Recently, three back-to-back published randomized clinical trials provided convincing results for the novel lipid-lowering agent Inclisiran. Inclisiran is a small interfering RNA (siRNA) that inhibits the hepatic translation proprotein convertase subtilisin-kexin type 9 (PCSK9), thereby upregulating the number of LDL-receptors on the hepatocytes. In the ORION-9 trial, 482 heterozygous familial hypercholesterolaemia (FH) patients were randomized to either 300 mg inclisiran sodium or matching placebo administered at baseline, 3 months later and then every 6 months for a total of four doses and showed a mean placebo-adjusted LDL-C reduction of 47.9% at the primary efficacy timepoint (Day 510) and a time-averaged LDL-C reduction of 44.3% over the 18-month trial.1 The ORION-10 and 11 had a similar design but included 1561 patients with cardiovascular disease (CVD) in the ORION-10 trial and 1617 patients with either cardiovascular disease or risk-equivalent disease in ORION-11 (type 2 diabetes, heterozygous FH, or 10-year 20% risk in the Framingham risk score) (Figure 1).2 Both trials showed an LDL-C reduction of at least 50% at the primary efficacy timepoint of Day 510 and a mean time-averaged LDL-C reduction of about 50% for the Inclisiran groups compared to placebo. In all three trials, Inclisiran reduced plasma PCSK9-levels with approximately 80% without any indication that this reduction attenuated over the duration of the trials. Moreover, Inclisiran also significantly lowered total cholesterol, non-high-density lipoprotein cholesterol (non-HDL-C), apolipoprotein B, and triglycerides and was associated with an 18.6–25.6% reduction in lipoprotein(a) [LP(a)] levels. HDL-C levels increased in the Inclisiran groups, but no differences in C-reactive protein (CRP) levels were found. Generally, Inclisiran had a favourable safety and tolerability profile—adverse events at the injection site (such as redness, bruising, or swelling) were more common in the inclisiran group than in the placebo group, but most were mild and none were severe or persistent.
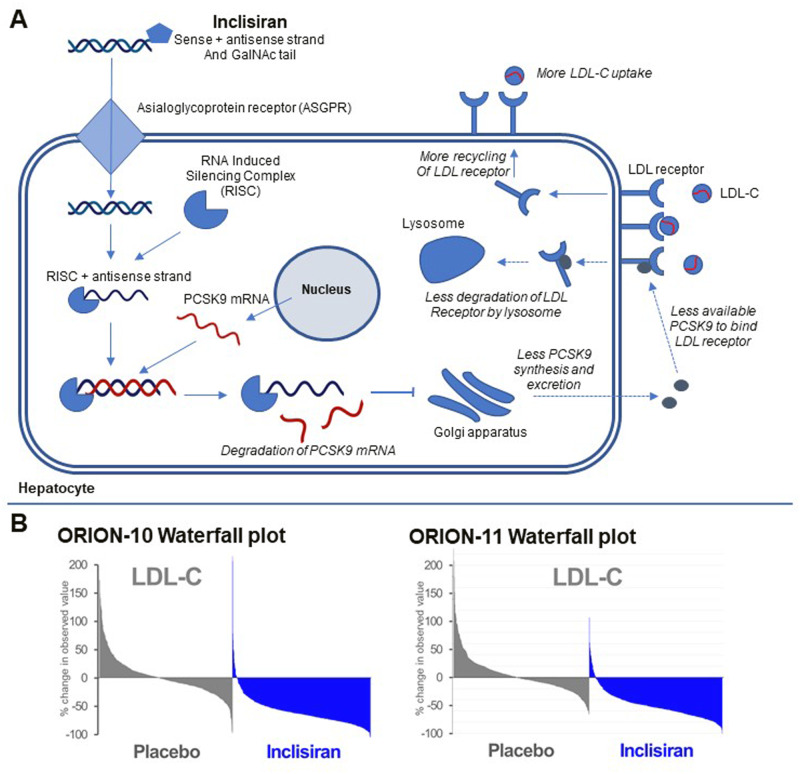
Figure 1.
