Abstract
Enormous societal challenges, such as feeding and providing energy for a growing population in a dramatically changing climate, necessitate technological advances in plant science. Plant cells are fundamental organizational units that mediate production, transport, and storage of our primary food sources and sequester a significant proportion of the world’s carbon. New technologies allow comprehensive descriptions of cells that could accelerate research across fields of plant science. Complementary to the efforts towards understanding the cellular diversity in human brain and immune systems, a Plant Cell Atlas that maps molecular machineries to cellular and subcellular domains, follows their dynamic movements, and describes their interactions would accelerate discovery in plant science and help solve imminent societal problems.
Keywords: Plant Cell Atlas, Proteomics, Data Science, Imaging, Nanotechnology, Single Cell Profiling
Plant Science Needed to Address Global Challenges
Climate change is the single biggest threat to global human health, and is expected to cause about a quarter of a million deaths globally from malnutrition, infectious diseases and heat stress (http://www.who.int/news-room/fact-sheets/detail/climate-change-and-health). We need innovations in science and technology to tackle global sustainability, food security and human health. Plants not only form the foundation of terrestrial ecosystems and the human food supply, but also provide renewable energy and essential medicines. Plant research is therefore fundamental to meeting these challenges [1]. Recent innovations in systems biology, sensors and biosensors, data science, artificial intelligence, gene editing, precision breeding and microbiome position us to make unprecedented discoveries in plant science to enable new solutions.
Information Gaps in Plant Cell Science and the Need for a Plant Cell Atlas
Cells were first described in slices of cork by Robert Hooke in 1665 [2]. Nearly 200 years later, Schleiden’s and Schwann’s investigations of plant and animal micro-anatomy [3, 4] led to the theory that these cells were in fact the fundamental organizational units of life [5]. A comprehensive understanding of plant cell structure and function at a molecular level is essential to uncover the mechanisms that plants use to produce the services we depend upon. Advances in genetics, molecular biology, biochemistry and microscopy have produced detailed pictures of important cellular components, pathways and mechanisms in higher plants, but many structural and functional features of plant cells remain to be understood at a molecular level and it is highly likely that important features and compartments remain to be discovered and elucidated. For example, plants synthesize a variety of natural products that are consumed for sustenance and medicinal purposes, but the cellular organization of many pathways is not understood and we do not know whether enzymes in a metabolic pathway are generally physically linked to provide a metabolic tunnel for efficient channeling of metabolites. Proteins have been observed to cluster into speckles in the nucleus and in microdomains in the cell membrane, but we do not know the composition, diversity and indeed function of most of these subcellular structures. Membrane trafficking pathways in plant cells are highly elaborated, but the diversity of trafficking compartments and their functional organization are poorly understood. Plant cells are connected by cell-cell channels (plasmodesmata), but little is understood of their molecular structure, biogenesis and regulation. These are just a few examples of broad unanswered questions that could impact many fields of basic and applied plant research. Finally, the functions of substantial proportions of genomes are unknown by either experimentation or prediction based on sequence similarity [6]. Knowledge about where these proteins reside in the cell, the other proteins they interact with, and how they behave as the environment changes will yield important clues about their functions and the mechanisms and pathways they are part of, including mechanisms pathways not previously discovered.
Plant Cell Atlas -Goals and Potential Impact
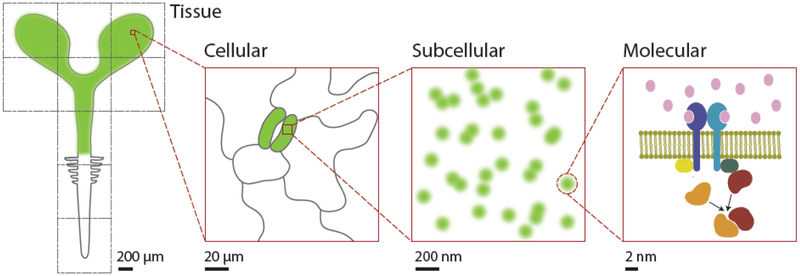
Here we propose the building of a Plant Cell Atlas (PCA), with the goal to create a community resource that comprehensively describes the state of the various cell types found in plants and incorporates information on nucleic acids, proteins, and metabolites at increasingly higher resolutions (Fig. 1). At its core, a PCA will map cellular and subcellular protein localization patterns, track the dynamics and various interactions between proteins, identify the molecular components of different cellular substructures, discern complete states and transitions of specialized cell types, and integrate these different types of data to generate testable models of cellular function [7]. What the integrated data should look like is a big open question (Box 1). The dynamic, spatio-temporal information contained in the human mitotic cell atlas [8] is one potential model. A related possibility is a virtual plant where users can zoom in and out from an organ down to the micro and nano scales where different objects are visible at each zoom level, like Google Maps (Fig. 1). Features that appear at any given level could be linked to genome databases where detailed, quantitative information can be obtained. Another possibility is to develop functional models of plant cells that enable simulations to be made at the level of signaling, growth, differentiation and metabolism. The PCA initiative will need to leverage emerging fields, such as data science, proteomics, single cell profiling, imaging and nanotechnology (see Glossary), along with data visualization innovations, to create a high-resolution, molecular, temporal and spatial map of the plant cell.
