Abstract
We present the case of a 45-year-old man with a known history of sarcoidosis who presented with double vision and headache. On examination, he was found to have left abducens and hypoglossal nerve palsy. CT and then MRI demonstrated extensive osseous lesions with a large expansile mass involving the clivus bone and sphenoid sinus. Laboratory data were remarkable for normocytic anaemia, low anion gap and elevated total protein which raised the suspicion for multiple myeloma. Subsequent protein electrophoresis and immunofixation illustrated monoclonal spike of IgG lambda present in the gamma zone. This was followed by a bone marrow biopsy that demonstrated plasma cells compromising around 80% of marrow cellularity. Left sphenoidal mass biopsy was consistent with plasmacytoma. Based on these findings, the patient was initially started on palliative radiation to shrink the intracranial tumour and is currently undergoing induction chemotherapy.
Keywords: malignant and benign haematology, cranial nerves, neurooncology, rheumatology
Background
Multiple myeloma (MM) is the neoplastic proliferation of plasma cells.1 It constitutes 17% of haematological malignancies in the USA and results in approximately 106 000 death per year worldwide.2 3 Renal impairment, hypercalcaemia, lytic bony lesions and anaemia are usually the initial manifestation of the disease and it rarely presents with neurological deficit.3 4 Plasmacytoma is a solid tumour with identical pathology to MM, which can occur in the bone (intramedullary) or arise outside the bone in soft tissue (extramedullary). Base of skull is a rare site for plasmacytomas, but if it is involved, the tumour can compromise the cranial nerves passing through this anatomical area.5 Several intracranial vascular, infective, neoplastic or inflammatory diseases can result in cranial nerve palsies. However, among rare causes, intracranial extension of MM and plasmacytoma should not be overlooked.6 Effective treatment options are available and a prolonged delay before diagnosis can lead to severe progression of the disease.1
Case presentation
A 45-year-old African-American man with a medical history consistent with sarcoidosis was admitted with a 2-day history of double vision and headaches. His diagnosis of sarcoidosis was not biopsy proven and was based on respiratory symptoms including cough and dyspnoea, diffuse cutaneous nodular lesions, weight loss and bilateral hilar lymphadenopathy on chest X-ray. He was treated with oral steroids and was symptom free for many months.
His current symptoms started a month before his presentation with bilateral shoulder and neck pain. He responded to a methylprednisolone dose pack given at the emergency room. However, a few days later, he developed double vision, left occipital and temporal headache that was constant, pounding and accompanied by nausea and vomiting. On admission, he was reporting about diplopia on primary gaze and when looking at left. He denied eye pain, red eye, flashes of light, floating spots, photosensitivity, symptoms of arthritis, respiratory problem or any skin lesions.
Sarcoidosis was the only medical condition in his past and he had not been taking any medication. Family history was only significant for hypertension in his mother. He denied smoking and was social on drinking habits. He is a widower, lives with daughter, unemployed and used to be a driver.
On presentation, the patient was afebrile (36.8°C), slightly hypertensive (144/80 mm Hg), with normal heart rate (79 beats/min) and respiratory rate (18 breaths/min). Left eye esotropia on primary gaze (figure 1A) was noted along with left eye limitation in abduction (figure 1B, C) and bilateral conjunctival pallor. Further cranial nerve examination revealed tongue deviation to the left side on protrusion (figure 2). He also had a flesh-coloured polypoid mass in the right posterior nasal cavity just inferior to the right middle turbinate, which appeared non-pulsatile. The remainder of the examination was normal.
Figure 1.
Left abducens nerve (cranial nerve VI) palsy. (A) Left eye esotropia on primary gaze due to weakness of left eye lateral rectus muscle. (B) Right eye abduction is unaffected. (C) Left eye abduction limitation on left gaze due to left lateral rectus muscle weakness.
Figure 2.

Left hypoglossal nerve (cranial nerve XII) palsy manifested by tongue deviation to the left.