(A) Simplified overview of mechanism of action by Inclisiran. Inclisiran is delivered to the hepatocyte through the asialoglycoprotein receptor (ASGPR). Its antisense strand then binds to the RNA Induced Silencing complex (RISC). The combination of RISC and the antisense stand then binds PCSK9 messenger RNA (mRNA), leading to degradation of PCSK9 mRNA and less PCSK9 protein synthesis. PCSK9 directs LDL receptor (LDLR) for degradation by the lysosome. Due to less PCSK9 protein, more LDLR can be recycled to the hepatic membrane for LDL-C uptake. (B) Waterfall plots for the ORION-10 and ORION-11 trials (with permission). These plots show the change in LDL-C between baseline and day 510 for each patient. Patients are ordered from the patients with the highest increase in LDL-C to the patient with the highest decrease in LDL-C.
These results mark an important milestone in both the development of lipid-lowering therapy as well as in the development of siRNA therapies. While siRNA therapies are currently approved for a rare disease, inclisiran is the first siRNA that has the potential to be used in the wide prevention of a common disease, atherosclerosis.
In clinical practice, >70% of patients with established atherosclerotic cardiovascular disease do not reach an LDL-C < 70 mg/dL. Poor adherence to currently available therapies is an important contributing factor and has been shown to be associated with an increased risk of CVD.3 Inclisiran’s infrequent dosing regimen may contribute to higher adherence—even complete adherence if inclisiran is administered by healthcare providers. In addition to the benefit of durability which enables an infrequent dosing regimen, the LDL-C reductions produced by inclisiran are substantial and similar to those of high-intensity statins—combining these treatments should contribute to substantial increase in the percentage of patients achieving their guideline-recommended LDL-C levels.4 Although these trials support the clinical application of inclisiran in hypercholesterolaemic patients, there are still open ends in research on PCSK9 per se and also on intra- or extracellular PCSK9 inhibition.
First, inclisiran inhibits the synthesis of PCSK9 within hepatocytes, as opposed to the extracellular inhibition by the currently approved PCSK9 monoclonal antibodies. Although the ORION trials show an effect on LDL-C and other lipids and lipoproteins that is similar to those seen with monoclonal antibodies, there is some evidence that PCSK9 also impacts lipid and lipoprotein metabolism through an intracellular mechanism.5 It is to be determined whether inclisiran exerts some of its lipid-lowering effects by modulating these intracellular pathways.
Moreover, even with the use of lipid-lowering therapy, a substantial residual risk on cardiovascular disease remains. This residual risk is thought to be caused by a combination of dyslipidaemia, dysglycaemia, hypertension, procoagulant status, and inflammation. Statins are shown to be effective in patients with a heightened inflammatory state as measured by increased hsCRP levels,6 and lower these levels by 37%—although it has not been confirmed that statin-induced CRP reductions contribute to their clinical effects beyond LDL-C reduction. Although PCSK9 inhibition does not lower CRP levels in clinical trials, including in the ORION trials,1,2,7pre-clinical studies provided substantial evidence for a connection between PCSK9 and inflammation. This has already been the subject of many reviews.7,8 Among others, pro-inflammatory molecules such as hepatocyte nuclear factor-1a, lipopolysaccharide, and tumour necrosis factor alpha are shown to induce PCSK9 in various cell lines.8 In addition, PCSK9 is also expressed in atherosclerotic plaques and in vascular areas with low shear stress, mainly in vascular smooth muscle cells (VSMCs). PCSK9 is associated with atherosclerotic plaque size and with apoptosis of these VSMCs, as well as with neo-intima proliferation.7 In patients with FH, PCSK9 inhibitors reduced the inflammatory phenotype of monocytes without a change in CRP.9 Furthermore, PCSK9 is reported to regulate scavenger receptor expression and plays a role in oxidized LDL-C uptake through a feedback loop with LOX-1, thereby contributing to foam cell formation.7 Finally, a PCSK9 loss-of-function variant was associated with beneficial clinical outcome in patients with septic shock, further providing evidence for a role of PCSK9 in inflammation.8 In summary, future studies could characterize the specific role of PCSK9 in inflammation.
