Abstract
Aims
Cardiovascular disease remains the greatest cause of mortality in Americans over 65. The stretch-activated transient receptor potential vanilloid-4 (TRPV4) ion channel is expressed in cardiomyocytes of the aged heart. This investigation tests the hypothesis that TRPV4 alters Ca2+ handling and cardiac function in response to increased ventricular preload and cardiomyocyte stretch.
Methods and results
Left ventricular maximal pressure (PMax) was monitored in isolated working hearts of Aged (24–27 months) mice following preload elevation from 5 to 20mmHg, with and without TRPV4 antagonist HC067047 (HC, 1 µmol/L). In preload responsive hearts, PMax prior to and immediately following preload elevation (i.e. Frank–Starling response) was similar between Aged and Aged+HC. Within 1 min following preload elevation, Aged hearts demonstrated secondary PMax augmentation (Aged>Aged+HC) suggesting a role for stretch-activated TRPV4 in cardiac hypercontractility. However, after 20 min at 20 mmHg Aged exhibited depressed PMax (Aged<Aged+HC) suggestive of TRPV4-dependent contractile dysfunction with sustained stretch. To examine stretch-induced Ca2+ homeostasis at the single-cell level, isolated cardiomyocytes were stretched 10–15% of slack length while measuring intracellular Ca2+ with fura-2. Uniaxial longitudinal stretch increased intracellular Ca2+ levels and triggered Ca2+ overload and terminal cellular contracture in Aged, but not Aged+HC. Preload elevation in hearts of young/middle-age (3–12 months) mice produced an initial PMax increase (Frank–Starling response) without secondary PMax augmentation, and cardiomyocyte stretch did not affect intracellular Ca2+ levels. Hearts of transgenic mice with cardiac-specific TRPV4 expression exhibited PMax similar to 3- to 12-month control mice prior to and immediately following preload elevation but displayed secondary PMax augmentation. Cardiomyocytes of mice with transgenic TRPV4 expression were highly sensitive to mechanical stimulation and exhibited elevated Ca2+ levels, Ca2+ overload, and terminal contracture upon cellular attachment and stretch.
Conclusion
TRPV4 contributes to a stretch-induced increase in cardiomyocyte Ca2+ and cardiac hypercontractility, yet sustained stretch leads to cardiomyocyte Ca2+ overload and contractile dysfunction in the aged heart.
Keywords: Calcium, Cardiomyocyte, Cardiac, Excitation–contraction coupling, TRP channel
Time for primary review: 22 days
1. Introduction
The ageing process fundamentally changes cardiac excitation–contraction coupling (ECC), causing alterations in cardiac function.1 Cardiomyocyte ECC initiates when sarcolemmal depolarization opens L-type Ca2+ channels (LTCC), triggering Ryanodine Receptor (RyR2) release of sarcoplasmic reticulum (SR) sequestered Ca2+ (Ca2+-induced Ca2+ release: CICR).2 Subsequent Ca2+i elevation initiates myofilament force development, cardiomyocyte shortening, and resultant cardiac systole. Cytosolic Ca2+ is removed through sarcoplasmic-endoplasmic reticulum Ca2+ ATPase (SERCA2a)-mediated reseque-stration in the SR and Na+/Ca2+ exchanger (NCX)-mediated extrusion at the sarcolemma, lowering Ca2+i and leading to reduced myofilament force, cardiomyocyte relaxation, and cardiac diastole.
Ageing associates with modifications to ECC components, including decreased SERCA2a expression3,4 and/or activity,5,6 leading to slower Ca2+ transient decay rates,7 and concomitantly diminished cardiomyocyte relaxation rate.5,6,8 Additionally, excessive RyR2 activity during diastole (SR Ca2+ ‘leak’) contributes to age-dependent increases in resting Ca2+i.9 These age-dependent modifications promote Ca2+ intolerance under conditions of stress and may increase the likelihood of cellular Ca2+ overload-mediated cardiac dysfunction.10,11 The transient receptor potential vanilloid-4 (TRPV4) non-selective cation channel (Ca2+:Na+ permeability ratio ∼6:1) has recently been shown to be expressed in cardiomyocytes of the aged heart12 and represents an additional sarcolemmal Ca2+ influx pathway that may contribute to cardiomyocyte Ca2+ overload following stress. Initially described as a cell osmoreceptor sensitive to hypoosmotic stimuli, further work indicates TRPV4 is sensitive to diverse stimuli including mechanical stretch.13
Although ageing associates with cardiac stiffness,14–17 cardiomyocytes within aged hearts exhibit sarcomere lengthening (i.e., stretch) in response to elevated ventricular filling pressures (i.e. increased preload).14 The present work tests the hypothesis that TRPV4 alters cardiomyocyte Ca2+ homeostasis and cardiac function in the aged heart following stretch. This study shows TRPV4 leads to sarcolemmal cation entry and increased levels of Ca2+ following stretch of isolated cardiomyocytes, and augments contractility transiently following preload elevation in hearts of aged mice. However, sustained cardiomyocyte stretch leads to TRPV4-dependent Ca2+ overload behaviour and sustained preload elevation leads to a decline in systolic and diastolic contractile performance in aged hearts. TRPV4 stretch-activation may play an important role in Ca2+ regulation/dysregulation in the aged heart and provide a potential therapeutic target in elderly populations.
2. Methods
2.1 Experimental animals
Male C57BL/6 mice were used at advanced ages of 24–27 months (Aged) and 32–35 months (Senescent). Male and female mice in 3–6 months (Young) and 7–12 months (Middle-age) age ranges were used for transgenic overexpression of TRPV4 in double-transgenic (DTg) MerCreMer × Tg(αMHC-loxp-mCherrySTOP-loxp-TRPV4-1td) mice. Mice were fed tamoxifen (500 mg/kg diet ad libitum for 1–2 weeks, followed by 1- to 2-month recovery on normal diet) to induce transgene expression (DTg:Tam). Successful recombination was confirmed by loss of mCherry fluorescence in isolated hearts (Excitation 510–540 nm, Emission <600 nm, NightSea, Lexington, MA, USA) or in isolated cardiomyocytes (LeicaSP5/MP, Excitation 543 nm, Emission 560–620 nm, see Supplementary material online, Figure S1 for details).12 Male and female mice of the same age ranges were used as controls and included DTg (−tamoxifen), single transgenic (±tamoxifen), and C57BL/6 (±tamoxifen) genotypes (defined collectively as CTL). Hearts were rapidly excised (<30 s) following anaesthesia via intraperitoneal sodium pentobarbital (60 mg/kg) or ketamine/xylazine (80/10 mg/kg) injection. Protocols involving animals were performed in accordance with the Animal Care and Use Committee of the University of Missouri and conform to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes or the current NIH guidelines.
