Emergency preparedness has been an emphasis for blood banks for the past several years, driven largely by the proliferation of high-profile mass shootings and the recognition that such a disaster could immediately impact blood bank operations.1,2 While most hospitals included a pandemic scenario into their emergency plans, for better or worse, lack of recent experience with pandemics made specific preparations difficult to identify and expert opinion on these matters remained highly theoretical. Highly detailed plans, such as those published by the AABB and Canada, had never been previously operationalized for a pandemic response.3,4 As a result, even the most well-prepared blood bankers found themselves working diligently to make frequent changes to operational plans as the coronavirus disease 2019 (COVID-19) pandemic unfolded in the United States in early 2020. In this article, we highlight “best practices” that have emerged during the pandemic, focusing on management of blood supply and blood bank operations, rapid incorporation of COVID-19 convalescent plasma into blood bank inventory, and changes to the approach to the patient requiring therapeutic apheresis.
Impact on Blood Banking Operations
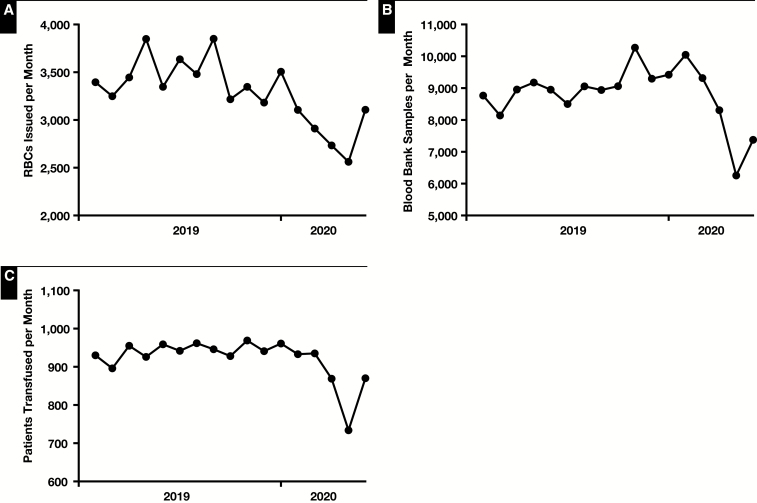
In the United States up to 80% of the blood supply is collected at mobile blood drives that are susceptible to cancellation or underperformance without strong support from the public.5 When large corporations, schools, and universities began closing in response to the need to promote social distancing, there was an immediate effect on blood donation. The American Red Cross, which is the largest single blood supplier in the United States, estimated that 4,600 blood drives were almost immediately cancelled, correlating in an estimated loss of 143,600 units of blood from the blood supply (Pampee Young, MD, PhD, American Red Cross email communication, March 18, 2020). In many areas, the changes to businesses and schools preceded major changes to hospital operations, such as cancellation of nonurgent elective surgery, and the blood banking community found itself stuck in the middle between, on the one hand, a rapidly developing blood supply shortage and, on the other, essentially normal hospital operations (eg, elective surgeries were ongoing, and changes were not yet made to transplant or oncology services). Many blood banks responded to this issue by doing everything possible to promote community blood donation at fixed donor sites, encourage blood suppliers to increase collection activities at hospitals, while lobbying with hospital administrators to reduce hospital operations or to at least warn the general hospital community that blood was quickly becoming a very scarce resource. In early late March 2020, the blood supply and demand stabilized, due to widespread cancellation of normal hospital operations, including cancellation of nonemergent surgeries and transplants, reduction in routine sickle cell disease transfusion volumes, and a sharp decline in elective cardiac surgery.6 As a result of these changes, demand for RBCs was significantly impacted and came largely into line with supply Figure 1. Critically, massive transfusion protocol trauma activations were markedly reduced during the pandemic, also suppressing transfusion utilization, especially of RBCs and platelets Table 1. An area of ongoing concern is coordination of resumption of normal patient activities with increased collection of blood components (Pampee Young, MD, PhD, American Red Cross email communication, May 1, 2020). Mobilization of the donor base will be a key part of any plans to resume normal volumes, as will coordination between blood suppliers and transfusion services, especially given that summer months are difficult on the blood supply even in the absence of a pandemic.
Figure 1.
Impact of COVID-19 on RBC transfusion volume (A), samples received in the blood bank (B), and patients transfused at The Johns Hopkins Hospital (C).
Table 1.
