse generator (Vercise PC; Boston Scientific, Marlborough, Massachusetts, USA), which was placed subclavicularly. Postoperative CT was coregistered to stereotactic MRI to evaluate the position of the electrodes (figure 1C, D). The stimulation was not turned on, and the patient was discharged the day after the operation.
Nine days postoperatively, we performed a monopolar review on ring-mode on the lower three levels of the electrode of the left Vc with frequency set to 99 Hz, pulse width to 100 µs (online supplementary file 1). The patient was blinded for the programming settings. We recorded the localisation and intensity of paresthesia’s reported by the patient at 0.5 mA, and subsequently increased the amplitude to 0.8 mA. Afterwards, we performed a directional review of the individual segments on the steerable electrode levels, recording the localisation and nature of the effect of DBS on the patient when turning on each segment at 0.5 mA. We then performed a new directional review in six directions on the level with the best results during the directional review; three directions allocating all stimulation to one segment and three directions allocating 50% of current to each of two adjacent segments using the multiple independent current control feature. During the monopolar review in ring mode, the best effect on both arm and leg was found on the second level from the bottom of the electrode, with segments numbered 2–3–4. Directional review on this level showed a differential effect on arm and leg depending on which directional contact was used (online supplementary file 1). In the same session, a monopolar review in ring mode of the electrode in the left PVG (with frequency set to 30 Hz, and pulse width to 450 µs) did not produce additional beneficial clinical effects, and no further directional testing was done.
bcr-2019-233254supp001.pdf (183.1KB, pdf)
Outcome and follow-up
DBS on the Vc electrode was set to contact 3, located at 12.5 mm lateral, 2.5 mm anterior and 2 mm inferior relative to PC, that is, in the ventral part of Vc. Stimulation was set at 0.5 mA at 99 Hz, pulse width of 100 µs, with a cycle of 30 s ON, 10 s OFF. Stimulation was turned off at night. At visit 3 weeks postoperative, the patientreported that the effect of DBS was waning. Amplitude was increased to 0.6 mA, and OFF time in the cycle was increased to 30 s. In the following week, the patient reported a good effect of DBS on the pain, but also bothersome paresthesia’s. The stimulation was lowered to 0.3 mA (6 weeks after surgery), and then to 0.1 mA (12 weeks after surgery). The patient’s maximum pain score was 5 and overall reported pain intensity reduced by almost 50%. Because of the good effects of Vc DBS, the PVG electrode remained OFF. At 16 months follow-up, cycling DBS was still set at 0.1 mA at contact 3, his pain intensity was still reduced by approximately 50%.
Discussion
The anatomical topology of Vc has been studied by measuring thalamic activity while providing sensory stimuli to the skin and deep tissue.9 These findings have led to recommendations on the anatomical DBS targets for patients with neuropathic pain syndromes. During implantation of DBS electrodes for CPSP, test stimulation is routinely used to determine the borders and anatomical topology of Vc.10 However, the intraoperative and postoperative treatments of CPSP remain challenging, because of a small target area in a complex of thalamic subregions with high functional diversity, often resulting in failure of treatment or low-threshold side-effects. To our knowledge, this is the first study showing directional threshold differences for producing sensory effects in arm and leg by Vc stimulation using an implanted directional DBS electrode. Threshold differences using directional DBS electrodes were also reported in patients undergoing DBS of the subthalamic nucleus for Parkinson’s disease.11–13 In addition, we showed that the use of only one small segment of a directional electrode can be enough to produce good clinical outcome when stimulating a very small and complex target area in the treatment of chronic pain. In our current surgical approach of CPSP patients, we try to maximise chances of good clinical outcome by implanting both a PVG and Vc electrode when test stimulation (at both targets) induces somatotopic coverage of the painful area. If directional Vc DBS is shown to be successful in more CPSP patients, we might consider omitting PVG test stimulation. Directional DBS may also be targeted to the thalamocortical tract in the posterior limb of the internal capsule, for example in cases where infarction destroyed a vital portion of Vc.14
In the current CPSP case, directional DBS allowed us to tailor the stimulation as much as possible to the patient’s needs, covering the full hemibody with pleasant paresthesias without causing bothersome side-effects due to spread of the current into adjacent structures.
Learning points.