2.2 Solutions
Modified Krebs–Henseleit buffer (KHB) for working heart perfusion contained the following (in mmol/L): 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11.1 Glucose, 0.4 Caprylic Acid, 1 Pyruvate, 0.5 Na EDTA, and 1.8 CaCl2. Physiological Saline Solution (PSS) for superfusion of cardiomyocyte stretch experiments contained the following (in mmol/L): 135 NaCl, 5 KCl, 10 Glucose, 10 HEPES, 1 NaHCO3, 1 MgCl2, and 2 CaCl2. For Mn2+ quench experiments CaCl2 was replaced with 2 mmol/L MnCl2.
2.3 Working heart experiments
Hearts were excised from anaesthetized mice and cannulated via aorta for retrograde coronary perfusion of oxygenated (95% O2/5% CO2) KHB at gravity-fed 60 mmHg afterload and pulmonary vasculature was ligated and incisions made in the right atrium and pulmonary artery. An incision was made in the left atrium and the atrium was cannulated and gravity-perfused with oxygenated KHB using a custom fabricated atrial cannula to control preload in working-heart mode. A 1.2-Fr SciSense pressure catheter was inserted into the left ventricle via the apex and the preparation was allowed to equilibrate at 5 mmHg preload for 10 min at 37°C. Inclusion criteria for working heart preparations at 5 mmHg included stable baseline rate and maximal pressure development (PMax) greater than afterload pressure. Following baseline measurements, the heart was subjected to elevated preload of 20 mmHg. Preliminary studies using an independent cohort of Aged animals revealed both preload responsive and preload non-responsive populations.18 Consequently, for this investigation we utilized initial preload-induced increases in PMax (i.e. Frank–Starling responses) as inclusion criteria for investigation of stretch-dependent responsiveness in Aged hearts (defined as ‘responsive’, PMax-derived group assignments in Supplementary material online, Figure S2). Mice of more advanced age (32–34 months, defined as Senescent) were used for comparison to preload non-responsive groups. For experiments involving transgenic expression of TRPV4, minimal differences in baseline (5 mmHg) or preload-induced (20 mmHg) function were observed between 3- to 6-month (Young) and 7- to 12-month (Middle-age) mice within the DTg:Tam group and also within the CTL group. Therefore, a 3–12 months age range was used to examine the effects of transgenic TRPV4 expression on functional parameters. TRPV4 antagonist HC067047 (HC) was continuously administered at 1 µmol/L before and after preload elevation in designated experiments. One to two seconds of peak PMax was obtained 1 min before preload elevation (5 mmHg), immediately after a ramp elevation (over ∼8 s) in preload (Frank–Starling), and within 1 min following preload elevation (Secondary). In instances where secondary PMax values were ≤ the Frank–Starling response, PMax values were collected at the mean time of peak secondary responses in responsive groups (t = 24 ± 3 s following onset of preload elevation in Aged; t = 17 ± 3 s following onset of preload elevation in DTg:Tam). Preparations with sustained non-steady-state pressure waveforms during the initial minute of preload elevation (Aged: 4/9, Aged+HC: 0/5, CTL: 2/13, DTg:Tam: 5/11) were excluded from analysis due to an inability to accurately quantify PMax. PMax and dP/dt Min were averaged for 1 min following 20-min preload elevation to derive the 20 Min time point.
2.4 Cardiomyocyte stretch experiments
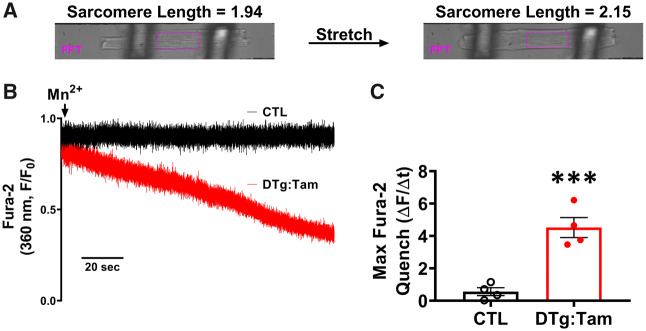
Enzymatically isolated left ventricular cardiomyocyte stretch experiments were performed in a rotating glass-bottomed chamber using an inverted microscope (Olympus, 40× oil-immersion objective) equipped with an IonOptix Myostretcher calcium and contractility acquisition system. Cardiomyocytes were loaded with 5 µmol/L Fura-2/AM for 25 min and washed in 1 mmol/L Ca2+ PSS for 45 min (±1 µmol/L HC067047) before transfer to chamber. Individual cardiomyocytes were attached between microfabricated glass rods coated with MyoTak (IonOptix) and lifted from chamber bottom. Cardiomyocytes were electrically field stimulated (1 Hz) while sarcomere length (Fast Fourier transform) and intracellular Ca2+ (Fura-2 340/380 ratio) were simultaneously monitored at 250 Hz. Following control measurements at slack length (value obtained from each cardiomyocyte prior to attachment), cardiomyocytes were stretched 10–15% of resting sarcomere length over ∼10 s using a variable length controller and held for 600 s or until cardiomyocytes exhibited terminal contracture. Maximum diastolic and systolic Ca2+ levels during stable ECC were monitored at each phase of the experimental protocol. To quantify non-steady state Ca2+ overload behaviour, maximum values observed during the protocol (excluding Ca2+ overload associated with terminal contracture) were also monitored and denoted as ‘Max’. Sarcomere shortening during Ca2+ overload and terminal contracture was not assessed due to non-uniform contractile behaviour which complicated Fast Fourier transform analysis. Manganese quench was performed in unstimulated cells under unattached, attached, or 10–15% stretch conditions. The PSS superfusion solution was replaced with 0 Ca2+, 2 mmol/L Mn2+ PSS and Ca2+-independent fura-2 quench (360 nm excitation, 510–565 nm emission) was assayed for 600 s. Change in fluorescence (ΔF) was assessed over 120 s intervals (Δt) and maximum rate [ΔF/Δt (ΔAU/120 s)] was utilized for analysis. All cardiomyocyte experiments were performed at room temperature (22–25°C).