Investigations
Laboratory data were remarkable for normocytic anaemia with haemoglobin: 88 (reference range: 135-175 g/L) and mean corpuscular volume: 92.0 (reference range: 80–96 µm3). Basic metabolic panel showed normal kidney function and electrolytes but with very low anion gap: 3 (reference range: 7–13 mEq/L). Total protein level was 13.8 (reference range: 5.5–9 g/dL). Erythrocyte sedimentation rate level was elevated up to 117 (reference range: 0–15 mm/hour) but C-reactive protein and ACE levels were normal. Chest X-ray was also unremarkable. Brain MRI without contrast demonstrated no recent infarct or other diffusion abnormality. However, extensive osseous lesions were seen in all structures with some extraosseous soft tissue involvement with no significant mass effect on brain parenchyma. Moreover, there was a large expansile lesion involving the clivus extending into the left sphenoid and cavernous sinus (figure 3).
Figure 3.
Brain MRI findings. (A) Sagittal section. Large expansile plasmacytoma lesion is involving the clivus with some soft tissue extension into the prepontine cistern. (B) Coronal section. Plasmacytoma involving bilateral cavernous sinus, more so on the left than on the right. (C) Axial section. Extension of the lesion to the sphenoid sinus.
Serum protein electrophoresis was done for the evaluation of MM and the outcome is as below in table 1 and figure 4.
Table 1.
Serum protein electrophoresis
| Fraction | g/dL | Reference range (g/dL) |
| Total protein | 13.8 | 5.5–9.0 |
| Albumin | 5.06 | 3.30–4.37 |
| Alpha 1 | 0.42 | 0.16–0.31 |
| Alpha 2 | 0.75 | 0.51–0.96 |
| Beta | 1.23 | 0.82–1.22 |
| Gamma | 6.33 | 0.86–1.98 |
Figure 4.

Serum protein electrophoresis. Homogenous band (monoclonal spike) present in gamma zone.
Free light chain assay showed free kappa: 0.38 (reference range: 0.33–1.94 mg/dL) and free lambda: 249 (reference range: 0.57–2.63 mg/dL), with extremely low kappa/lambda ratio.
Further immunofixation demonstrated that IgG lambda band present in the gamma zone.
Imaging for skeletal involvement illustrated diffusely abnormal bone marrow with multiple lytic lesions affecting all visualised bones in the body including ribs, spine with T5 compression fracture (figures 5 and 6).
Figure 5.
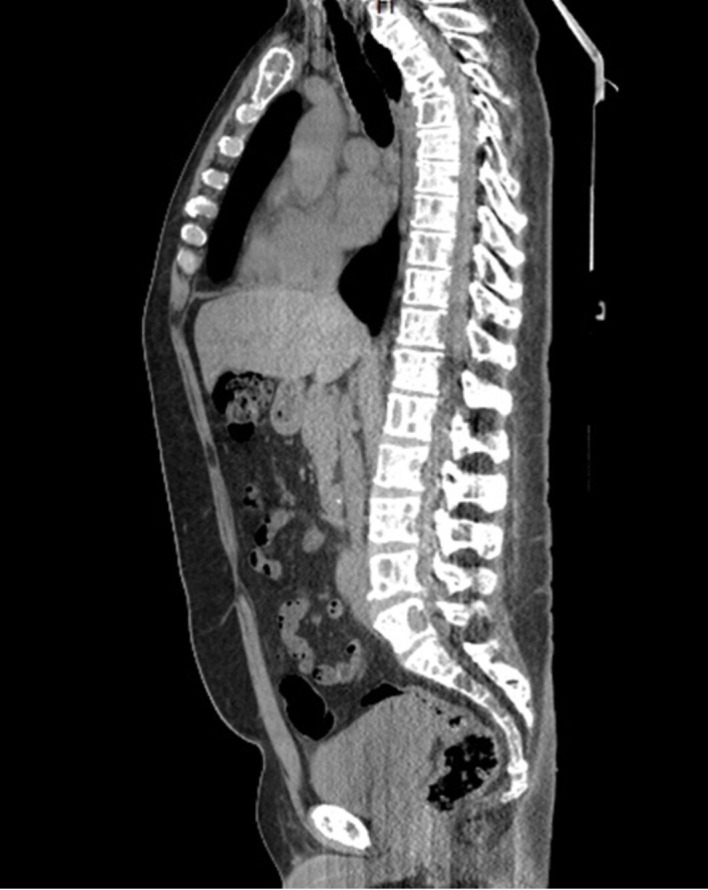
Diffuse multiple myeloma lytic lesions involving all the visualised bones on CT scan.
