Abstract
A reaction-based sensor (NAS-1) showed a high affinity and sensitivity to HSO3- via a nucleophilic addition reaction in the aqueous media, giving dual signals from absorption and emission spectra. NAS-1 was successfully applied in RK13 epithelial cells to detect HSO3- in a cellular environment.
Introduction
Sulfur dioxide (SO2), long known as a pollutant and toxicant, is typically in equilibrium with sulfite (SO32−) and (bisulfite) HSO3− in aqueous solutions and in biological systems [1–2]. In nature, SO2 is present in the atmosphere due to volcanic emissions and fossil fuel combustion, and its conversion to HSO3− poses a threat to ecosystems by inducing acid rain [3,4]. SO2 also is considered dangerous to humans at certain concentrations. SO2 derivatives SO3− and HSO3− are found in a ratio of 3:1 m/m in physiological environments [5]. Exposure to sulfites is known to cause asthmatic and allergic reactions in humans [6–7]. Other health issues including lung cancer, cardiovascular disease, neurological disorders, migraines, and strokes [8–11] are also associated with SO2 inhalation and/or consumption. Neurological defects occur when sulfites disrupt thiol levels, resulting in an inhibition of redox balance [12–13]. In addition to external sources of SO2, endogenous SO2 levels that are exceptionally high are associated with disease [5]. Furthermore, oral consumption of sulfites can lead to gastrointestinal issues [6–7]. While it is known that sulfites have toxic characteristics, it also has been determined that sulfites may have beneficial effects on the body at certain concentrations. The vasodilating and anti-atherogenic effects of SO2, SO3−, and HSO3− can fight hypertension [2]. SO2 has been considered a gasotransmitter, in addition to the three standing gasotransmitters: nitric oxide, carbon monoxide, and hydrogen sulfide [4]. The physiological roles of endogenous SO3− and HSO3− include maintaining the body’s sulphur levels, working as cardiovascular messengers, and regulating lipid metabolism and insulin levels [12]. Moreover, bisulfite has been widely used in foods, beverages, and pharmaceuticals as a preservative due to its antimicrobial and anti-oxidant properties, which prevent browning and bacterial growth [14–15]. However, due to its potential negative side effects, bisulfite levels in food are often limited [15]. The Joint FAO/WHO Expert Committee on Food Additives recommends a daily intake of no more than 0.7 mg of bisulfite per kg of body weight [16].
Since there are many unique properties and applications of bisulfite, developing a robust method for its quantitative detection is highly desired [17]. In the past years, many fluorescence based approaches have been reported for the detection of HSO3− due to their high sensitivity, simple operation, and nondestructive characteristics [18–20]. Although intensive efforts have been put into developing fluorescent chemosensors, the high selectivity and sensitivity are still pose a challenge for sensor design [21]. Due to the nucleophilicity of HSO3−, many bisulfite sensors are designed on the basis of organic reactions, including Michael addition, deprotection of a levulinate group, and reactions with aldehyde, which significantly enhance the selectivity of bisulfite sensors [22–25].
In our group, we recently reported a fluorescence sensor for the detection of HSO3−, based on a nucleophilic addition reaction, but not a typical Michael addition reaction [26]. We found that an electron withdrawing group (-NO2) plays a critical role for the nucleophilic addition reaction, even not directly connected to the double bond. To verify this discovery further, we developed a derivative of 1,8-naphthalimide (NAS-1), in which a phenyl group was used to isolate the electron withdrawing group (-CF3) from a double bond. Meanwhile, another molecule (NAS-1) with an electron donating group (-OCH3) was prepared for comparison.
Apparatus
All reagents used for synthesis and measurements were purchased from Sigma-Aldrich (MO, USA), Fisher Scientific (USA), TCI (USA), Alfa Aesar (USA) and Acres Organics (USA) in analytical grade and were used as received, unless otherwise stated. Absorbance spectra were collected via Cary Series Uv-vis Spectrophotometer (Agilent Technologies). Fluorescence measurements were all performed by using a FluoroMax-4 Spectrofluorometer (Horiba Jobin Yvon, USA). All fluorescence spectra were recorded in a 1 cm quartz cuvette. The excitation and emission slits were set at 2 nm. 1H and13C NMR spectra were recorded via Bruker Avance III HD 400 Spectrometre (1H 400 MHz, l3C 100 MHz) Bruker 400 Ultra-Shield spectrometer at room temperature.
Materials and methods for cell imaging
RK13 Cell Culture.
