Abstract
Proliferative lesions of the Brunner’s glands are uncommonly encountered lesions of the small intestine, originating from the deeply seated mucosal and submucosal Brunner’s glands, mainly in the duodenum. The vast majorities of these lesions are benign and include Brunner’s glands hyperplasia (adenomas/nodules) and hamartomas. The etiology and pathogenesis of these lesions are not fully understood, and the diagnosis can sometimes be challenging. We report a case of Brunner’s gland hamartoma in a 57-year-old man who presented with chronic dyspepsia, hematemesis and weight loss. Endoscopic and radiological investigations show a submucosal polypoid lesion at the first part of the duodenum. Routine endoscopic biopsies demonstrated normal duodenal mucosa. The lesion considered endoscopically unresectable and was surgically resected. Frozen section examination and intraoperative consultation showed unremarkable duodenal mucosa and histologically bland Brunner’s glands.
Keywords: Brunner's gland hamartoma, adenoma, hyperplasia, duodenal polyps, frozen section
INTRODUCTION
Brunner's glands (BGs) are zymogens and mucin-secreting acinar glands located in the deep mucosa and submucosal, mainly in the first and second parts of the duodenum, to less extent in the jejunum and rarely in the distal ileum [1]. These glands develop during the 13–14 week of gestation and are functionally and structurally similar to gastric pyloric glands. In the duodenum, the concentration of the BGs decreases with advancing age [2]. Proliferative lesions of the BGs include hyperplasia, adenoma (BGs nodule), hamartoma (BGH) and extremely rarely adenocarcinoma [3]. Benign proliferative lesions of the BG account for 5–10% of all benign duodenal masses and less than 1% of all gastrointestinal tumors [4]. These lesions are often identified incidentally during endoscopy, can be intraluminal or polypoid/exophytic, sessile or pedunculated and can be treated by endoscopic or surgical resection [5]. The diagnosis of BGs proliferative lesions is usually straightforward. However, recognizing proliferative lesions of the BGs can be challenging in a frozen section examination and may potentially alter an intraoperative clinical decision. Here, we report a case of BGH that caused partial pylorus obstruction and discuss the diagnostic challenges of this lesion.
CASE REPORT
A 57-year-old man was referred because of 4 months history of dyspepsia, 4 kg weight loss and one episode of coffee emesis. Physical examination was unremarkable. Laboratory investigations were within normal limits. Computed tomography (CT) demonstrated a round soft tissue density lesion in the proximal duodenum, measuring 2 × 4 cm (Fig. 1a and b).
Figure 1.
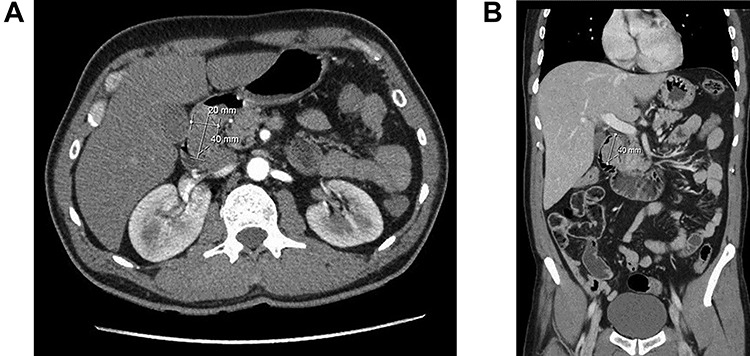
Computed tomography of duodenal Brunner’s gland hamartoma. (a) Axial plane. (b) Coronal plane.
Upper gastrointestinal endoscopic examination revealed a polypoid submucosal lesion in the first part of the duodenum (Fig. 2a and b). The mass was freely mobile, intermittently projected, moving back and forth through the pylorus causing intermittent obstruction. Endoscopic ultrasound revealed hypoechoic submucosal lesion measuring 2.9 × 2.8 cm (Fig. 3).
Figure 2.
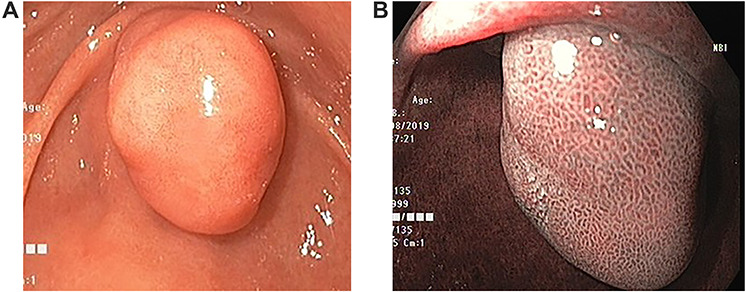
Endoscopic images. (a) Large duodenal submucosal lesion protruding through the pylorus to gastric antrum. (b) Narrow Band Imaging with normal tubulovillous mucosal patterns with regular vessels.