Studies with other therapies that lower LDL-C by upregulating the LDL receptor have shown discrepant effects on the incidence of type 2 diabetes (T2D). Although statins are shown to increase the risk of T2D,10 a meta-analysis of 39 randomized controlled trials (RCTs) with either the PCSK9 inhibitor evolocumab or alirocumab did not show an increase in the risk of T2D.11 However, Mendelian Randomization demonstrated earlier that LDL-C reduction due to genetic variants in the PCSK9 locus did increase the risk of T2D.12 The exact mechanism through which statins and PCSK9 inhibitors could increase the risk for T2D is not clear, but it is tempting to speculate that upregulation of the LDL receptor on beta-cells in the pancreas might lead to lipotoxicity and premature cell death.
Finally, Inclisiran is an agent that makes use of a GalNAc-tail to ensure its hepatic delivery through the asialoglycoprotein receptor (ASGPR).5 ASGPR is a transmembrane protein that consists of two subunits, ASGR1 and ASGR2, and is mainly expressed by hepatocytes. ASGR1 expression was shown to be directly correlated with protein levels, which could impact drug-delivery through ASGPR.13 However, pre-clinical models with up to 50% reduced ASGPR receptor expression showed no impairment in the uptake of GalNAc-siRNA conjugates, which may explain the homogeneous response to inclisiran in the ORION trials.14 The subunit ASGR1 was shown to be the most critically involved subunit. Human models using variations in ASGR1 could be used to assess the drug-delivery capacity of ASGPR.
In summary, the recent publications of three RCTs investigating inclisiran reaffirm the efficacy of this compound in reducing LDL-C and show that its long therapeutic half-life comes with a favourable side-effect profile. Here, we highlighted some of the questions and opportunities for further research, including the effects of intracellular inhibition of PCSK9; the impact of inflammation on CVD and the role of PCSK9 specifically; the uncertain connection between PCSK9 inhibition and type 2 diabetes; and the association between ASGPR protein function and drug-delivery efficacy for Inclisiran and other GalNAc-linked compounds.
Conflict of interest: A.J.C. has nothing to disclose. J.J.P.K., has received consulting fees from Akcea Therapeutics, AstraZeneca, CiVi Biopharma, Corvidia Therapeutics, CSL Behring, Daiichi Sankyo, Draupnir Bio, Esperion, Gemphire Therapeutics, Madrigal Pharmaceuticals, Matinas BioPharma, NorthSea Therapeutics, Novo Nordisk, Novartis, Regeneron Pharamaceuticals, REGENXBIO, Staten Biotechnology, and 89bio.
Authors

Biography: Dr. Arjen Cupido is a physician and a PhD candidate in cardiovascular genetics at the Academic Medical Center, University of Amsterdam. After his graduation with a double bachelor’s in Interdisciplinary Sciences and Medicine, he received his medical training at the Academic Medical Center, University of Amsterdam. His research interests include the use of Big Data and large-scale genetics for risk stratification and therapeutic target identification and validation. By combining basal and clinical skills, he aims to contribute to the field of Precision Medicine. In 2018, he received the AMC Young Talent grant to spend time at the University of Cambridge, where he received training in genetics and Mendelian Randomization. He is prospected to further his studies in the clinical application of large-scale genetics and -omics data at the University of California, Los Angeles in the fall of 2020.