RK13 (Rabbit Kidney Epithelial) cells were maintained at 37˚C with 5% CO2 in T-25 and T-75 flasks with RPMI medium supplemented with 5% heat inactivated FBS. The RK13 cells were trypsinized by adding 3mL of trypsin to each flask and incubating at 37˚C with 5% CO2 for 5-10 minutes. When the cells appeared to be dislodging from the surface of the flask, they were collected and 3 mL of supplemented RPMI added, the cells were then counted. The cells were diluted to 104 cells/mL and 1 mL of cell suspension was added to each well of a glass bottom 6-well plate. The cells were allowed to incubate for 48 hours before the sensor (NAS-1) and quencher (HSO3−) were tested.
Sensor and quencher testing and confocal microscopy.
After 48 hours of incubation, the media was aspirated from the wells. The following treatments were performed: supplemented RPMI only, 10 mM NAS only, 50 mM HSO3−only, and 10 mM NAS-1 + 50 mM HSO3− For the 10 mM NAS-1 + 50 mM HSO3− treatment group, the 10 mM NAS-1 was allowed to incubate for 10 minutes before the 50 mM HSO3− was added. Imaging was done using an Olympus FV3000 Confocal Laser Scanning Microscope (excitation 405 nm, emission 485-585 nm).
Synthesis
NSA-1 and NSA-2 were synthesized from commercially available 4-bromo-1,8-naphthalic anhydride via a two-step reaction.
6-bromo-2-(2-morpholinoethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (a). 4-bromo-1,8-naphthalic anhydride (1.11g, 4.0 mmol) was mixed with 2-morpholinoethanamine (1.04g, 8.0 mmol) in ethanol (40 mL) under reflux for 4 hrs. After cooling the reaction mixture to room temperature, a yellow solid was collected using suction filtration. The crude product was purified by column chromatography (silica gel 200-400 mesh, 60 Å) eluted by EtOAc to yield a white solid (1.38g, 89%). Melting point (m.p.): 165-167 °C. 1H NMR (300 MHz, CDCl3) δ: 2.6 (t, J= 4.5 Hz, 4H), 2.7 (t, J= 6.6 Hz, 2H), 3.7 (t, J=4.6 Hz, 4H), 4.3 (t, J=6.9 Hz, 2H), 7.8 (t, J=7.9 Hz, 1H), 8.0 (d, J=7.8 Hz, 1H), 8.4 (d, J=7.8 Hz, 1H), 8.5 (d, J=8.5 Hz, 1H), 8.6 (d, J=7.2 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ: 37.4, 53.8, 56.2, 67.1, 122.2, 123.1, 128.1, 129.0, 130.3, 130.6, 131.1, 131.3, 132.0, 133.3, 163.8.
(E)-2-(2-morpholinoethyl)-6-(4-(trifluoromethyl)styryl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (NAS-1).
6-hydroxy-2-(2-morpholinoethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (1) A mixture of a (194 mg, 0.5 mmol), (E)-(4-(trifluoromethyl)styryl)boronic acid (140 mg, 0.65 mmol), Pd(Ph)3 (28.9 mg), and t-BuOK (61.5mg) were refluxed in 2-propoanol (5 mL) for 2 hours at 80 °C. After cooling to room temperature, the reaction mixture was poured into cold water and the mixture was extracted using CH2Cl2. The crude product was purified by column chromatography (silica, 220-400 mesh). Hexane and methanol (9:1) were used as elution solvents. A yellow solid (NAS-1) was obtained as the product (161 mg, 67%). 1H-NMR (400 MHz, CDCl3) δ: 2.6-3.0 (m, 4H), 3.8 (t, J=6.7 Hz, 4H), 4.4 (t, J=6.8 Hz, 2H), 7.4 (d, J=16.6 Hz, 1H), 7.7-7.8(m,4H), 7.8 (t, J=8.3 Hz, 1H), 7.9-8.1 (m, 2H), 8.5-8.7 (m, 3H). 13C-NMR (100 MHz, CDCl3) δ: 29.8, 53.2, 53.5, 56.0, 121.9, 122.7, 123.1, 124.4, 125.4, 125.9, 126.2, 127.1, 127.3, 128.8, 129.7, 130.2, 130.4, 130.7, 131.3, 131.5, 133.9, 140.0, 141.0, 164.2, 164.4. TOF EI+: M+ m/z 480.1661 (calcd.), 480.1702(found).
(E)-2-(2-morpholinoethyl)-6-(4-(trifluoromethyl)styryl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (NAS-2).