Figure 3.
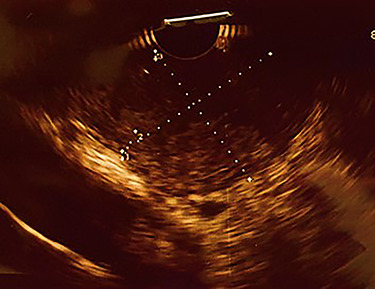
Endoscopic ultrasound, showing heterogeneous hypoechoic subepithelial lesion.
Endoscopic polypectomy to remove the mass was not attempted due to its size and location. Histological examination of multiple endoscopic biopsies was unremarkable. Thus, the patient underwent laparoscopic transduodenal exploration and excision without any complications. Frozen section (FS) examination demonstrated submucosal, nodular glandular proliferation with mild architectural distortion and focally admixed with scattered irregularly shaped duct-like structures. Focal equivocal mild cytonuclear atypia is noted in BGs and ductal structures. Malignancy could not be excluded and a definite diagnosis was deferred.
Grossly, the mass was tan brown, firm polypoid with a stalk and measured 4 × 2.5 × 2 cm. The external surface was delicately trabeculated but showed no papillary configurations. Serial sectioning of the mass showed tan, firm, solid myxoid cut surfaces with no evidence of hemorrhage or necrosis. The lesion was well defined and showed no ulceration of the overlying mucosa and no evidence of infiltrative growth pattern. Light microscopic analysis showed well-defined submucosal, polypoid proliferative BGs lesion with morphological features of BG hamartoma (Fig. 4a and b). It consisted of variably sized nodules of tightly packed bland BGs, separated by fibromuscular septa of variable thickness. The BGs were lined by a single layer of bland cuboidal epithelial cells that had small, basely located normochromic, round nuclei, indistinct nucleoli, moderate amount of clear to light eosinophilic cytoplasm and delicate cell membranes. No mitotic figures were seen. Scattered, irregularly shaped duct-like structures with no cytonuclear atypia were identified (Fig. 4b). However, no infiltrative growth pattern or extension of the lesion into the muscularis propria was seen. Focally, the lesion extended into and effaced the overlying duodenal mucosa (Fig. 4c).
Figure 4.
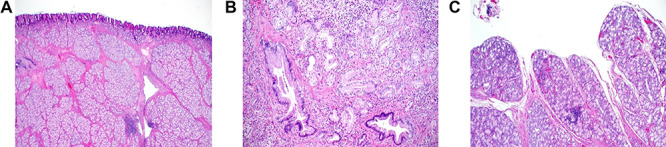
Histopathology of Brunner’s gland hamartoma. (a) The submucosal lesion consisted of lobules of proliferating bland Brunner’s glands, separated by fibromuscular septa; scattered lymphoid follicles were evident (Hematoxylin and Eosin × 100). (b) Irregularly-shaped ductal structures were present, mainly at the deep aspect of the lesion (Hematoxylin and Eosin × 400). (c) Focal papillary architecture was evident on the surface of the lesion (hematoxylin and eosin × 200).
DISCUSSION
Proliferative lesions of the BGs are uncommon small intestinal lesions, encountered primarily in the proximal duodenum and account for approximately 5–10% of all duodenal masses and less than 1% of all gastrointestinal tumors [4]. These lesions include hyperplasia/adenoma (nodule/polyp), hamartoma (BGH) and extremely rarely adenocarcinoma [3]. The histopathological distinction between these lesions is arbitrary, and there is a substantial degree of overlapping and interchangeability among the pathological nomenclature of BGs hyperplastic and hamartomatous lesions. Thus, considerable confusion and overlapping in the diagnosis exist and accurate estimation of the prevalence of these lesions is difficult. Generally, BG hyperplastic polyps are small (0.5–2.0 cm), asymptomatic, can be multiple and discovered incidentally during endoscopic examination, while BGH are usually solitary and larger (>2 cm) [5].
The etiology and pathogenesis of BG hyperplastic and hamartomatous lesions are not fully understood. The suggested causes are those associated with chronic local irritation, hyperchlorhydria, H. pylori infection, and pancreatic exocrine insufficiency [4]. Brunner’s gland proliferative lesions can present at any age, but commonly observed between the ages of 50 and 60 years and affect both sexes equally [6]. While small hyperplastic lesions are asymptomatic, large BGH can have a diverse clinical presentation and patients with BGH can present with vomiting, epigastric pain, gastrointestinal tract bleeding, intestinal intussusception or obstruction, pancreatitis and obstructive jaundice [1, 7].