Figure 1.
Various scales of the plant cell components that need to be mapped and integrated in the Plant Cell Atlas
Outstanding Questions Box.
How does a new cell type arise over evolutionary scales?
How is long-term growth maintained in stem cells and the meristem?
What is the diversity of plant cell responses to pathogens and other stresses and how does the diversity of response relate to an individual cell’s resistance?
What property enables certain plant cells to embark on regeneration?
What is the diversity and function of intracellular trafficking compartments?
How do plant cells coordinate their developmental maturation in the meristem?
How is the plant plasma-membrane organized, both structurally and dynamically, at the nano to the microscale?
How is the interface between the plasma-membrane and the cell wall organized and what functions does this interface mediate for development and defense?
How is biosynthesis and function of the cell wall organized and regulated?
How does cellular scale organization and function arise from nanoscale organization and function?
How are cell-cell channels regulated and functionally diversified?
What is the full complement of intercellular signals and their effect on neighboring cells?
How do plant cells establish and position functional domains at their periphery to regulate cytoskeletal organization, morphogenesis, polarized intercellular communication and developmental patterning?
What are the best mechanisms of funding this initiative?
What are the best ways to engage the general public with the initiative?
Which organism(s) and cell type(s) are most appropriate to start the initiative with?
How can position information be mapped to cell type information from single cell profiling analyses?
What is the best way to visualize and access the integrated data?
A Plant Cell Atlas will facilitate on basic, long-standing questions in biology (Box 1) that relate directly to grand challenges in food security and climate change. For example, how do we get the maximal output of specialized plant cells that produce useful products (e.g., nutrients, oils, natural products) while reducing environmental impact? In addition, a Cell Atlas can provide insights into how viral or bacterial pathogens change cell properties, addressing critical issues such as how to minimize herbivory and pathogens under a stressful environments? Efforts to understand cellular specialization in C4 plants can, for example, address issues of efficiency of crops and help feed a growing population while reduce agriculture’s carbon footprint. And, basic questions about the totipotency of plant cells relate directly to long-term growth in plants and the ability to take advantage of gene editing techniques by efficient regeneration of a variety of crops.
Technological Components of the PCA
To build a PCA, several methodologies will need to be employed and integrated. They include large-scale gene tagging and transformation to reveal the cellular and subcellular localization of proteins, imaging technologies to understand the dynamic behaviors of those proteins, large-scale proteomics to understand the interactions among proteins and the cellular machines they comprise, single-cell profiling to assess the variety and range of cell types, cell states and their transitions, and finally analytic and visualization tools to ingrate and model these data to generate testable models of cellular function (Fig. 2). We envisage the creation of the PCA as proceeding in stages, starting with an initial tool building and proof of concept stage, a build-out phase where these tools are deployed to build up initial maps and networks of information, and then a phase where multiple projects across the community contribute to and build the Atlas, using infrastructure created and expanded in phases one and two. In the sections below, we highlight some recent advances as well as limitations in key technologies needed to establish PCA as a community.
Figure 2.