2.5 Statistical analysis
Data are presented as individual observations and sample means (x̄) ± standard error of the sample mean (SEM). n indicates number of cells sampled from N animals. Cell values (n) were averaged per animal to obtain a single N for statistical testing, with the exception of cell survival studies which were presented and analysed using n. Summary data were analysed using two-tailed Student’s t-test [homoscedastic (F-test of variance not different) or heteroscedastic (F-test of variance significantly different) as appropriate], Mann–Whitney test for non-parametric samples, log-rank (Mantel–Cox) test, or mixed-effects analysis (Holm–Sidak post hoc multiple comparisons test) as appropriate to experimental design and indicated by figure legend. Data were analysed using Microsoft Excel 2016, LabChart 8.1, SigmaPlot 12, and GraphPad 8.02.
3. Results
3.1 Preload-induced secondary hypercontractility in hearts of aged mice
Left intraventricular maximal pressure (PMax) was assayed in isolated working hearts of preload responsive Aged mice during preload elevation from 5 to 20 mmHg (representative traces, Figure 1A). Although preload elevation and the associated Frank–Starling response was complete within ∼8 s, a Secondary phase of PMax augmentation was observed in Aged hearts (Figure 1A and D, Secondary) that peaked 24 ± 3 s following onset of preload elevation. Recent data suggest the mechanosensitive cation channel TRPV4 plays a role in age- and stimulus-dependent cardiac hypercontractility.12 To ascertain the role of TRPV4 in preload-dependent hypercontractility, Aged hearts were pretreated with the TRPV4 small molecule inhibitor HC067047 (Aged+HC). No differences in PMax were observed between Aged and Aged+HC during either the 5 mmHg (Figure 1B) or Frank–Starling (20 mmHg, Figure 1C) periods. However, hearts of Aged exhibited significant Secondary hypercontractility compared to Aged+HC (Figure 1D). Relaxation rates (dP/dt Min) were not significantly different between groups (Supplementary material online, Figure S3). Change-in-value (Δ) analysis calculated from 5 mmHg to Frank–Starling time periods revealed a similar Frank–Starling PMax response between groups (Supplementary material online, Figure S4). However, changes in PMax calculated from Frank–Starling to Secondary periods were present in Aged and absent in Aged+HC (Figure 1E). These results suggest TRPV4 contributes to secondary hypercontractility following acute elevation of preload in hearts of Aged mice.
Figure 1.
TRPV4 inhibition prevents secondary hypercontractility following preload elevation in hearts of Aged mice. (A) Example left intraventricular pressure trace prior to (5 mmHg), during (START to END, denoted by arrows), and following (Frank–Starling and Secondary) preload elevation from 5 to 20 mmHg in an Aged heart. (B–E) Maximum pressure (PMax) prior to (5 mmHg, B) or immediately following (Frank–Starling, C) preload elevation in isolated working hearts of Aged (black closed, N = 5) and Aged treated with the TRPV4 antagonist HC067047 (Aged+HC, 1 µmol/L, green open, N = 5). (D) Secondary PMax responses within 60 s following preload elevation in hearts of Aged and Aged+HC. (E) Change-in-value (Δ) from Frank–Starling to Secondary (ΔSecondary-F/S) in hearts of Aged and Aged+HC (D, *P = 0.0272 vs. Aged, homoscedastic t-test, E, **P = 0.0083 vs. Aged, heteroscedastic t-test).
3.2 Cardiac function following sustained preload elevation in hearts of Aged mice
In contrast to findings at the Secondary (i.e., within seconds) time point, following 20 min of sustained preload elevation (20 min) hearts of Aged mice exhibited decreased PMax (Figure 2A) and slower dP/dt Min (Figure 2B) compared to Aged+HC hearts. These findings demonstrate that while TRPV4 may be initially beneficial to cardiac function, sustained stretch-dependent TRPV4 activation results in cardiac dysfunction. Control experiments were also performed on Senescent mice (32–35 months) for comparison to the sub-population of Aged hearts that were preload non-responsive (Supplementary material online, Figures S2, S5, S6). In addition to being non-responsive to preload elevation (Supplementary material online, Figure S2), these two groups did not exhibit secondary hypercontractility (Supplementary material online, Figure S5) or functional decline following sustained preload elevation (Supplementary material online, Figure S6).
Figure 2.
TRPV4 inhibition improves cardiac function following sustained preload elevation in hearts of Aged mice. PMax (A) and dP/dt Min (B) 20 min following sustained preload elevation in Aged hearts in the absence (black closed, N = 5) and presence (green open, N = 5) of HC067047 (Aged+HC, 1 µmol/L). (A, **P = 0.0049 vs. Aged, heteroscedastic t-test; B, *P = 0.0473 vs. Aged, homoscedastic t-test).
3.3 Stretch-induced cation influx and Ca2+ homeostasis in cardiomyocytes of Aged mice
To isolate the effects of stretch-activated sarcolemmal cation entry at the cellular level, manganese quench of fura-2 fluorescence was examined in cardiomyocytes enzymatically isolated from hearts of Aged mice. Measurements were obtained after attachment to the MyoStretcher cell stretching device and following 10–15% stretch from resting sarcomere length (Figure 3A), in the absence and presence of TRPV4 inhibition with HC067047. Manganese quench rate was minimal prior to cardiomyocyte stretch, and similar between Aged and Aged+HC (Supplementary material online, Figure S7A). However, following stretch an increase in manganese quench rate was observed in Aged but not Aged+HC (Figure 3B and C). The contribution of TRPV4 to cardiomyocyte Ca2+ homeostasis (fura-2 340/380 ratio) was monitored in electrically stimulated (1 Hz) cardiomyocytes of Aged mice prior to and following 10–15% stretch in the absence and presence of HC067047 (Figure 3D). In Aged, there were no changes in diastolic or systolic Ca2+ levels during stable ECC immediately (<10 s) after stretch (Figure 3Di and ii, E and F). However, with sustained stretch, cardiomyocytes of Aged exhibited an elevation in diastolic and systolic Ca2+ (Figure 3E and F, Max), and non-steady-state Ca2+ overload behaviour (Figure 3Diii) which preceded terminal cellular contracture (red TC, time course shown in Figure 3G, summary data in Figure 3H). TRPV4 antagonism with HC067047 prevented stretch-induced changes in diastolic and systolic Ca2+ (Figure 3E and F, Max), as well as the terminal contracture (Figure 3G and H). Cardiomyocyte Ca2+ data therefore implicate TRPV4 mediation of stretch-activated sarcolemmal Ca2+ entry and cellular Ca2+ overload in the aged heart.