Figure 6.

Spine MRI. Significant wedge compression deformity involving the T4 vertebral body (yellow arrow). Extraosseous soft tissue (plasmacytoma) is seen in the epidural space at T4–T5 and T5–T6 with mild mass effect on the thecal sac without cord compression (red arrow).
Bone marrow biopsy showed more than 80% cellular bone marrow with decreased trilineage haematopoiesis. Plasma cells were increased mostly in sheets compromising around 80% of total marrow cellularity (figure 7). The karyotype was 46, XY. Fluorescence in situ hybridisation testing revealed positivity for monosomy 13 and 14, deletion of 17p13, IGH/14q32 gene rearrangement and gain of chromosomes 1q21.3/CKS1B, 9 and 15.
Figure 7.
Bone marrow biopsy histopathology. (A) Plasma cells are increased and most of them are neoplastic with large cells and prominent nucleoli. The findings are consistent with diagnosis of plasma cell myeloma. (B) Immunohistochemical stain showing strong and diffuse positivity for lambda light chains.
Left sphenoidal mass biopsy with transsphenoidal resection was performed and the findings were consistent with MM and plasmacytoma (figure 8).
Figure 8.
Sphenoidal mass biopsy histopathology. (A) Sheets of atypical plasma cells with large and dark nucleoli and ample cytoplasm with perinuclear huff are observed. The findings are consistent with plasmacytoma. (B) Strong and diffuse positivity for lambda on immunohistochemical stain.
Differential diagnosis
Initially, neurosarcoidosis, vasculitis and stroke were between differential diagnoses. Neurosarcoidosis can happen in 5%–10% of patients with sarcoidosis. It should never be overlooked in a patient with previous medical history of sarcoidosis presenting with cranial mononeuropathy.7 Following the neuroimaging, primary central nervous system neoplasia or secondary metastasis of other cancers became more probable. Putting the imaging results along with the normocytic anaemia, low anion gap and high total protein and low albumin levels raised MM to the top of the differential diagnosis list.
Treatment
The remainder of the patient’s hospital course was uneventful. He was discharged on day 12 with scheduled outpatient follow-up with radiation oncology. Patient underwent cranial irradiation for the large sphenoid/clival mass as well as the T4–T6 plasmacytoma. Thereafter, he was started on chemotherapy with carfilzomib, lenalidomide, elotuzumab and dexamethasone combination as his first-line systemic therapy.
Outcome and follow-up
He has tolerated the therapy well without any complications after three cycles of treatment. Diplopia is resolved and disconjugate gaze is not noticeable. However, he continues to have tongue deviation to the left. IgG level is 0.64 g/dL following completion of third cycle of chemotherapy, compared with 4.04 g/dL at the time of diagnosis, representing an approximate 84% reduction with treatment. The patient is being followed for starting the next cycle of chemotherapy. The further plan will be autologous haematopoietic stem cell transplantation.
Discussion
MM is the malignant proliferation of plasma cells. The diagnosis is usually suspected if the patient presents with one or more of the following features; bone pain, unexplained anaemia, symptomatic or asymptomatic hypercalcaemia and acute renal failure.1 Diplopia or other neurological symptoms as the initial manifestation of MM is not common.4 However, taking into consideration of other clinical and paraclinical clues like elevated total protein level, low anion gap can help to navigate the workup towards evaluation of plasma cell dyscrasia. MM diagnosis can be accompanied by a significant delay (>6 months) in more than 40% of patients. It has been shown that more than 6 months of delay in diagnosis is associated with higher number of complications and also decreased disease-free survival.1 Treatment of MM is based on risk stratification and bone marrow transplant eligibility.3 Combination of cranial irradiation, surgery and chemotherapy has been tried in patients with intracranial involvement of MM and plasmacytoma. Nevertheless, there is limited experience about the definitive therapeutic management due to rarity of occurrence.5 6 8 9
Literature review shows a few other case reports with acute cranial nerve palsy due to intracranial extension of MM and plasmacytoma. Kalwani et al5 reported a 69-year-old man who presented with bilateral abducens nerve palsy. MRI revealed solitary tumour at base of skull within the clivus. Endoscopic biopsy showed sheets of plasma cells and confirmed the diagnosis of solitary intracranial plasmacytoma. Another rare clinical scenario was depicted by Zaino et al6 where a 78-year-old man with a history of MM was admitted with unilateral horizontal diplopia and ptosis. Workup revealed intracranial extension of MM to right sphenoid sinus, extending towards right orbital fissures with right third and sixth cranial nerve compressions. Cranial radiation in both cases resulted in significant improvement of symptoms.