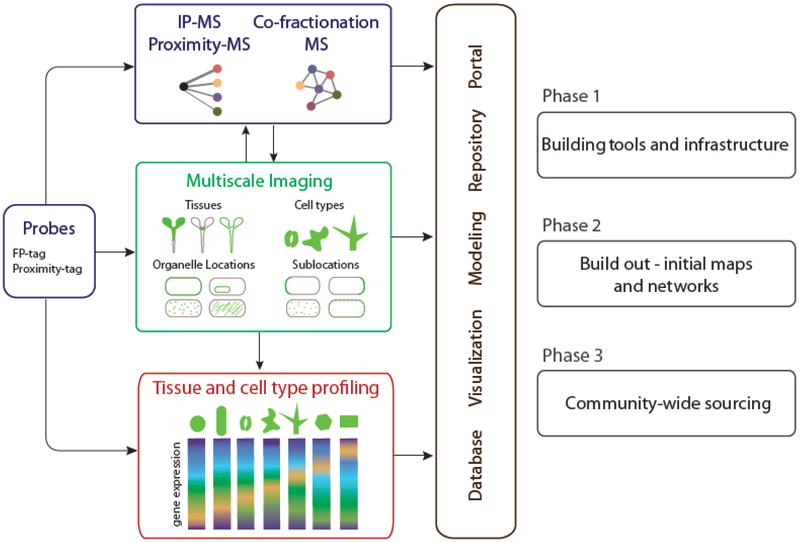
Core activities, relationships and phases for building a Plant Cell Atlas community. The diagram focuses on activities related to proteins but the broader Plant Cell Atlas initiative will include other molecules including RNA’s, cell wall components and metabolites.
Gene tagging, transformation and expression
Visualizing the in vivo localization patterns of proteins requires protein tagging with markers, most commonly fluorescent tags [9]. For a PCA project, tags need to be sufficiently bright to allow detection of small protein complexes, monomerized to avoid artifacts by self-association, and not easily attenuated by protonation of the fluorophore. The latter is important because many plant organelles are acidic. Several fluorescent proteins have been engineered to meet these criteria, including mCitrine [10]. The choice of where the fusion is made and the nature of the peptide link between the tag and target can also be critical. A reasonable proposal [11] is to employ a flexible peptide linker for all fusions and to use sequence analysis to assess the likelihood N- or C- terminal targeting to direct the choice of which terminus to tag. Overexpression can mask many localization patterns and produce significant artifacts if the machineries that process and localize proteins get saturated. Therefore, stable transformations using native expression sequences are greatly preferred. To preserve native expression, in situ tagging would be ideal, such as have been accomplished using CRISPR in animal cells [12], but efficient in situ tagging remains to be demonstrated in higher plants. Although transient expression can be compromised by overexpression, it can also allow for much greater throughput. A controllable promoter to titrate expression, such as a steroid inducible promoter, could mitigate the overexpression problem. Exciting new methods have been developed using nanoparticles to aid cellular transformation, including electrospray [13], magnetofection [14] and nanostraw-mediated electroporation [15]. If adapted to plant tissue, these techniques may allow for large-scale transient transformation of probes into differentiated plant tissue, and even extend studies to a wide range of species with specialized cell types.
Imaging
In vivo and in planta observation of fluorescently-tagged proteins requires sensitive detection, rapid acquisition, and optical sectioning. Instruments such as spinning disk confocal microscopes that use highly efficient detectors (e.g. electron-multiplying charge-coupled device (EMCCD) and scientific complementary metal-oxide-semiconductor (sCMOS)) and multipoint scanning currently meet these needs. The ability to mitigate the light scattering effects of highly refractile cells walls, especially for analysis of cells below the epidermis, is also important for imaging plant tissues. Using water, glycerin, and the recently developed silicon oil objectives can improve results when imaging cells through thick plant tissue. Many new and advanced imaging modalities, including super-resolution microscopies [9] to resolve optical probes below the diffraction limit, will enable the visualization of novel complexes and interactions. While super resolution microscopies reveal new biological detail using light imaging, a revolution in CryoEM techniques, including correlated fluorescence-CryoEM, add molecular information to three dimensional electron microscopy imaging, bringing visualization of molecular organization in cells to the nanoscale [9, 16]. Imaging can also explore protein-protein interactions and dynamic protein relationships in living cells using advanced techniques including cross-correlation spectroscopy (CCS) [17], scanning fluorescence correlation spectroscopy (FCS) [18], Forster resonance energy transfer (FRET) [19], bioluminescence energy transfer (BRET) [20] and bimolecular fluorescence complementation (BiFC) [21]. All these data will be valuable in helping to identify potential protein partners, complexes and networks to build a comprehensive PCA.