Impact of COVID-19 on the Number of Massive Transfusion Protocol (MTP) Related Blood Transfusions at The Johns Hopkins Hospital
| Year | MTP RBCs | MTP Plasma | MTP Platelets | MTP Cryoprecipitate |
|---|---|---|---|---|
| 2018 | 2,378 | 2,373 | 537 | 160 |
| 2019 | 2,916 | 2,473 | 575 | 148.5 |
| 2020a | 1,558 | 1,378 | 346 | 89 |
aAnnualized based on January to May 2020 data.
As blood donation was encouraged, blood bankers were suddenly inundated with concern that blood transfusions could transmit severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and the transfusion medicine community was asked to provide reassurance against this possibility. Extrapolation from previous experience with SARS-CoV, Middle Eastern respiratory syndrome, and influenza, and with the strong backing of statements by AABB, the Centers for Disease Control and Prevention, and the Food and Drug Administration (FDA), as well as the preliminary experience of other areas that were afflicted by COVID-19 prior to its wide spread in the United States, blood bankers were able to convince most stakeholders that the true risk to the blood supply was not SARS-CoV-2 itself, but rather social distancing practices resulting in an interruption to the critically needed blood supply.5,7,8 To assist with donor recruitment, the FDA eased several donor deferral criteria pertaining to low-risk HIV-associated behaviors, travel-related malaria risk mitigation, and Creutzfeldt-Jakob or variant Creutzfeldt-Jakob Disease Table 2.9-11 The available evidence and experience with COVID-19 continues to confirm that SARS-CoV-2 is not transmitted by transfusion.
Table 2.
Summary of Major April 2020 Updates to Food and Drug Administration Guidance Regarding Blood Donor Eligibility
| Deferral Reason | Previous Deferral Requirement | Updated Deferral Requirement |
|---|---|---|
| Male donor with recent sexual contact with male partner | 12 mo | 3 mo9 |
| Female donor with recent sexual contact with male who had male sexual contact | 12 mo | 3 mo9 |
| Recent tattoo or piercing | 12 mo | 3 mo9 |
| Travel to malaria endemic area | 12 mo | 3 mo10 |
| Theoretical risk of Creutzfeldt-Jakob disease or variant Creutzfeldt-Jakob disease due to European travel or military service | Lifetime | Eliminated many restrictions that previously applied to military personnel, individuals treated with bovine insulin, and time spent in many European countries11 |
An area of secondary concern was that potential blood donors could come into contact with SARS-CoV-2 while participating in a blood drive. This was immediately anticipated by the blood suppliers, many of whom quickly stopped using small confined areas to collect blood (such as trailers) and began asking potential blood drive sponsors to provide large spaces that could allow donors to maintain at least a 6-ft distance from each other and, as much as possible, from collection staff. In addition, blood suppliers began to publicize the cleaning activities that they used to disinfect donor areas.
Meanwhile, within the hospital, there began to be concern that units of blood could theoretically promote transmission of SARS-CoV-2 if they were returned from the immediate area of a COVID-19 patient to the blood bank and subsequently reissued to a different patient. In many large centers, it is known that blood may be issued to 3 to 4 patients and returned prior to issue to the patient who is ultimately transfused, but data are not kept on this phenomenon and there are no specific regulatory standards or benchmarks that apply. The bags themselves are made of a soft, permeable plastic that cannot be cleaned without the possibility of introducing the cleaning solution to the blood itself. Prior to COVID-19, many transfusion services already had best practices in place to prevent avoidable exposure of units of blood to potentially biohazardous areas, such as asking transfusionists to avoid opening coolers in patient areas until the decision to transfuse was final. However, due to the airborne nature of SARS-CoV-2, as well as the potential for large numbers of cases in various treatment wards, hospitals considered various methods to more definitively address this problem. Possibilities included mandating the destruction of any units of blood that came into the direct patient area for a COVID-19 patient (which is difficult to tolerate at baseline, and even worse during a blood shortage); issuing all blood units in plastic bags, with instructions to not open the outer plastic bag unless the decision to transfuse was final, and to wipe down the outer bag with an approved hospital disinfectant wipe prior to returning to the blood bank; and changing guidance to clinical personnel that is printed on the outside of validated blood transportation coolers Image 1. These activities required changes to internal blood bank operations, as well as strong communication to the general hospital audience during a time when information flow was already extremely high.
Image 1.
COVID-19–specific handling instructions added to the validated blood bank coolers at The Johns Hopkins Hospital.