Figure 3.
TRPV4 inhibition prevents stretch-induced Ca2+ overload in cardiomyocytes of Aged mice. Transmitted light images (A) and representative traces (B) of Mn2+ quench of Fura-2 fluorescence in stretched cardiomyocytes of Aged hearts in the absence (black) and presence (green) of HC067047 (Aged+HC, 1 µmol/L). Traces normalized to initial fluorescence (F0), arrow indicates Mn2+ addition. (C) Maximum change-in fluorescence values (ΔAU/120 s) post-Mn2+ addition in Aged (black closed, n = 7/N = 5, 11.4 + 0.8% stretch) and Aged+HC (green open, n = 5/N = 5, 11.9 ± 0.6% stretch). (D) Example Fura-2 Ca2+ traces from electrically field stimulated (1 Hz, stimuli denoted by circles) enzymatically isolated cardiomyocytes of Aged under baseline-attached (i), stretched (ii), and pre-overload (iii) conditions (stretch denoted by arrowhead, Aged: 12.0 ± 0.8% stretch, Aged+HC: 12.8 ± 1.0% stretch, TC = terminal contracture). (E and F) Diastolic Ca2+ (E) and peak Ca2+ (F) at attachment or following stretch (stable ECC Ca2+ transients) in cardiomyocytes of Aged (n = 9/N = 7) and Aged+HC (n = 9/N = 8). Non-steady state Ca2+ overload quantified as maximum values during the protocol (Max). (G and H) Cell survival time (G) and time to terminal contracture or end of protocol (H, dashed line at t = 600 s indicates end of protocol) of cardiomyocytes of Aged (n = 12/N = 7) and Aged+HC (n = 10/N = 8). Gray field in (G) indicates variable, non-linear time discontinuity between cardiomyocyte attachment and stretch initiation. Values in C, E, and F are shown per animal (N); values in G and H are shown per cell studied (n) (C, *P = 0.0416 vs. Aged, heteroscedastic t-test; E, ***P = 0.0009 vs. Aged, mixed-effects analysis; F, ***P = 0.0003 vs. Aged, mixed-effects analysis; H, ****P < 0.0001 vs. Aged, homoscedastic t-test).
3.4 Preload-induced secondary hypercontractility in hearts of TRPV4 transgenic mice
To more specifically, address the contribution of TRPV4 to stretch-induced contractile function without the multifactorial ageing phenotype, isolated working hearts of 3- to 12-month mice with tamoxifen inducible TRPV4 overexpression (DTg:Tam) were subjected to elevated, sustained preload while measuring left intraventricular pressure. While 5 mmHg (Figure 4A) and Frank–Starling (Figure 4B) PMax values were not different between CTL and DTg:Tam hearts, DTg:Tam exhibited a Secondary PMax response compared to CTL (Figure 4C). Relaxation rates (dP/dt Min) were not significantly different between groups at 5 mmHg, Frank–Starling, and Secondary phases (Supplementary material online, Figure S8). Change-in-value (Δ) analysis calculated from 5 mmHg to Frank–Starling time periods revealed a similar Frank–Starling PMax response between CTL and DTg:Tam groups (Supplementary material online, Figure S9). Consistent with findings in Aged (Figure 1E), changes in PMax calculated from Frank–Starling to Secondary periods were observed in hearts of DTg:Tam mice (Figure 4D). However, in contrast to findings in Aged (Figure 2), hearts of DTg:Tam mice maintained enhanced cardiac performance with higher PMax and faster dP/dt Min vs. CTL after 20 min of preload elevation (Figure 5A and B). In sum, TRPV4 exerts age-independent initial inotropy followed by age-dependent loss of contractile function with sustained preload elevation.
Figure 4.
Transgenic cardiomyocyte TRPV4 expression leads to secondary hypercontractility following preload elevation. (A–D) PMax in isolated working hearts of CTL (black open, N = 11) or DTg:Tam (red closed, N = 6) mice prior to (5 mmHg, A) or immediately following preload elevation (Frank–Starling, B). (C) Secondary PMax responses within 60 s following preload elevation in hearts of CTL and DTg:Tam mice. (D) Change-in-value (Δ) from Frank–Starling to Secondary (ΔSecondary-F/S) in hearts of CTL and DTg:Tam mice (C, *P = 0.0278 vs. CTL, homoscedastic t-test, D, **P = 0.0086 vs. CTL, homoscedastic t-test).
Figure 5.
Transgenic cardiomyocyte TRPV4 expression leads to sustained systolic and diastolic performance following preload elevation. PMax (A) and dP/dt Min (B) 20 min following sustained preload elevation in hearts of CTL (black open, N = 11) and DTg:Tam (red closed, N = 6) mice (A, **P = 0.0093 vs. CTL, homoscedastic t-test; B, *P = 0.0312 vs. CTL, homoscedastic t-test).
3.5 Stretch-induced cation influx and Ca2+ homeostasis in cardiomyocytes of TRPV4 transgenic mice
Sarcolemmal cation entry was examined using manganese quench of fura-2 in cardiomyocytes enzymatically isolated from hearts of CTL and DTg:Tam mice (Figure 6A and B). Manganese quench rate was minimal in cardiomyocytes of CTL mice, irrespective of mechanical state including post 10–15% cardiomyocyte stretch. On the contrary, cardiomyocytes of DTg:Tam exhibited an increase in quench rate following both mechanical attachment (Supplementary material online, Figure S7B) and following stretch (Figure 6C). Consistent with these findings, cardiomyocyte Ca2+ handling was stable in electrically stimulated (1 Hz) cardiomyocytes of CTL mice prior to attachment (Figure 7D and E), after attachment (Figure 7Ai, D and E), and following mechanical stretch (Figure 7Aii, iii, D and E). Cardiomyocytes of DTg:Tam exhibited stable Ca2+ handling following electrical stimulation (Supplementary material online, Figure S10, Figure 7D and E). However, after mechanical attachment ∼50% of cardiomyocytes of DTg:Tam exhibited Ca2+ overload behaviour (Figure 7Biv, F) and the remainder transitioned into Ca2+ overload and terminal cellular contracture immediately following stretch (Figure 7Cv, F and G). In cardiomyocytes of DTg:Tam, TRPV4 antagonism with HC067047 eliminated attachment-induced cation entry (Supplementary material online, Figure S11), and delayed Ca2+ overload and terminal contracture following stretch (Supplementary material online, Figure S12).
Figure 6.