Deep brain stimulation (DBS) treatment of central poststroke pain remains challenging, because of a small target area in a complex of thalamic subregions with high functional diversity.
The use of only one-directional segment of an electrode can be enough to produce good clinical outcome when stimulating a very small and complex target area.
Directional DBS increases the therapeutic possibilities of electrical stimulation in a complex functional target area.
Footnotes
Contributors: TRtB and PvdM were involved in the treatment of the patient, data acquisition, interpretation of the data and writing of the manuscript. HA and RS critically revised the paper and participated in discussions about the case.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: TRtB has received non-financial support from Boston Scientific in the form of a travel grant. RS acts as an independent advisor for Medtronic, Elekta, and Boston Scientific.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Déjerine J, Roussy G. Le syndrome Thalamique. Rev Neurol 1906;14:521–32. [Google Scholar]
- 2.Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol 2009;8:857–68. 10.1016/S1474-4422(09)70176-0 [DOI] [PubMed] [Google Scholar]
- 3.Leijon G, Boivie J, Johansson I. Central post-stroke pain--neurological symptoms and pain characteristics. Pain 1989;36:13–25. 10.1016/0304-3959(89)90107-3 [DOI] [PubMed] [Google Scholar]
- 4.Pereira EAC, Aziz TZ. Neuropathic pain and deep brain stimulation. Neurotherapeutics 2014;11:496–507. 10.1007/s13311-014-0278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alves RV, Asfora WT. Deep brain stimulation for Dejerine-Roussy syndrome: case report. Minim Invasive Neurosurg 2011;54:183–6. 10.1055/s-0031-1280833 [DOI] [PubMed] [Google Scholar]
- 6.MARK VH. Clinical aspects of stereotactic thalamotomy in the human. Arch Neurol 1960;3:351 10.1001/archneur.1960.00450040001001 [DOI] [PubMed] [Google Scholar]
- 7.Ward M, Mammis A. Deep brain stimulation for the treatment of Dejerine-Roussy syndrome. Stereotact Funct Neurosurg 2017;95:298–306. 10.1159/000479526 [DOI] [PubMed] [Google Scholar]
- 8.Frizon LA, Yamamoto EA, Nagel SJ, et al. Deep brain stimulation for pain in the modern era: a systematic review. Neurosurgery 2019;10 10.1093/neuros/nyy552 [DOI] [PubMed] [Google Scholar]
- 9.Lenz FA, Dostrovsky JO, Tasker RR, et al. Single-Unit analysis of the human ventral thalamic nuclear group: somatosensory responses. J Neurophysiol 1988;59:299–316. 10.1152/jn.1988.59.2.299 [DOI] [PubMed] [Google Scholar]
- 10.Kiss ZHT, Anderson T, Hansen T, et al. Neural substrates of microstimulation-evoked tingling: a chronaxie study in human somatosensory thalamus. Eur J Neurosci 2003;18:728–32. 10.1046/j.1460-9568.2003.02793.x [DOI] [PubMed] [Google Scholar]
- 11.Steigerwald F, Müller L, Johannes S, et al. Directional deep brain stimulation of the subthalamic nucleus: a pilot study using a novel neurostimulation device. Mov Disord 2016;31:1240–3. 10.1002/mds.26669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TAK, Djilas M, Nowacki A, et al. Analysis of patient-specific stimulation with segmented leads in the subthalamic nucleus. PLoS One 2019;14:e0217985. 10.1371/journal.pone.0217985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dembek TA, Reker P, Visser-Vandewalle V, et al. Directional DBS increases side-effect thresholds-A prospective, double-blind trial. Mov Disord 2017;32:1380–8. 10.1002/mds.27093 [DOI] [PubMed] [Google Scholar]
- 14.Franzini A, Messina G, Levi V, et al. Deep brain stimulation of the posterior limb of the internal capsule in the treatment of central poststroke neuropathic pain of the lower limb: case series with long-term follow-up and literature review. J Neurosurg 2019:1–9. 10.3171/2019.5.JNS19227 [DOI] [PubMed] [Google Scholar]
- 15.Hassler RG, Rolf G, Mundinger F, et al. Adapted by permission from Springer Nature : Stereotaxis in Parkinson syndrome: clinical-anatomicalcontributions to its pathophysiology. Springer-Verlag, 1979: 315. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2019-233254supp001.pdf (183.1KB, pdf)