As the extent of illness caused by the virus became clearer, the number of possible treatment modalities under investigation literally exploded. Overnight, demand drugs with relatively narrow therapeutic applications—and unproven effect in the treatment or prevention of COVID-19—suddenly exploded.12 In this context, convalescent plasma, which has been employed for treatment of emerging infectious disease outbreaks for over 100 years—and had been shown to be of no benefit for treatment of Ebola virus and of promise but uncertain efficacy for treatment of SARS and H1N1 influenza—suddenly became of extreme interest to the scientific community and to the public.13-16 Early experience from China, which featured case reports of small numbers of patients treated without controls, was viewed as promising despite the low quality of the underlying studies.17 Blood bankers, the majority of whom had never previously handled a unit of convalescent plasma, were suddenly viewed as content matter experts for this promising, but ultimately unproven treatment, and multiple clinical trials, as well as other access pathways, were planned in a matter of weeks.18 At the time of this writing, there remained a lack of consensus on the efficacy of convalescent plasma in the treatment of COVID-19, and availability of convalescent plasma was gradually improving but uneven. Initial safety data, published in preprint format, appears to support the continued application of convalescent plasma to patients with severe disease.19 Peer reviewed efficacy data are eagerly anticipated. Placebo-controlled clinical trials are enrolling, and there is a national debate as to whether placebo-controlled, randomized clinical trials should be prioritized above assured access to convalescent plasma via expanded access. Proponents of the randomized clinical trials identify that this approach is the most likely to determine with scientific certainty whether or not convalescent plasma is effective and preventing severe morbidity or death. Advocates of expanded access note that information gathered during clinical trials will only apply to COVID-19 and not to future emerging pathogens, limiting the import of the clinical trial findings and creating an imperative to provide compassionate access to any patient who could theoretically benefit.
Impact on Therapeutic Apheresis
Initially when considering the impact to the apheresis and cellular therapy units, the first concern was to minimize the risk of exposing patients with compromised immune systems to SARS-CoV-2. Centers across the country were advised to restrict visitors to the units as well as restrict any staff members with respiratory symptoms and where feasible utilize telemedicine visits in place of in-person visits. Patients and health care workers were advised to wear masks as well as health care workers with face-to-face care of the patients. All patients who could safely defer cellular therapies were advised to do so. In the event transplant could not be postponed, patients were advised to self-isolate for up to 14 days prior to receiving any cellular therapies as well as undergo COVID-19 testing to ensure the donor candidate is negative. Any candidates found to be positive were recommended to defer treatment until asymptomatic and negative by PCR on 2 occasions at least 1 week apart. In the event a patient had close contact with a COVID-19–positive person, deferral of cellular therapy is recommended for 14 to 21 days and COVID-19 testing of the candidate became negative.20 Donors of stem cells should self-isolate or at least avoid crowded locations for 21 days before donation. If donors have returned from travel of an area with community transmission, the recommendation is to defer collection for up to 4 weeks.21
To accommodate these requirements, units communicated with each provider to defer routine therapies or to increase the intervals between treatments to accommodate maintaining safe distance between patients. Patients undergoing therapeutic plasma exchange (TPE) for neurologic conditions were advised to maintain current treatments but to remain vigilant regarding social distancing and good hand hygiene.22 Photopheresis therapies for cutaneous T-cell lymphoma and stable graft-vs-host disease were deferred for treatments or converted from every other week to 1 treatment monthly.
Importantly, patients with sickle cell anemia (SCA) were most impacted as antigen-matched units became scarce as mobile blood drives were cancelled nationwide due to the closure of businesses, universities, and schools. The apheresis unit was tasked to work with the SCA teams to review all patients and, where possible, convert patients to simple transfusion. Those patients who had a history of cerebrovascular accident required more thoughtful examination, including review of hemoglobin fractionations over time and, where appropriate, increased the intervals between exchanges or partial exchanges were performed.23 Providers also considered starting or increasing hydroxyurea in an effort to maintain lower levels of hemoglobin S.