6-hydroxy-2-(2-morpholinoethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (1) A mixture of a (194 mg, 0.5 mmol), (4-methoxystyryl)boronic acid (116 mg 0.65 mmol), Pd(Ph)3 (28.9 mg), and t-BuOK (61.5mg) were refluxed in 2-propoanol (5 mL) for 2 hours at 80 °C. After cooling to room temperature, the reaction mixture was poured into cold water and the mixture was extracted using CH2Cl2. The crude product was purified by column chromatography (silica, 220-400 mesh). Dichloromethane and ethyl acetate (2:1) were used as elution solvents. A yellow solid (NAS-1) was obtained as the product (173 mg, 72%). 1H-NMR (400 MHz, CDCl3) δ: 2.6 (t, J=6.9 Hz, 4H), 2.7 (t, J=6.7 Hz, 2H), 3.7 (t, J=6.8 Hz, 4H), 3.9 (s, 3H), 4.4 (t, J=6.6 Hz, 2H), 7.0 (d, J=8.7 Hz, 2H), 7.3 (d, J=17.7 Hz, 1H), 7.6 (d, J=7.2 Hz, 2H), 7.7-7.9 (m, 2H), 8.0 (d,J=8.1 Hz, 1H), 8.5-8 7 (Μ, 3H). 13C-NMR (100 MHz, CDCl3) δ: 53.6, 55.5, 56.0, 66.3, 105.7, 119.8, 121.5, 122.8, 126.9, 127.2, 128.1, 128.7, 129.0, 129.8, 130.3, 130.9, 131.3, 133.1, 133.9, 134.4, 147.4, 158.5, 154.5, 164.6. TOF EI+:M+ m/z 442.1893 (calcd.), 442.1788(found).
Results and Discussion
In our previous studies, an extra conjugation moiety or a substituent with different electronic properties (electron withdrawing or electron donating) appended at position 4 on the naphthalene ring significantly affected the photophysical properties of 1,8-naphthalimides [27]. Based on these findings, two derivatives of 1,8-naphthalimide (NAS-1 and NAS-2) with an electron withdrawing group (-CF3) and an electron donating group (-OCH3) respectively, were synthesized via Suzuki coupling reaction (Scheme 1). The photophysical properties and sensing ability of NAS-1 and NAS-2 have been investigated in aqueous media.
Scheme 1:

The synthetic route to prepare NAS-1 and NAS-2.
As polar molecules, NAS-1 and NAS-2 were able to completely dissolve in the aqueous media containing H2O (1 mM phosphate buffer at pH 7.4) /DMSO (4:6), which was used as the media to conduct measurements in this project. The maximum absorption of NAS-1 and NAS-2 were observed at 398 and 418 nm respectively. The emission peaks were shown at 491 nm and 602 nm. NAS-2 gave a relatively higher quantum yield (0.023) than NAS-1 (0.011). The Push-Pull electric effect in NAS-2 contributed a long emission (602 nm) and large Stokes-shift (184 nm) (Table 1) [28].
Table 1:
The photophysical properties of NAS-1 and NAS-2
| Absorption (nm) | Emission (nm) | Ɛ (M−1cm−1) | Ф | |
|---|---|---|---|---|
| NAS-1 | 390 | 491 | 10987 | 0.011 |
| NAS-2 | 418 | 602 | 11212 | 0.023 |
Based on the previous investigation in our group, HSO3−, could be used as a nucleophile to react with a highly conjugated molecule via a nucleophilic addition reaction, which has been proved as an efficient sensing mechanism to quantitatively detect HSO3− [29]. The electron-withdrawing group in a conjugation system played a critical role for the addition reaction. Thus, NAS-1 containing a strong electron-withdrawing group (-CF3) on 1,8-naphthalimide was synthesized via a Suzuki coupling reaction, being expected to quantitatively measure HSO3−. Meanwhile, NAS-2 with a strong electron-donating group (-OCH3) was also synthesized as the comparison.