Transgenic cardiomyocyte TRPV4 expression increases sarcolemmal cation entry. Transmitted light images (A) and representative traces (B) of Mn2+ quench of Fura-2 fluorescence in stretched cardiomyocytes of CTL (black) and DTg:Tam (red) mice. Traces normalized to initial fluorescence (F0), with arrow indicating addition of Mn2+. (C) Maximum change-in fluorescence values (ΔAU/120 s) post-Mn2+ addition in CTL (black open, n = 8/N = 4, 10.9 ± 0.8% stretch) and DTg:Tam (red closed, n = 4/N = 4, 10.1 ± 0.3% stretch) (C, ***P = 0.0010 vs. CTL, homoscedastic t-test).
Figure 7.
Transgenic cardiomyocyte TRPV4 expression leads to stretch-induced Ca2+ overload. (A) Example Fura-2 Ca2+ traces from electrically field stimulated (1 Hz, stimuli denoted by circles) enzymatically isolated cardiomyocytes of CTL (A, black) under baseline-attached (i), stretched (ii), and sustained stretch conditions (iii). (B and C) Example Fura-2 Ca2+ traces of cardiomyocytes of DTg:Tam (B and C, red) exhibiting Ca2+ oscillations following attachment (B, iv) or non-steady state excitation-contraction coupling following electrical field stimulation and stretch (C, v). Cardiomyocytes of DTg:Tam hearts exhibited either post-attachment Ca2+ oscillations and overload (n = 5/9) or immediate stretch-induced Ca2+ overload and terminal contracture (C, v, n = 4/9, TC = terminal contracture). (D and E) Diastolic Ca2+ (D) and peak Ca2+ (E) in unattached, attached, or stretched cardiomyocytes (stable ECC Ca2+ transients) of CTL (black open, n = 4/N = 4, 13.3 ± 1.0% stretch) and DTg:Tam (n = 4/N = 4) mice. Non-steady state Ca2+ overload quantified as maximum values during the protocol (Max). DTg:Tam cells exhibited non-steady-state behaviour immediately post-attachment/stretch and no data are presented for Stable ECC (#). (F and G) Cell survival time (F) and time to terminal contracture or end of protocol (G, dashed line at t = 600 s indicates end of protocol) in CTL (n = 7/N = 4) and DTg:Tam (n = 9/N = 7 in F; n = 4/N = 4 in G due to n = 5 cells exhibiting attachment-induced overload as shown in F). Gray field in (F) indicates variable, non-linear time discontinuity between cardiomyocyte attachment and stretch initiation. Values in D and E are shown per animal (N); values in F and G are shown per cell studied (n) (D, **P = 0.0022 vs. CTL Max, heteroscedastic t-test; E, **P = 0.0071 vs. CTL Max, heteroscedastic t-test; G, ****P < 0.0001 vs. CTL, heteroscedastic t-test).
4. Discussion
4.1 TRPV4 and transient secondary hypercontractility
Roles for transient receptor potential ion channels are rapidly integrating into ventricular myocyte signal transduction and ion homeostasis canon, as cardiac functions for TRPC,19,20 TRPM,21–23 TRPP,24–26 TRPA,27 and TRPV28–30 subfamily channels have been elucidated. The present investigation extends this literature to include TRPV4 and cardiomyocyte stretch, representing a fundamental physiological stimulus of the heart. At the isolated cardiomyocyte level, stretch led to TRPV4-dependent increases in sarcolemmal cation entry, elevation in intracellular Ca2+, and predisposition to cellular Ca2+ overload. At the organ level, increased ventricular preload led to TRPV4-dependent secondary hypercontractility observed within 1 min following preload elevation. This secondary response was therefore kinetically distinct from the Frank–Starling Response (classically defined as the immediate response following preload elevation) and the Anrep/Stress-Stimulated Contractility (SSC)/Slow Force Response (SFR, multiple minutes).31 These data support a novel role for TRPV4 in transient cardiac hypercontractility within 1 min following increased preload, representing a unique kinetic phenomenon in the aged heart.
4.2 TRPV4 and cardiac dysfunction following sustained preload elevation
While hearts of Aged mice exhibited a TRPV4-dependent increase in contractile function early after preload elevation (Aged > Aged+HC, Figure 1D), there was a reversal of the contractile phenotype following sustained preload challenge (Aged < Aged+HC, Figure 2A). In addition, relaxation speed was slower in Aged compared to Aged+HC at the 20 Min time point, suggesting TRPV4 may also contribute to diastolic dysfunction in Aged mice (Figure 2B). Integrated with isolated cardiomyocyte findings, these data argue that increased ventricular preload and cardiomyocyte stretch lead to TRPV4-mediated Ca2+ entry, subsequent increased cardiomyocyte myofilament force production, and enhanced ventricular pressure development. This model is consistent with previous findings of a TRPV4-dependent increase in SR Ca2+ content, RyR Ca2+ release, and Ca2+ transient amplitude during ECC following transient application of mild (∼50 mOsm) hypoosmotic stress to cardiomyocytes of Aged mice.12 However, with sustained stretch, cardiomyocytes of Aged are unable to effectively sequester/extrude additional Ca2+, transitioning into cellular Ca2+ overload (Figure 3D) with associated whole heart systolic (PMax, Figure 2A) and diastolic (dP/dt Min, Figure 2B) dysfunction at 20 min. In contrast, whole hearts of the younger DTg:Tam mice with transgenic TRPV4 expression maintained enhanced systolic (PMax, Figure 5A) and diastolic (dP/dt Min, Figure 5B) function at 20 min compared to hearts of CTL mice. These findings imply age-dependent susceptibility to TRPV4 Ca2+ entry and systolic and diastolic dysfunction. We did observe non-steady-state pressure waveforms in DTg:Tam mice following preload elevation, and this behaviour was severe enough to preclude accurate measurement of steady-state peak pressure development in 5/11 DTg:Tam hearts. These whole heart findings are consistent with isolated cell studies which demonstrated exquisite sensitivity of DTg:Tam cardiomyocytes to attachment to the Myostretcher device. This sensitivity was indicated by the post-attachment rate of Manganese quench (Supplementary material online, Figure S7) as well as by post-attachment non-steady state Ca2+ overload behaviour (Figure 7B–F). It is important to note that the sensitivity to attachment was mediated by TRPV4 as it was prevented by HC067047 (Supplementary material online, Figures S11 and S12) and was absent in cardiomyocytes of CTL mice expressing minimal TRPV4 (Figure 7A, Supplementary material online, Figure S7B).12 A limitation of the present investigation was the inability to rigorously classify and quantify arrhythmia using pressure waveform analysis. Future investigations using electrocardiogram measurements will determine if TRPV4 plays a role in arrhythmogenesis as has been shown for other TRP channels.20,32
4.3 Two-hit hypothesis in the aged heart
Experimental data indicate cardiomyocytes of the aged heart are predisposed to Ca2+ influx-induced Ca2+ overload.11 This predisposition supports the ‘two-hit’ hypothesis wherein two (or more) independent aberrations of cellular homeostasis lead to pathological outcomes.33 Ageing associates with increased reactive oxygen species production, decreased cardiomyocyte energetic capacity,34 impairment of SERCA2a-mediated Ca2+ sequestration,6,35 and enhancement of RyR2 diastolic leak.9 Such factors in the presence of sustained TRPV4 Ca2+ influx may lead to futile Ca2+ cycling, elevated diastolic Ca2+, and diastolic dysfunction (dP/dt Min, Figure 2B) during increased cardiac demand. Such aberrations impair systolic performance due to uncoordinated ECC, slower systolic contraction and delayed relaxation, and limited diastolic filling resulting in decreased systolic performance at the whole organ level over time (Figure 2A). This work shows that TRPV4 mediates initially beneficial Ca2+ elevation which devolves into deleterious excessive Ca2+ load in the aged heart.