There were initially reports of TPE being done in COVID-19 patients with florid infections who developed sepsis, pneumonia, acute respiratory distress syndrome, and multisystem organ failure, most likely the result of cytokine storm with endothelial damage, inflammation, and hypercoagulability.24-27 In 1 report, 3 patients underwent TPE in a single center with reported recovery in all 3 patients after TPE. The report does not comment on the number of TPE treatments required and other medications utilized for these patients, but in a follow-up letter to the editor, the 3 patients are explained in more detail. Surprisingly, the follow-up letter revealed that only 1 patient who developed an antiphospholipid syndrome during his COVID-19 infection was treated with TPE successfully with an improvement in symptoms after 3 treatments. The other 2 patients were in fact treated with continuous renal replacement therapy and not TPE. Recently there was another report of a single patient undergoing TPE for COVID-19; this patient also received a combination of therapies, including intravenous immunoglobulin and steroids, and therefore the contribution of apheresis to recovery is difficult to determine.24
Based on American Society for Apheresis guidelines, the use of TPE in multisystem organ failure is listed as a category III, indicating that this is used in patients who have failed medical therapy and is used as a rescue therapy and is most effective early in the course of treatment. Randomized controlled trials for sepsis with multisystem organ failure have yielded mixed results. Exchange procedures must be performed with donor plasma, and the average length of time for performing the procedure is up to 14 days.28 Currently, the risk to the apheresis nurse is high considering the length of time to perform the procedure. Some institutions have advocated extended tubing for both dialysis and apheresis instruments so the nurse performing the procedure can connect the patient to the instrument and then remain outside of the patient’s room for the duration of the procedure. In larger institutions, the IL-6 agonist, tocilizumab, has been used to treat the cytokine storm and florid inflammatory process in patients with fulminant COVID-19 infections. One theoretical risk of TPE is alteration of the coagulation cascade, which is already perturbed in many advanced cases of COVID-19 and is associated with a high degree of morbidity and mortality. This risk should be carefully weighed, especially when considering that the literature contains significant advocacy for extensive plasma exposure to COVID-19 patients, some with no actual patient experience to support the proposed practice.27
Although early on in the pandemic it was believed that children were very unlikely to become seriously ill, as the pandemic continued some children were disturbingly found to suffer from a multisystem inflammatory syndrome. This condition is marked by fever, shock, and acute heart failure and has been managed with various combinations of immunosuppressive medical therapy, including intravenous immunoglobulin, steroids, anakinra, and infliximab.29-31 The role of transfusion, as well as the role of TPE, remains unclear as of this writing.
Finally, and of note, there are reports of using blood “purification” filters as a component of apheresis therapy to reduce proinflammatory cytokines such as IL-3, IL-6, IL-10, and other chemical markers of inflammation. Experience with such inline filters, which are typically integrated downstream of where plasma is separated from whole blood in the apheresis device, are again largely limited to sepsis with multiorgan failure and have shown mixed, at best, outcomes in reducing mortality.28,32 Nonetheless, the FDA did issue an emergency use authorization (EUA) for a blood filtration product, the Depuro D2000 Adsorption Cartridge, for the treatment of complications of COVID-19.33 Per the FDA EUA release, the Depuro filtration system should be applied only to patients 18 years of age or older admitted to intensive care units with confirmed COVID-19 and definitive or imminent respiratory failure. As of this writing, the device is not commercially available for routine use but is being studied in clinical trials per a communication from the manufacturer received by the authors.
Summary
Overall, the hospital transfusion service and the blood suppliers have responded to the COVID-19 health crisis by working with the broader medical center to understand the effect of social distancing on supply, alerting the public of the need to donate blood, and working with recovered patients and regulators to safely collect convalescent plasma. At the same time, efforts have been taken to assure safe, continued access to apheresis treatments for patients who are apheresis- or transfusion-dependent, such as patients with myasthenia gravis and sickle cell disease complicated by previous stroke. The role of TPE for the treatment of COVID-19 remains uncertain at this time, and, similar to what is ongoing with convalescent plasma, if this therapy is pursued it should be done as a part of a carefully constructed clinical trial.
References
- 1. Lozada MJ, Cai S, Li M, et al. The Las Vegas mass shooting: an analysis of blood component administration and blood bank donations. J Trauma Acute Care Surg. 2019;86:128-133. [DOI] [PubMed] [Google Scholar]
- 2. Glynn SA, Busch MP, Schreiber GB, et al. Effect of a national disaster on blood supply and safety: the September 11 experience. JAMA. 2003;289:2246-2253. [DOI] [PubMed] [Google Scholar]
- 3. AABB. Disaster Operations Handbook v 2.0.2008. https://www.aabb.org/programs/disasterresponse/Documents/disastophndbkv2.pdf. Accessed June 8, 2020.
- 4. National Advisory Committee on Blood and Blood Products. The national plan for management of shortages of labile blood components.2017. https://www.nacblood.ca/resources/shortages-plan/index.html. Accessed June 8, 2020.