Proteomics
Technical innovations in proteomics, especially large-scale methods for affinity purification/mass spectrometry (AP-MS) [22, 23], co-fractional/mass spectrometry (CF-MS) [24, 25], and 2-hybrid screening [26, 27] have allowed for the collection of protein-protein data on an unprecedented scale. These studies have revealed new components of known protein complexes and, excitingly, entirely new complexes whose functions remain to be determined [28]. Traditionally, large-scale protein interaction assays required high protein-protein affinity to identify interaction partners. However, many functionally important protein interactions are not of high affinity, such as kinase-substrate interactions. A family of methods known as proximity labeling utilizes an enzyme to label nearby proteins with a high affinity molecular tag. This technique enables detection of weak protein interactions and even positional proximity, allowing the possibility to explore spatial and functional relationships among proteins that were inaccessible by previous methods [29, 30]. New possibilities for determining organellar proteomes have opened up by targeting the labeling enzyme itself to the organelle [31]. If combined with cell type specific expression, this technique could also enable determination of organelle proteomes by cell-type or even cell-state. Building maps of protein localization together with interaction data will be strongly synergistic and mutually reinforcing.
RNA Sequencing
Single-cell RNAseq profiling and related analyses that interrogate chromatin, DNA, and even protein levels present new opportunities for defining complex cellular states. These techniques have evolved rapidly from sequencing hundreds of cells [32-34] to combinatorial barcoding techniques that permit processing of a million cells in one experiment [35, 36]. The dramatic increase in sampling scale can be used to overcome increased noise [37], enhancing the ability to fine map cellular states and define the transcriptional differences among cellular subtypes. Additionally, the ability to conduct sequence-enabled single-cell molecular analyses provides new opportunities to address plant-specific questions, such as what cell-specific regulatory responses mediate the ability of the plant cell to alter their state during development and in response to external cues [38, 39]. One key advantage of single-cell analyses at the genome scale is the ability to discover previously undescribed heterogeneity in cellular states that allow a description of the early regulatory responses in coping with stress or developmental transitions.
The indeterminate growth of plant meristems means that cells at all developmental stages are present in a single organ, providing a progression of cellular maturation in a single set of samples. This property is ideal for single-cell-seq approaches that reconstruct developmental trajectories from single-cell assays. Models that take advantage of this fine-scale time series could be used to identify key steps in meristem organization that, for example, control the specification of cells that load nutrients or mediate growth to optimize yield. In crop physiology, the function of differentiated cells is often a critical question. Along those lines, newer combinatorial barcoding techniques have demonstrated the feasibility of labeling mRNAs in single nuclei [36]. The latter technique offers some promise to assay cells that have been recalcitrant to cell wall digestion, like mature plant cells or those with specialized secondary cell walls.
Data Science
Advances in computing and communications technologies have opened an era of big data and artificial intelligence, which is transforming all sectors of our societies and cultures [40, 41]. The PCA should leverage and extend big data technologies such as parallel computing, data management infrastructure, data analytics, and machine learning. The PCA initiative has various applications for machine learning (ML), including detecting and delineating compartments, tracking movements, and extracting morphological characteristics. The rapid increase in throughput, scale and dimensionality of image capture will enable us to chart out the plant cell at unprecedented detail but will require having image processing tools that are easy to use, adaptable, and able to take in multiple dimensions and parameters. ML is ideally suited for this type of task [42]. From the PCA initiative, we will likely see many new cellular compartments and structures, which may not easily be defined by a few parameters. Also, we may identify various states or novel subdomains of known compartments. Since there would be no prior knowledge for these novel structures and compartments, unsupervised ML methods need to be applied. These include simple clustering to categorize data, active learning [42] and neural-network based deep learning [40] to identify new structures and compartments [43].
Innovation in data visualization will be critical in every phase of the project such as data acquisition, quantification, pattern recognition and interpretation. Much progress has been made towards visualizing high dimensional data in the last decade [44]. An innovative way of visualizing complex data at multiple scales is semantic zooming. Unlike geometric zooming that increases objects in size, semantic zooming changes the content of the object at different scales. Semantic zooming has been successfully implemented by Google Maps and, while some efforts have been made in biology [7, 45, 46], the full potential of this tool has yet to be applied. The PCA project would be an ideal vehicle for semantic zooming (Fig. 1). In order to semantically connect objects at different scales, the entities need to be organized in a hierarchy. The Cellular Component domain of Gene Ontology [47] and Cell Ontology [48] would serve as a great starting point for mapping these entities.