4.4 Functional interactions between TRPV4 and other ion channels
The TRP channel superfamily is notable for the prevalence of heterotetramer formation both within and between families.36 TRPV4 forms heterotetrameric channels with other TRP channel members, most notably TRPC1 and TRPP2.37–40 Interestingly, co-assembly of TRPV4 and TRPC1 in cell culture increases both the Ca2+ selectivity and single-channel conductance of the heteromeric channel vs. the respective homomeric channels.41 Should such interactions occur in the ventricular myocardium, TRPV4 would provide a high-flux Ca2+ entry pathway via both homomeric assembly as well as by co-assembling with endogenous low-flux TRP channel isoforms, shifting their channel properties towards greater Ca2+ selectivity and higher Ca2+ flux. Heteromerization of TRPV4 with other TRP channels may also explain why the TRPV4-specific inhibitor HC067047 was remarkably effective at preventing trans-sarcolemmal divalent cation entry in response to stretch (Figure 3C). The contribution of other stretch-sensitive TRP channels (e.g., TRPC family) would be reduced by HC067047 if these subunits functioned in heterotetrameric complexes with TRPV4.40,42 In addition to aforementioned protein–protein interactions, TRPV4 may also functionally couple to Ca2+-activated channels or transporters via local Ca2+-dependent signalling. Prominent candidate channels expressed in the myocardium under normal or diseased states include NCX,20 TRPM4,22,23 RyR,43 and the small-conductance Ca2+-activated potassium channel.44–46 Future work will investigate potential consequences of such coupling in the aged heart.
4.5 Preload responsive and preload non-responsive populations in aged hearts
Previous investigations in aged (24–28 months C57BL/6) mice revealed variability in sarcomere lengthening in response to defined changes in ventricular pressure,14 which manifest as preload responsive and preload non-responsive populations in working heart preparations.18 Such findings allowed for a priori inclusion criteria to be established for hypothesis testing in the present study, which similarly revealed both preload responsive and preload non-responsive populations in an independent cohort of animals (Supplementary material online, Figure S2). Though age-dependent increases in passive and active stiffness are observed owing to factors intrinsic and extrinsic (e.g. interstitial fibrosis47) to the cardiomyocyte,48 the majority of Aged hearts were able to stretch and augment function in response to elevated preload. Non-responsive Aged hearts were qualitatively similar to those of Senescent mice of more advanced age, presumably owing to accelerated progression of the aforementioned factors.
4.6 Limitations and future directions
Working heart preparation: The ex vivo working heart preparation is ideal for the present investigation as it allows for experimental control of left ventricular preload. Nevertheless, it has several limitations. The perfusate Ca2+ concentration of 1.8 mmol/L is higher than free extracellular Ca2+ levels found in vivo and may exacerbate Ca2+ overload and cardiac dysfunction. Crystalloid perfusion solutions have decreased oxygen carrying capacity compared to blood. Time control experiments revealed our experimental preparation was stable under baseline conditions (5 mmHg) for the duration of the protocol (data not shown). However, limited oxygen carrying capacity associated with perfusion solutions may lead to mild ischaemia under scenarios of high metabolic demand and contribute to time-dependent cardiac functional decline observed following preload elevation.49Sensitivity to mechanical attachment: Compared to cardiomyocytes of Aged mice with endogenous TRPV4 expression, cardiomyocytes of DTg:Tam mice were very sensitive to attachment to the Myostretcher device and ∼50% of sampled cells exhibited Ca2+ overload following attachment. The remainder of DTg:Tam cardiomyocytes transitioned into Ca2+ overload immediately after onset of longitudinal stretch. Attachment-induced membrane deformation likely represents a sufficient stimulus for TRPV4 channel activation, and differences in functional behaviour between DTg:Tam and Aged groups may be due to relative TRPV4 expression (∼2 fold greater in DTg:Tam vs. Aged12) or age-associated cortical stiffening.14,15
5. Conclusions
Integrated with earlier work,14 these results point to diverse forms of dysfunction in the aged heart. One is described by a loss in the Frank–Starling mechanism and an inability to respond to ventricular preload with increased pressure development. Another is described by a maintained Frank–Starling response with TRPV4-dependent secondary hypercontractility that, when sustained, leads to a progressive loss in ventricular systolic and diastolic function. Elucidating mechanisms of age-dependent susceptibility to sustained stretch will allow precise targeting and prevention of cardiac dysfunction in the future. Prevention of TRPV4 stretch activation may hold therapeutic promise in further investigations of age-dependent cardiac dysfunction.50
Supplementary Material
Acknowledgements
The authors would like to acknowledge the intellectual contributions of John Jones, Joel Robinett, and Zahra Nourian to the final manuscript.
Conflict of interest: A.B.V. employed by IonOptix Scientific Instruments following initial manuscript submission. Other authors have nothing to disclose.
Funding
This work was supported by the National Institutes of Health F31HL140882 (to A.B.V.), F31HL147559 (to D.P.), K01AG041208 (to T.L.D.), and R01HL136292 (to T.L.D.).