Biography: Dr. John Kastelein is Emeritus Professor at the Academic Medical Center, University of Amsterdam with a research focus in genetics of cardiovascular disease. After his medical degree and his specialty training in internal medicine in Amsterdam, he received training in medical genetics, lipidology, and molecular biology at the University of British Columbia. He is well known for the extreme genetics approach, where the study of rare human disorders associated with premature coronary disease can be used in understanding the aetiology and pathophysiology of heart disease in general. In his research efforts, he identified and validated numerous therapeutic targets and he has founded several companies focusing on the development of therapeutic agents. He has directed over 55 postdoctoral theses and published over 1200 papers in peer reviewed journals, including Nature Genetics, The Lancet, JAMA, Circulation, and the New England Journal of Medicine. Prof. Kastelein has received the 2014 Anitschkov Prize from the European Atherosclerosis Society and the 2014 Huibregtsenprize for best academic researcher in the Netherlands. He also received the 2010 Lifetime Achievement Award from the Dutch Heart foundation and the 2010 ZonMW parel prize for his research. Nowadays, Prof. Kastelein is an operating partner at Forbion Capital Management and he is acting chief medical officer of Staten Biotechnology Inc. He also holds a board position at NorthSea Therapeutics.
References
- 1. Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med2020;382:1520–1530. [DOI] [PubMed] [Google Scholar]
- 2. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ, Kastelein JJP.. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020;382:1507–1519. [DOI] [PubMed] [Google Scholar]
- 3. Perreault S, Dragomir A, Blais L, Bérard A, Lalonde L, White M, Pilon D.. Impact of better adherence to statin agents in the primary prevention of coronary artery disease. Eur J Clin Pharmacol 2009;65:1013–1024. [DOI] [PubMed] [Google Scholar]
- 4. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J2020;doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed]
- 5. Nishikido T, Ray KK.. Inclisiran for the treatment of dyslipidemia. Expert Opin Investig Drugs 2018;27:287–294. [DOI] [PubMed] [Google Scholar]
- 6. Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ.. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 7. Ding Z, Pothineni NVK, Goel A, Lüscher TF, Mehta JL.. PCSK9 and inflammation: role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc Res 2020;116:908–915. [DOI] [PubMed] [Google Scholar]
- 8. Tuñón J, Badimón L, Bochaton-Piallat M-L, Cariou B, Daemen MJ, Egido J, Evans PC, Hoefer IE, Ketelhuth DFJ, Lutgens E, Matter CM, Monaco C, Steffens S, Stroes E, Vindis C, Weber C, Bäck M.. Identifying the anti-inflammatory response to lipid lowering therapy: a position paper from the working group on atherosclerosis and vascular biology of the European Society of Cardiology. Cardiovasc Res 2019;115:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernelot Moens SJ, Neele AE, Kroon J, van der Valk FM, Van den Bossche J, Hoeksema MA, Hoogeveen RM, Schnitzler JG, Baccara-Dinet MT, Manvelian G, de Winther MPJ, Stroes ESG.. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J 2017; 8:1584–1593. [DOI] [PubMed] [Google Scholar]
- 10. Noto D, Giammanco A, Barbagallo CM, Cefalù AB, Averna MR.. Anti-PCSK9 treatment: is ultra-low low-density lipoprotein cholesterol always good? Cardiovasc Res 2018;114:1595–1604. [DOI] [PubMed] [Google Scholar]
- 11. Guedeney P, Giustino G, Sorrentino S, et al. Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J 2019;doi: 10.1093/eurheartj/ehz430. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt AF, Swerdlow DI, Holmes MV, et al. PCSK9 genetic variants and risk of type 2 diabetes: a Mendelian randomisation study Lancet Diabetes Endocrinol 2017;5:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Witzigmann D, Quagliata L, Schenk SH, Quintavalle C, Terracciano LM, Huwyler J.. Variable asialoglycoprotein receptor 1 expression in liver disease: implications for therapeutic intervention. Hepatol Res 2016;46:686–696. [DOI] [PubMed] [Google Scholar]
- 14. Willoughby JLS, Chan A, Sehgal A, Butler JS, Nair JK, Racie T, Shulga-Morskaya S, Nguyen T, Qian K, Yucius K, Charisse K, van Berkel TJC, Manoharan M, Rajeev KG, Maier MA, Jadhav V, Zimmermann TS.. Evaluation of GalNAc-siRNA conjugate activity in pre-clinical animal models with reduced asialoglycoprotein receptor expression. Mol Ther 2018;26:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]