Open source initiatives such as ImageJ and FIJI [49]have greatly enabled useful sharing of image analysis tools and have significantly increased access to image analysis. However, these tools for image processing and quantification are still largely manually driven using macros and plug-ins that are customized for particular datasets or experimental setups. Customization and automation can be performed by modifying the open source code and integrating these tools with environments like Matlab [50]. However, it is often difficult to find the right software or analysis functions; even if they exist, they can still be challenging to adapt and implement in other environments. Further, these solutions are typically not optimized and are inefficient, limiting their application to large-scale datasets and computationally intense analyses. Therefore, there are big opportunities for software engineering to play a pivotal role in the PCA initiative.
Concluding remarks and future perspectives
To successfully implement the PCA initiative, we need to build a community that brings together scientists from fields such as imaging, nanotechnology, single-cell profiling, data science and proteomics (Fig. 3). The community needs a forum, perhaps via a website and social media links, to connect various projects whose datasets could contribute to the PCA network. The community also needs to tackle topics such as data sharing and release policy, public and internal data tracking, and data visualization tools. Connecting with projects like the Human Cell Atlas [51] and OpenWorm (www.openworm.org) to learn from their experiences would be important. An obvious first step is to convene a workshop to bring in key stakeholders and scientists to discuss the vision, approaches, and bottlenecks. How to engage the public in this initiative should be an important part of the discussion.
Figure 3.
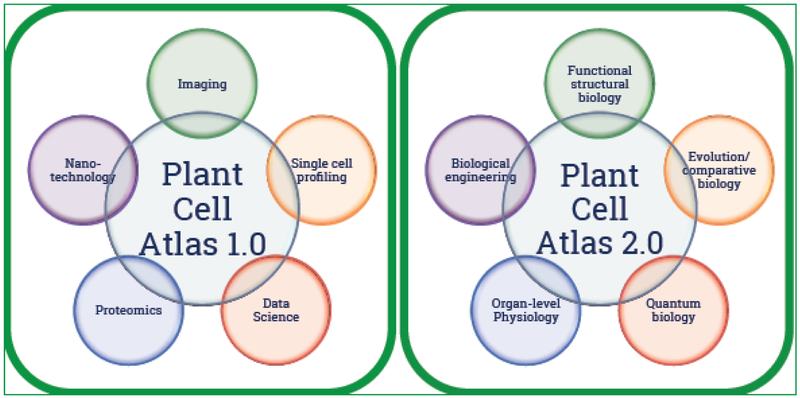
Fields needed to establish the Plant Cell Atlas communities
Going beyond the initial phase, we envisage the next generation PCA to encompass emerging technologies (Fig.3). For example, functional structural biology such as X-ray Free Electron-Laser [52], large-volume imaging [53], and dynamic Transmission Electron Microscopy [54] have the potential to transform the way we visualize the molecular events inside cells. Also, the diversity of cellular structures and functions across the spectrum of species would require evolutionary and comparative biology. Exciting developments in biological engineering could make engineering artificial organelles or using biological machineries and structures as inspiration for manufacturing synthetic systems possible [55]. Going beyond genes and proteins, metabolites should be incorporated into the PCA. Finally the next generation PCA would enable the probing of organ- and organism-level physiology at molecular resolutions, enabled by modeling relationships and functions between cells, which would open doors for modeling plant behavior under various environmental scenarios (see also outstanding questions).
Highlights.
Plant cells are the fundamental organizational unit that mediate production, transport, and storage of our primary food sources and sequester a significant proportion of the world’s carbon.
A comprehensive and quantitative understanding of how plant cells are organized and function is critical in advancing plant science.
Recent advances in microscopy, mass spectrometry, artificial intelligence, sequencing, and nanotechnology make this the right time for the creation of a Plant Cell Atlas initiative.
Acknowledgements
We thank Heather Meyer, Hannah Vahldick, Benjamin Jin, and Suryatapa Jha for their comments on the manuscript. Work in the Rhee lab is supported by the Carnegie Institution for Science endowment, grants from the National Science Foundation (IOS-1546838, IOS-1026003), Department of Energy (DE-SC0008769, DE-SC0018277), and National Institutes of Health (1U01GM110699-01A1). The Ehrhardt lab is supported by the Carnegie Institution for Science endowment and grants from the National Institututes for Health (RO1-GM123259-01), National Science Foundation (NSF MCB-1413254), and the Department of Energy (DE-AR0000825).