Translational perspective
Ageing associates with altered cardiomyocyte Ca2+ handling and intolerance to elevated levels of intracellular Ca2+. As a mechanosensitive ion channel expressed with age, transient receptor potential vanilloid-4 (TRPV4) may contribute to intracellular Ca2+ elevation during situations of increased cardiac demand. Pharmacologic blockade of TRPV4 Ca2+ influx may therefore relieve intracellular Ca2+ stress in aged hearts and promote Ca2+ homeostasis. Phase II clinical trials to date have demonstrated safe use of TRPV4 inhibitors, and TRPV4 antagonism may represent a novel intervention to improve cardiomyocyte Ca2+ homeostasis in elderly populations.
References
- 1. Feridooni HA, Dibb KM, Howlett SE.. How cardiomyocyte excitation, calcium release and contraction become altered with age. J Mol Cell Cardiol 2015;83:62–72. [DOI] [PubMed] [Google Scholar]
- 2. Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd ed Dordrecht: Kluwer Academic Publishers; 2001. [Google Scholar]
- 3. Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J.. AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell 2010;9:592–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cain BS, Meldrum DR, Joo KS, Wang J-F, Meng X, Cleveland JC, Banerjee A, Harken AH.. Human SERCA2a levels correlate inversely with age in senescent human myocardium. J Am Coll Cardiol 1998;32:458–467. [DOI] [PubMed] [Google Scholar]
- 5. Qin F, Siwik DA, Lancel S, Zhang J, Kuster GM, Luptak I, Wang L, Tong X, Kang YJ, Cohen RA, Colucci WS.. Hydrogen peroxide–mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc 2013;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu A, Narayanan N.. Effects of aging on sarcoplasmic reticulum Ca2+-cycling proteins and their phosphorylation in rat myocardium. Am J Physiol 1998;275:H2087–H2094. [DOI] [PubMed] [Google Scholar]
- 7. Isenberg G, Borschke B, Rueckschloss U.. Ca2+ transients of cardiomyocytes from senescent mice peak late and decay slowly. Cell Calcium 2003;34:271–280. [DOI] [PubMed] [Google Scholar]
- 8. Lim CC, Apstein CS, Colucci WS, Liao R.. Impaired cell shortening and relengthening with increased pacing frequency are intrinsic to the senescent mouse cardiomyocyte. J Mol Cell Cardiol 2000;32:2075–2082. [DOI] [PubMed] [Google Scholar]
- 9. Zhu X, Altschafl BA, Hajjar RJ, Valdivia HH, Schmidt U.. Altered Ca2+ sparks and gating properties of ryanodine receptors in aging cardiomyocytes. Cell Calcium 2005;37:583–591. [DOI] [PubMed] [Google Scholar]
- 10. Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BHL, Hewett TE, Robbins J, Houser SR, Molkentin JD.. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest 2007;117:2431–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hano O, Bogdanov KY, Sakai M, Danziger RG, Spurgeon H. A, Lakatta EG.. Reduced threshold for myocardial cell calcium intolerance in the rat heart with aging. Am J Physiol 1995;269:H1607–H1612. [DOI] [PubMed] [Google Scholar]
- 12. Jones JL, Peana D, Veteto AB, Lambert MD, Nourian Z, Karasseva NG, Hill MA, Lindman BR, Baines CP, Krenz M, Domeier TL.. TRPV4 increases cardiomyocyte calcium cycling and contractility yet contributes to damage in the aged heart following hypoosmotic stress. Cardiovasc Res 2019;115:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White JPM, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I.. TRPV4: molecular conductor of a diverse orchestra. Physiol Rev 2016;96:911–973. [DOI] [PubMed] [Google Scholar]
- 14. Nance ME, Whitfield JT, Zhu Y, Gibson AK, Hanft LM, Campbell KS, Meininger GA, McDonald KS, Segal SS, Domeier TL.. Attenuated sarcomere lengthening of the aged murine left ventricle observed using two-photon fluorescence microscopy. Am J Physiol Heart Circ Physiol 2015;309:H918–H925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lieber SC, Aubry N, Pain J, Diaz G, Kim S-J, Vatner SF.. Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am J Physiol Heart Circ Physiol 2004;287:H645–H651. [DOI] [PubMed] [Google Scholar]
- 16. Pacher P, Mabley JG, Liaudet L, Evgenov OV, Marton A, Haskó G, Kollai M, Szabó C.. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol 2004;287:H2132–H2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schulman SP, Lakatta EG, Fleg JL, Lakatta L, Becker LC, Gerstenblith G.. Age-related decline in left ventricular filling at rest and exercise. Am J Physiol 1992;263:H1932–H1938. [DOI] [PubMed] [Google Scholar]
- 18. Veteto AB, McDonald KS, Domeier TL.. Preload-induced changes in systolic and diastolic performance in the young and aged murine heart. Biophys J Biophysical Society 2016;110:296a. [Google Scholar]
- 19. Seo K, Rainer PP, Shalkey Hahn V, Lee D-I, Jo S-H, Andersen A, Liu T, Xu X, Willette RN, Lepore JJ, Marino JP, Birnbaumer L, Schnackenberg CG, Kass DA.. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc Natl Acad Sci USA 2014;111:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doleschal B, Primessnig U, Wolkart G, Wolf S, Schernthaner M, Lichtenegger M, Glasnov TN, Kappe CO, Mayer B, Antoons G, Heinzel F, Poteser M, Groschner K.. TRPC3 contributes to regulation of cardiac contractility and arrhythmogenesis by dynamic interaction with NCX1. Cardiovasc Res 2015;106:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoffman NE, Miller BA, Wang J, Elrod JW, Rajan S, Gao E, Song J, Zhang X-Q, Hirschler-Laszkiewicz I, Shanmughapriya S, Koch WJ, Feldman AM, Madesh M, Cheung JY.. Ca2+ entry via TRPM2 is essential for cardiac myocyte bioenergetics maintenance. Am J Physiol Heart Circ Physiol 2015;308:H637–H650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guinamard R, Demion M, Magaud C, Potreau D, Bois P.. Functional expression of the TRPM4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension 2006;48:587–594. [DOI] [PubMed] [Google Scholar]
- 23. Mathar I, Kecskes M, Mieren G. V D, Jacobs G, Camacho Londoño JE, Uhl S, Flockerzi V, Voets T, Freichel M, Nilius B, Herijgers P, Vennekens R.. Increased β-adrenergic inotropy in ventricular myocardium from TRPM4−/− mice. Circ Res 2014;114:283–294. [DOI] [PubMed] [Google Scholar]