In another clinical vignette, Newman et al4 described a 73-year-old woman with MM who got partial treatment for several years, later presenting with dysarthria. Further workup demonstrated multiple lytic lesions adjacent to the occipital condyle and clivus. The underlying cause of dysarthria was impingement of the hypoglossal nerve within the hypoglossal canal which is at the base of occipital condyle. Untreated MM can result in severe lytic bone destruction through different cytokines activation. Specifically, interleukin 1, by activating receptor activator of nuclear factor kappa-B ligand plays an important role in osteoclast stimulation and osteoblastic activity reduction. This is the reason that patients with MM with bone disease require treatment with bisphosphonates apart from standard therapy. Targeted local irradiation or surgical intervention can also be part of therapeutic strategy depending on the severity of bone involvement.10 11
MM rarely involves central nervous system directly including brain parenchyma, pia and dura mater.12 Leptomeningeal MM (LMM) is thought to be from either haematogenous spread of malignant plasma cells or direct invasion of contiguous lytic bone lesions in the skull or spine. Pak et al8 reviewed 11 MM cases with leptomeningeal involvement and cranial nerve palsies. They reviewed nine patients with one cranial nerve palsy (second cranial nerve was most commonly involved) and two patients suffered from several cranial nerve palsies (third, fifth and seventh nerves were affected in both of them). Cerebrospinal fluid analysis was positive for malignant plasma cells in all cases. All of the reviewed patients underwent intrathecal chemotherapy and unfortunately, prognosis for most of them was poor with mean survival of 3.5 months. However, a review of 109 patients with LMM by Nieuwenhuizen and Biesma9 showed that cranial irradiation in these patients is associated with longer survival.
Diplopia can also occur when MM involves extraocular muscles. Saffra et al13 presented a known case of MM who had already undergone spinal radiation, followed by chemotherapy and stem-cell transplantation. The patient was 3 months into her clinical disease remission when she developed diplopia and proptosis. However, MRI showed eccentric enlargement of the right lateral rectus muscle with an intramuscular mass as the underlying cause of patient’s presentation.
Although epidemiologic studies have shown higher risk of lymphoproliferative diseases in patients with sarcoidosis,14 development of MM in patients with sarcoidosis is limited to case reports.15 Different explanations for the association between these two diseases have been proposed. One possible answer is that the uninterrupted state of inflammatory stimulation in sarcoidosis not only induces polyclonal hypergammaglobulinaemia but also prolongs B cell and plasma cell half-life. This process can predispose genetic mutation and ultimately haematological malignancy.16 17 Even though MM is the primary malignancy of B cells, abnormality of T cells has also been observed in this disease. Th17 and Treg cells have been another suggested linkage between sarcoidosis and MM.18 But still these explanations remain uncertain and require further investigations.
Learning points.
Critical need to enhance the awareness of multiple myeloma and associated conditions within medical communities due to increasing worldwide incidence of multiple myeloma.
Multiple myeloma can present with cranial nerve palsy due to either base of skull lytic lesions causing nerve impingement or leptomeningeal involvement or metastatic intracranial mass lesion.
Plasmacytoma is a solid tumour with identical pathology to multiple myeloma and if involves the base of skull, it can compromise the cranial nerves course.
Keeping high suspicion and looking at the whole clinical and paraclinical picture can help to reduce the delay before diagnosing multiple myeloma and starting the treatment.
There is a higher risk of lymphoproliferative diseases in patients with sarcoidosis. However, the mechanism of association is not clearly defined yet.