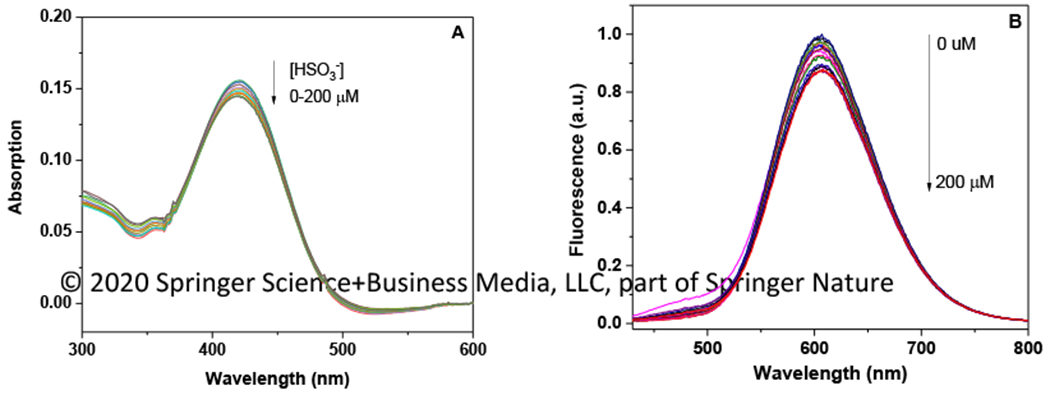
After incubating NAS-1(1.0 × 10−5 M) with different amount of HSO3− (0-200 μM) in the aqueous media (PBS buffer (pH 7.4)/DMSO (4:6)) at 25 °C for 15 min, the absorption and emission spectra were collected (Figure 1). In the presence of HSO3−, the maximum absorption of NAS-1 at 390 nm showed a decrease, and a new absorption peak at 360 nm appeared. The ratiometric change in the absorption spectra clearly indicated a nucleophilic addition reaction triggered by HSO3−, which interrupted the conjugated structure of NAS-1 and consequently caused the significant change of absorption spectra. In the 1H NMR spectrum, the signal of H in double bond decreased with the additon of HSO3−, indicating the interruption of cojugation. the In emission spectra, the fluorescence intensity at 491 nm was significantly decreased with the addition of HSO3−. The maximum quenching (78%) was reached after an addition of 120 μM HSO3−, which was consistent to the absorption titration, supporting the fact that a nucleophilic addition reaction occurred. Moreover, the fluorescence intensity at 491 nm showed a proportional correlation to the concentration of HSO3−, indicating NAS-1 could be used as a fluorescence approach to quantitatively detect HSO3−. The detection limit (3δ/k) for HSO3− was calculated to be 3.2 nM based on fluorescence titration at 491 nm. The fluorescence intensity at 491 nm showed an excellent correlation to the concetration of HSO3 in the range of 0-100 μM (y = −0.0059 x + 0.988, R2= 0.993).
Figure 1:

The titration spectra (absorption/emission) of NAS-1 (1.0 × 10−5 M) after incubating with HSO3−, (0-200 μM) for 15 min in PBS buffer (pH 7.4)/DMSO (4:6) at 25 °C (λex=390 nm).
As a reaction-based fluorescence approach, the sensitivity and reliability of NAS-1 strongly relied on the responding time (reaction time). The kinetics study for NAS-1 was conducted by using HSO3−, with different concentration (50 μM, 100 μM, and 150 μM) in the media of PBS buffer (pH 7.4)/DMSO (4:6) at 25 °C. As shown in Figure 3, the fluorescence emission of NAS-1 at 491 nm was gradually quenched in the presence of HSO3−, but the quenching scale varied according to the concentration of HSO3−. In the presence of 50 μM HSO3−, the fluorescence was continuously quenched in 25 min, indicating the low reaction rate. However, with increasing concentration of HSO3− to 150 μM, the maximum quenching (80%) was observed at 10 min, which can be considered as an acceptable responding time for reaction-based sensors.
Figure 3.

The kinetic study of NAS-1 (1.0 × 10−5 M) with different amount of HSO3− (50 μM, 100 μM, and 150 μM) in PBS buffer (pH 7.4)/DMSO (4:6) at 25 °C (λex=390 nm).