Histopathological distinction between hyperplastic and hamartomatous lesions of BGs is blurred and both lesions tend to be submucosal polypoidal growth of excessive BGs. In our opinion, the presence of fibromuscular and adipose tissue components, lymphoid aggregates, cystic dilatation of BGs and ductal and pancreatic heterotopic elements renders the lesion hamartomatous. Pathological diagnosis of these lesions by a routine endoscopic biopsy is difficult due to their submucosal nature, and they should be included in the differential diagnosis of any submucosal mass, which includes duplication cyst, leiomyoma, gastrointestinal stromal tumors, neuroendocrine tumors and lymphoma [8].
Frozen section diagnosis of BGH in certainty can be difficult in some cases as an exclusion rare case of adenocarcinoma arising from BGs requires a histopathological analysis of the entire lesion [3]. Thus, caution must be practiced during FS examination, especially that dysplasia was previously reported in BGH and can be easily overlooked [9].
The vast majority of Brunner’s gland hyperplasia and BGH are asymptomatic, incidentally discovered and treated conservatively because their neoplastic potential is low. However, large or symptomatic lesions should be removed. Endoscopic resection is preferred for accessible and pedunculated lesions. If endoscopy method fail or if there is a suspicion of malignancy, surgical removal by open or laparoscopic transduodenal exploration and excision, which is proven to be safe, effective and cause less adverse effect and complication [10].
AUTHOR CONTRIBUTIONS
All authors contributed equally to this article.
Contributor Information
Mohammed A Bakir, College of Medicine, Alfaisal University, Riyadh, Saudi Arabia.
Mohammed Y AlYousef, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
Fahad I Alsohaibani, Department of Medicine, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia.
Khaled O Alsaad, Department of Pathology and Laboratory Medicine, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia.
Conflict of interest statement
The authors declared no potential conflict of interest with respect to the research, authorship and/or publication of this article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Ethical approval
Our institution does not require ethical approval for reporting individual cases or case series.
REFERENCES
- 1. Henken EM, Forouhar F. Hamartoma of Brunner's gland causing partial obstruction of the ileum. J Can Assoc Radiol 1983;34:73–4. [PubMed] [Google Scholar]
- 2. Fuller JW, Maj MC, Cruse CW, Williams JW. Hyperplasia of Brunner's glands of the duodenum. Am Surg 1997;43:246–50. [PubMed] [Google Scholar]
- 3. Mochizuki T, Fujikuni N, Nakadoi K, Nakahara M, Tanabe K, Yonehara S, et al. . Adenocarcinoma of the duodenum arising from Brunner's gland resected by partial duodenectomy: a case report. Surg Case Rep 2019;5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Destek S, Gul VO. Brunner's gland hyperplasias and hamartomas in association with helicobacter pylori. Can J Gastroenterol Hepatol 2019;2019:6340565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yi L, Cheng Z, Qiu H, Yang J, Wang T, Liu K. A giant Brunner's gland hamartoma being treated as a pedunculated polyp: a case report. BMC Gastroenterol 2019;19:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levine JA, Burgart LI, Batts KE, Wang KK. Brunner's gland hamartomas: clinical presentation and pathological features of 27 cases. Am J Gastroenterol 1995;90:290–4. [PubMed] [Google Scholar]
- 7. Rieth DG, Abbott GF, Gray G. Duodenal intussusception secondary to Brunner's gland hamartoma. A case report. Gastrointest Radiol 1977;2:13–6. [DOI] [PubMed] [Google Scholar]
- 8. Walden DT, Marcon NE. Endoscopic injection and polypectomy for bleeding Brunner's gland hamartoma: case report and expanded literature review. Gastrointest Endosc 1998;47:403–7. [DOI] [PubMed] [Google Scholar]
- 9. Rath MM, Mohapatra D, Mohanty SK, Shantisudha S. Brunner's gland hamartoma with dysplasia, presenting as multiple duodenal polyps: an unexplored entity with literature review. Indian J Pathol Microbiol 2019;62:290–2. [DOI] [PubMed] [Google Scholar]
- 10. Khawaja HT, Deakin M, Colin-Jones DG. Endoscopic removal of a large ulcerated Brunner's gland adenoma. Endoscopy 1986;18:199–201. [DOI] [PubMed] [Google Scholar]