Glossary
- Active learning
an ML framework where the learner algorithm prioritizes data that will be most beneficial for training typically based on the degree of uncertainty and iteratively improves itself from experimental or manual annotation of the unlabeled data
- Deep learning
A type of machine learning approach, which uses neural networks and relies on large amount of data to predict patterns and classify things
- Machine learning
A type of artificial intelligence approach in computer science that uses statistics and information theory to predict entities and relationships from examples
- Nanotechnology
science, engineering and technology conducted at scales of less than 100 nanometers
- Semantic zooming
vitalizing qualitative changes of information content as a function of scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones AM et al. (2008) The impact of Arabidopsis on human health: diversifying our portfolio. Cell 133, 939–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooke R (1665) Micrographia, or, Some physiological descriptions of minute bodies made by magnifying glasses : with observations and inquiries thereupon. . Printed for James Allestry [Google Scholar]
- 3.Schleiden MJ (1838) Contributions to our Knowledge of Phytogenesis. Arch. Anat. Physiol. Wiss. Med 13, 137–176 [Google Scholar]
- 4.Schwann T (1838) Ueber die Analogic in der Structur und dem Wachsthum der Thiere und Pflanzen. Neue Not Geb Nat Heil Jan:33–36; 1838;Feb:25–29; 1838;Apr:21–23 [Google Scholar]
- 5.Schwann T (1839) Mikroskopische Untersuchungen über die Uebereinstimmung in der Struktur und dem Wachsthum der Thiere und Pflanzen. Verlag der Sander'schen Buchhandlung; [PubMed] [Google Scholar]
- 6.Rhee SY and Mutwil M (2014) Towards revealing the functions of all genes in plants. Trends Plant Sci. 19, 212–221 [DOI] [PubMed] [Google Scholar]
- 7.Waese J et al. (2017) ePlant: Visualizing and Exploring Multiple Levels of Data for Hypothesis Generation in Plant Biology. Plant Cell 29, 1806–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Y et al. (2018) Experimental and computational framework for a dynamic protein atlas of human cell division. Nature 561, 411–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw SL and Ehrhardt DW (2013) Smaller, faster, brighter: advances in optical imaging of living plant cells. Annu. Rev. Plant Biol 64, 351–375 [DOI] [PubMed] [Google Scholar]
- 10.Shaner NC et al. (2005) A guide to choosing fluorescent proteins. Nature Methods 2, 905–909 [DOI] [PubMed] [Google Scholar]
- 11.Tian GW et al. (2004) High-throughput fluorescent tagging of full-length Arabidopsis gene products in planta. Plant Physiol. 135, 25–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts B et al. (2017) Systematic gene tagging using CRISPR/Cas9 in human stem cells to illuminate cell organization. Mol. Biol. Cell 28, 2854–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abyadeh M et al. (2017) Electrosprayed chitosan nanoparticles: facile and efficient approach for bacterial transformation. International Nano Letters 7, 291–295 [Google Scholar]
- 14.Zhao X et al. (2017) Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nature Plants 3, 956–964 [DOI] [PubMed] [Google Scholar]
- 15.Cao Y et al. (2018) Universal intracellular biomolecule delivery with precise dosage control. Science Advances 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanetti G et al. (2013) The structure of the COPII transport-vesicle coat assembled on membranes. Elife 2, e00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwille P et al. (1997) Dual-color fluorescence cross-correlation spectroscopy for multicomponent diffusional analysis in solution. Biophys. J 72, 1878–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrasek Z et al. (2010) Scanning FCS for the characterization of protein dynamics in live cells. Methods Enzymol. 472, 317–343 [DOI] [PubMed] [Google Scholar]
- 19.Forster T (1948) Zwischenmolekulare Energiewanderung und Fluoreszenz. Annalen der Physik 437, 55–75 [Google Scholar]
- 20.Xie Q et al. (2011) Bioluminescence resonance energy transfer (BRET) imaging in plant seedlings and mammalian cells. Methods Mol. Biol 680, 3–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerppola TK (2008) Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu. Rev. Biophys 37, 465–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hein MY et al. (2015) A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 163, 712–723 [DOI] [PubMed] [Google Scholar]