- 5. Gehrie EA, Frank SF, Goobie SM. Balancing supply and demand for blood during the COVID-19 pandemic. Anesthesiology. 2020. https: 10.1097/ALN.0000000000003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horton A. Blood banks narrowly averted a supply crisis. But they’re “worried about four weeks from now.” The Washington Post. March 31, 2020. https://www.washingtonpost.com/health/2020/03/31/blood-donation-coronavirus/. Accessed June 8, 2020. [Google Scholar]
- 7. AABB. Update: impact of 2019 novel coronavirus on blood donation.2020. http://www.aabb.org/advocacy/regulatorygovernment/Documents/Impact-of-2019-Novel-Coronavirus-on-Blood-Donation.pdf. Accessed June 8, 2020.
- 8. AABB. AABB COVID-19 toolkit.2020. http://www.aabb.org/advocacy/regulatorygovernment/Documents/COVID-19-Toolkit.pdf. Accessed June 8, 2020.
- 9. United States Food and Drug Administration. Revised recommendations for reducing the risk of human immunodeficiency virus transmission by blood and blood products.2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/revised-recommendations-reducing-risk-human-immunodeficiency-virus-transmission-blood-and-blood. Accessed June 8, 2020.
- 10. United States Food and Drug Administration. Revised recommendations to reduce the risk of transfusion-transmitted malaria 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/revised-recommendations-reduce-risk-transfusion-transmitted-malaria. Accessed June 8, 2020.
- 11. United States Food and Drug Administration. Recommendations to reduce the possible risk of transmission of Creutzfeldt-Jakob disease and variant Creutzfeldt-Jakob disease by blood and blood components: guidance for industry.2020. https://www.fda.gov/media/124156/download. Acessed June 8, 2020.
- 12. Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020;24:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yeh KM, Chiueh TS, Siu LK, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soo YO, Cheng Y, Wong R, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10:676-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020. doi: 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joyner M WR, Fairweather D, Senefeld J, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. medRxiv. 2020. doi: 10.1101/2020.05.12.20099879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. European Society for Blood and Marrow Transplantation. Coronavirus disease COVID-19: EBMT recommendations update March 23, 2020.2020. https://www.ebmt.org/sites/default/files/2020-03/EBMT%20COVID-19%20guidelines%20v.4.3%20%282020-03-23%29.pdf. Accessed June 8, 2020.
- 21. WMDA donor medical suitability recommendations. Novel coronavirus—SARS-CoV-2 & COVID-19.2020. https://share.wmda.info/pages/viewpage.action?pageId=344866320#/. Accessed June 8, 2020.
- 22. International MGC-WG, Jacob S, Muppidi S, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andemariam B. Guidelines aim to ensure proper care of patients with sickle cell disease amid COVID-19 pandemic.2020. https://www.healio.com/news/hematology-oncology/20200402/guidelines-aim-to-ensure-proper-care-of-patients-with-sickle-cell-disease-amid-covid19-pandemic. Accessed June 8, 2020.
- 24. Shi H, Zhou C, He P, et al. Successful treatment of plasma exchange followed by intravenous immunogloblin in a critically ill patient with 2019 novel coronavirus infection. Int J Antimicrob Agents. 2020:105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keith P, Day M, Perkins L, et al. A novel treatment approach to the novel coronavirus: an argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit Care. 2020;24:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang L, Zhai H, Ma S, et al. Efficacy of therapeutic plasma exchange in severe COVID-19 patients. Br J Haematol. 2020. doi: 10.1111/bjh.16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kesici S, Yavuz S, Bayrakci B. Get rid of the bad first: therapeutic plasma exchange with convalescent plasma for severe COVID-19. Proc Natl Acad Sci U S A. 2020. 10.1073/pnas.2006691117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the Writing Committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34:171-354. [DOI] [PubMed] [Google Scholar]
- 29. Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis Soc. 2020. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belhadjer Z, Meot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 31. Dolinger MT, Person H, Smith R, et al. Pediatric Crohn’s disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J Pediatr Gastroenterol Nutr. 2020. doi: 10.1097/MPG.0000000000002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schadler D, Pausch C, Heise D, et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: a randomized controlled trial. PLoS One. 2017;12:e0187015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. United States Food and Drug Administration. Regulatory letter to Terumo BCT, Inc.2020. https://www.fda.gov/media/136834/download. Accessed June 8, 2020.