Footnotes
Contributors: OY, JK, IS and HAS all contributed towards fact findings, investigating the patient, proofreading and discussing other similar literature. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kariyawasan CC, Hughes DA, Jayatillake MM, et al. Multiple myeloma: causes and consequences of delay in diagnosis. QJM 2007;100:635–40. 10.1093/qjmed/hcm077 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.Cowan AJ, Allen C, Barac A, et al. Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol 2018;4:1221–7. 10.1001/jamaoncol.2018.2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman NB, Puthenpura V, Mischell S, et al. Hypoglossal nerve mononeuropathy as the first presenting symptom of progressing multiple myeloma. World J Oncol 2017;8:15–17. 10.14740/wjon1000w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalwani N, Remenschneider AK, Faquin W, et al. Plasmacytoma of the clivus presenting as bilateral sixth nerve palsy. J Neurol Surg Rep 2015;76:e156–9. 10.1055/s-0035-1554930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaino D, Rufa A, Gozzetti A, et al. Sixth nerve and superior division of third nerve palsy due to intracranial extension of multiple myeloma. A diagnostic challenge and differential diagnosis. Neurol Sci 2018;39:593–4. 10.1007/s10072-017-3167-3 [DOI] [PubMed] [Google Scholar]
- 7.Terushkin V, Stern BJ, Judson MA, et al. Neurosarcoidosis: presentations and management. Neurologist 2010;16:2–15. 10.1097/NRL.0b013e3181c92a72 [DOI] [PubMed] [Google Scholar]
- 8.Pak N, Shakki Katouli F, Radmard AR, et al. Multiple cranial nerve palsy concomitant with leptomeningeal involvement in multiple myeloma: a case report and review of literature. Int J Hematol Oncol Stem Cell Res 2018;12:8–13. [PMC free article] [PubMed] [Google Scholar]
- 9.Nieuwenhuizen L, Biesma DH. Central nervous system myelomatosis: review of the literature. Eur J Haematol 2008;80:1–9. 10.1111/j.1600-0609.2007.00956.x [DOI] [PubMed] [Google Scholar]
- 10.Terpos E, Ntanasis-Stathopoulos I, Gavriatopoulou M, et al. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J 2018;8:7. 10.1038/s41408-017-0037-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hameed A, Brady JJ, Dowling P, et al. Bone disease in multiple myeloma: pathophysiology and management. Cancer Growth Metastasis 2014;7:CGM.S16817. 10.4137/CGM.S16817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yellu MR, Engel JM, Ghose A, et al. Overview of recent trends in diagnosis and management of leptomeningeal multiple myeloma. Hematol Oncol 2016;34:2–3. 10.1002/hon.2185 [DOI] [PubMed] [Google Scholar]
- 13.Saffra N, Gorgani F, Panasci D, et al. Diplopia and proptosis due to isolated lateral rectus plasmacytoma in a patient with multiple myeloma. BMJ Case Rep 2019;12. 10.1136/bcr-2018-229178. [Epub ahead of print: 08 Jul 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boffetta P, Rabkin CS, Gridley G. A cohort study of cancer among sarcoidosis patients. Int J Cancer 2009;124:2697–700. 10.1002/ijc.24261 [DOI] [PubMed] [Google Scholar]
- 15.Tiago Serra J, Martinho A, Paixao Duarte F, et al. Sarcoidosis and multiple myeloma: a case report and literature review. Case Rep Hematol 2019;2019:4586265. 10.1155/2019/4586265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sen F, Mann KP, Medeiros LJ. Multiple myeloma in association with sarcoidosis. Arch Pathol Lab Med 2002;126:365–8. [DOI] [PubMed] [Google Scholar]
- 17.Dachs R, Horn A, Koornhof H, et al. Double pathology, sarcoidosis associated with multiple myeloma: a case report. J Bone Oncol 2014;3:61–5. 10.1016/j.jbo.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loke WSJ, Herbert C, Thomas PS. Sarcoidosis: immunopathogenesis and immunological markers. Int J Chronic Dis 2013;2013:1–13. 10.1155/2013/928601 [DOI] [PMC free article] [PubMed] [Google Scholar]