- 23.Huttlin EL et al. (2015) The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell 162, 425–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkwood KJ et al. (2013) Characterization of native protein complexes and protein isoform variation using size-fractionation-based quantitative proteomics. Mol. Cell. Proteomics 12, 3851–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havugimana PC et al. (2012) A census of human soluble protein complexes. Cell 150, 1068–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolland T et al. (2014) A proteome-scale map of the human interactome network. Cell 159, 1212–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rual JF et al. (2005) Towards a proteome-scale map of the human protein-protein interaction network. Nature 437, 1173–1178 [DOI] [PubMed] [Google Scholar]
- 28.Drew K et al. (2017) Integration of over 9,000 mass spectrometry experiments builds a global map of human protein complexes. Mol. Syst. Biol 13, 932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han S et al. (2018) Proximity labeling: spatially resolved proteomic mapping for neurobiology. Curr. Opin. Neurobiol 50, 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CL and Perrimon N (2017) Proximity-dependent labeling methods for proteomic profiling in living cells. Wiley Interdisciplinary Reviews: Developmental Biology 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rees JS et al. (2015) Protein Neighbors and Proximity Proteomics. Mol. Cell. Proteomics 14, 2848–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picelli S et al. (2013) Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nature Methods 10, 1096–1098 [DOI] [PubMed] [Google Scholar]
- 33.Hashimshony T et al. (2012) CEL-Seq: single-cell RNA-Seq by multiplexed linear amplification. Cell Rep. 2, 666–673 [DOI] [PubMed] [Google Scholar]
- 34.Jaitin DA et al. (2014) Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science 343, 776–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg AB et al. (2017) Scaling single cell transcriptomics through split pool barcoding. bioRxiv [Google Scholar]
- 36.Cao J et al. (2017) Comprehensive single-cell transcriptional profiling of a multicellular organism. Science 357, 661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegenhain C et al. (2017) Comparative Analysis of Single-Cell RNA Sequencing Methods. Mol. Cell 65, 631–643 e634 [DOI] [PubMed] [Google Scholar]
- 38.Gifford ML et al. (2008) Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. U. S. A 105, 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dinneny JR et al. (2008) Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320, 942–945 [DOI] [PubMed] [Google Scholar]
- 40.Ching T et al. (2018) Opportunities and obstacles for deep learning in biology and medicine. J. R. Soc. Interface 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Driscoll A et al. (2013) 'Big data', Hadoop and cloud computing in genomics. J. Biomed. Inform 46, 774–781 [DOI] [PubMed] [Google Scholar]
- 42.Sommer C and Gerlich DW (2013) Machine learning in cell biology - teaching computers to recognize phenotypes. J. Cell Sci 126, 5529–5539 [DOI] [PubMed] [Google Scholar]
- 43.Christiansen EM et al. (2018) In Silico Labeling: Predicting Fluorescent Labels in Unlabeled Images. Cell 173, 792–803 e719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu S et al. (2017) Visualizing High-Dimensional Data: Advances in the Past Decade. IEEE Transactions on Visualization and Computer Graphics 23, 1249–1268 [DOI] [PubMed] [Google Scholar]
- 45.Zhukova A and Sherman DJ (2015) Mimoza: web-based semantic zooming and navigation in metabolic networks. BMC Syst. Biol 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Z et al. (2007) Towards zoomable multidimensional maps of the cell. Nat. Biotechnol 25, 547–554 [DOI] [PubMed] [Google Scholar]
- 47.The Gene Ontology, C. (2017) Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 45, D331–D338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diehl AD et al. (2016) The Cell Ontology 2016: enhanced content, modularization, and ontology interoperability. Journal of Biomedical Semantics 7, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schindelin J et al. (2012) Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiner MC et al. (2017) ImageJ-MATLAB: a bidirectional framework for scientific image analysis interoperability. Bioinformatics 33, 629–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Regev A et al. (2017) The Human Cell Atlas. Elife 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johansson LC et al. (2017) A Bright Future for Serial Femtosecond Crystallography with XFELs. Trends Biochem. Sci 42, 749–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji N et al. (2016) Technologies for imaging neural activity in large volumes. Nat. Neurosci 19, 1154–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell GH et al. (2014) Time resolved electron microscopy for in situ experiments. Applied Physics Reviews 1, 041101 [Google Scholar]
- 55.Wintle BC et al. (2017) A transatlantic perspective on 20 emerging issues in biological engineering. Elife 6 [DOI] [PMC free article] [PubMed] [Google Scholar]