- 24. Kuo IY, Campbell SG, Russell KS, Kwaczala AT, Ehrlich BE, Nguyen L.. Decreased polycystin 2 expression alters calcium-contraction coupling and changes β-adrenergic signaling pathways. Proc Natl Acad Sci USA 2014;111:16604–16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tian X, Estrada M, Somlo S, Ehrlich BE, Anyatonwu GI.. Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc Natl Acad Sci USA 2007;104:6454–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Volk T, Schwoerer A, Thiessen S, Schultz J, Ehmke H.. A polycystin-2-like large conductance cation channel in rat left ventricular myocytes. Cardiovasc Res 2003;58:76–88. [DOI] [PubMed] [Google Scholar]
- 27. Andrei SR, Sinharoy P, Bratz IN, Damron DS.. TRPA1 is functionally co-expressed with TRPV1 in cardiac muscle: co-localization at z-discs, costameres and intercalated discs. Channels 2016;10:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rubinstein J, Lasko VM, Koch SE, Singh VP, Carreira V, Robbins N, Patel AR, Jiang M, Bidwell P, Kranias EG, Jones WK, Lorenz JN.. Novel role of transient receptor potential vanilloid 2 in the regulation of cardiac performance. Am J Physiol Heart Circ Physiol 2014;306:H574–H584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aguettaz E, Lopez JJ, Krzesiak A, Lipskaia L, Adnot S, Hajjar RJ, Cognard C, Constantin B, Sebille S.. Axial stretch-dependent cation entry in dystrophic cardiomyopathy: involvement of several TRPs channels. Cell Calcium 2016;59:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwata Y, Ohtake H, Suzuki O, Matsuda J, Komamura K, Wakabayashi S.. Blockade of sarcolemmal TRPV2 accumulation inhibits progression of dilated cardiomyopathy. Cardiovasc Res 2013;99:760–768. [DOI] [PubMed] [Google Scholar]
- 31. Seo K, Rainer PP, Lee D, Hao S, Bedja D, Birnbaumer L, Cingolani OH, Kass DA.. Hyperactive adverse mechanical stress responses in dystrophic heart are coupled to transient receptor potential canonical 6 and blocked by cGMP–protein kinase G modulation. Circ Res 2014;114:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simard C, Sallé L, Rouet R, Guinamard R.. Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle. Br J Pharmacol 2012;165:2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lorin C, Vögeli I, Niggli E.. Dystrophic cardiomyopathy: role of TRPV2 channels in stretch-induced cell damage. Cardiovasc Res 2015;106:153–162. [DOI] [PubMed] [Google Scholar]
- 34. Dai D-F, Rabinovitch PS, Ungvari Z.. Mitochondria and cardiovascular aging. Circ Res 2012;110:1109–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Q, Wu S, Li S-Y, Lopez FL, Du M, Kajstura J, Anversa P, Ren J.. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol 2007;292:H1398–H1403. [DOI] [PubMed] [Google Scholar]
- 36. Hofmann T, Schaefer M, Schultz G, Gudermann T.. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci 2002;99:7461–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G.. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 2008;182:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ma X, Cao J, Luo J, Nilius B, Huang Y, Ambudkar IS, Yao X.. Depletion of intracellular Ca2+ stores stimulates the translocation of vanilloid transient receptor potential 4-C1 heteromeric channels to the plasma membrane. Arterioscler Thromb Vasc Biol 2010;30:2249–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du J, Ma X, Shen B, Huang Y, Birnbaumer L, Yao X.. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J 2014;28:4677–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Greenberg HZE, Carlton-Carew SRE, Khan DM, Zargaran AK, Jahan KS, Vanessa Ho W-S, Albert AP.. Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced nitric oxide production and vasorelaxation in rabbit mesenteric arteries. Vascul Pharmacol 2017;96–98:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma X, Nilius B, Wong J-Y, Huang Y, Yao X.. Electrophysiological properties of heteromeric TRPV4-C1 channels. Biochim Biophys Acta 2011;1808:2789–2797. [DOI] [PubMed] [Google Scholar]
- 42. Ma Y, Zhang P, Li J, Lu J, Ge J, Zhao Z, Ma X, Wan S, Yao X, Shen B.. Epoxyeicosatrienoic acids act through TRPV4-TRPC1-KCa1.1 complex to induce smooth muscle membrane hyperpolarization and relaxation in human internal mammary arteries. Biochim Biophys Acta 2015;1852:552–559. [DOI] [PubMed] [Google Scholar]
- 43. Earley S, Heppner TJ, Nelson MT, Brayden JE.. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 2005;97:1270–1279. [DOI] [PubMed] [Google Scholar]
- 44. Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, Xu Y, Nie L, Vázquez AE, Young JN, Glatter KA, Chiamvimonvat N.. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol 2005;289:H2714–H2723. [DOI] [PubMed] [Google Scholar]
- 45. Terentyev D, Rochira JA, Terentyeva R, Roder K, Koren G, Li W.. Sarcoplasmic reticulum Ca2+ release is both necessary and sufficient for SK channel activation in ventricular myocytes. Am J Physiol Heart Circ Physiol 2014;306:H738–H746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chua S-K, Chang P-C, Maruyama M, Turker I, Shinohara T, Shen MJ, Chen Z, Shen C, Rubart-von der Lohe M, Lopshire JC, Ogawa M, Weiss JN, Lin S-F, Ai T, Chen P-S.. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res 2011;108:971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Biernacka A, Frangogiannis NG.. Aging and cardiac fibrosis. Aging Dis 2011;2:158–173. [PMC free article] [PubMed] [Google Scholar]
- 48. Loffredo FS, Nikolova AP, Pancoast JR, Lee RT.. Heart failure with preserved ejection fraction. Circ Res 2014;115:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giles AV, Sun J, Femnou AN, Kuzmiak-Glancy S, Taylor JL, Covian R, Murphy E, Balaban RS.. Paradoxical arteriole constriction compromises cytosolic and mitochondrial oxygen delivery in the isolated saline-perfused heart. Am J Physiol Heart Circ Physiol 2018;315:H1791–H1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goyal N, Skrdla P, Schroyer R, Kumar S, Fernando D, Oughton A, Norton N, Sprecher DL, Cheriyan J.. Clinical pharmacokinetics, safety, and tolerability of a novel, first-in-class TRPV4 ion channel inhibitor, GSK2798745, in healthy and heart failure subjects. Am J Cardiovasc Drugs 2019;19:335–342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.