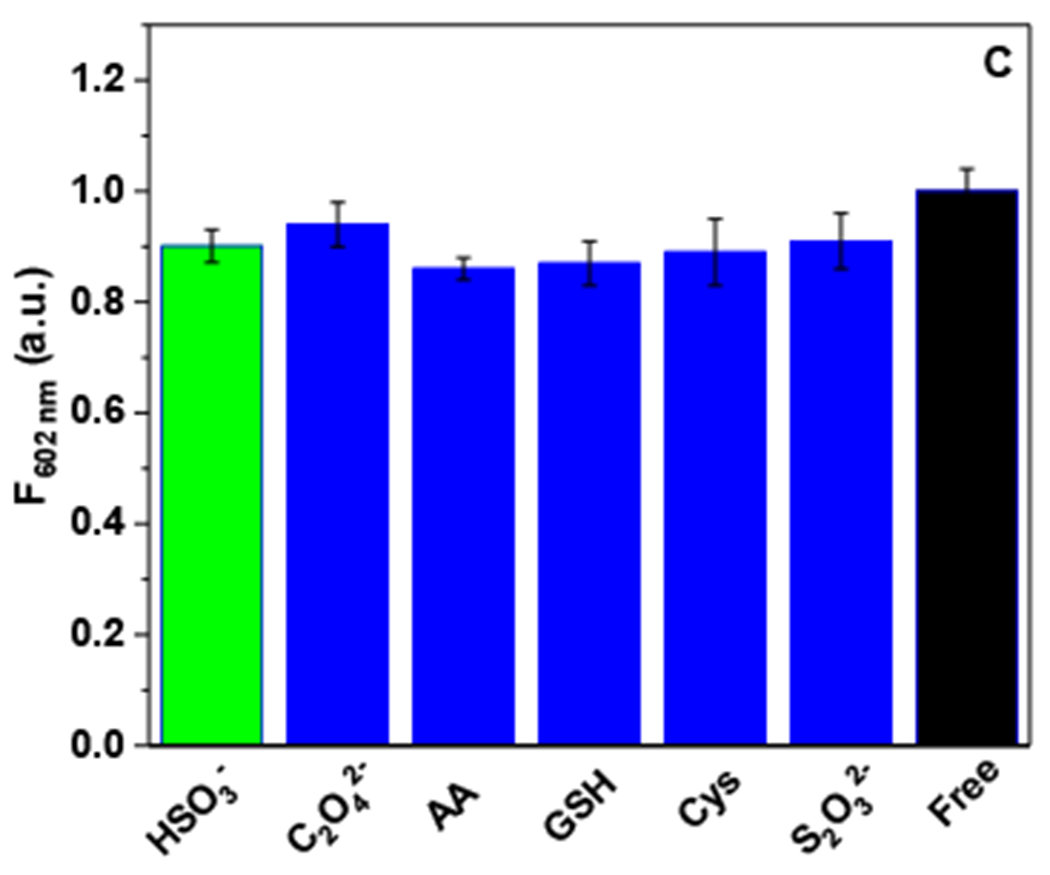
To investigate the selectivity of NAS-1, several bio-abundant reagents including C2O42−, ascorbic acid, GSH, Cys, and S2O32− have been examined. NAS-1 (1.0 × 10−5 M) was incubated individually with HSO3−, C2O42−, ascorbic acid, GSH, Cys, and S2O32− (150 μM) for 10 min in PBS buffer (pH 7.4)/DMSO (4:6) at 25 °C. The fluorescence spectra with a maximum emission at 491 nm were collected with an excitation at 390 nm. As shown in the Figure 4, NAS-1 showed a remarkable quenching in the presence of HSO3−, indicating a high reaction rate. Compared to HSO3−, other bio-abundant species with a nucleophilicity, including C2O42−, ascorbic acid, GSH, Cys, and S2O32−, displayed a slight quenching (around 10%). These results clearly indicated the high affinity of NAS-1 to HSO3−, over other nucleophiles, which allowed NAS-1 to quantitatively detect HSO3−, as a highly selective sensor in the aqueous environment.
Figure 4:

The fluorescence emission change of NAS-1 (1.0 × 10−5 M) after incubation with HSO3−, C2O42−, ascorbic acid, GSH, Cys, and S2O32− (150 μM) for 10 min in PBS buffer (pH 7.4)/DMSO (4:6) at 25 °C (λex=390 nm).
To evaluate the sensing ability of NAS-1 for detection HSO3− in a living system, cell imaging was conducted by using RK13 epithelial cells. After incubating with NAS-1 (10 mM) for 10 min, RK13 epithelial cells is mixed with HSO3−1 (50 mM), The supernatant was removed by aspiration, and the cells were visualized using an Olympus FV3000 Confocal Laser Scanning Microscope (excitation 405 nm, emission 485-585 nm). As shown in Figure 5A and 5C, RK13 epithelial cells and HSO3− do not show fluorescence, but NAS-1 gives a strong emission (Figure 5B), With addition of HSO3−, a strong fluorescence quenching is observed (Figure 5D), which indicated that the NAS-1 was still able to react to HSO3− as a sensor to quantitatively measure the concentration of HSO3− in a complicated cellular environment.
Figure 5.

Confocal microscopy in RK13 epithelial cells. (A) RK13 cells without treatment in RPMI media only; (B) RK13 cells with 10 mM NAS-1; (C) RK13 cells with 50 mM HSO3− quencher; (D) RK13 cells with 10 mM NAS-1 followed by 50 mM HSO3− quencher. (λem =405 nm, λem =485-585 nm)
As the comparison, a molecule (NAS-2) with an electron donating group (-OCH3) has been synthesized and investigated by using the same methods as used for NAS-1. NAS-2 showed a longer emission (602 nm) and high quantum yield (0.023) than NAS-1 due to the Push-Pull effect. Since NAS-1 shows high sensitivity and selectivity to HSO3− reflected by change of absorption and emission spectra based on a nucleophilic addition reaction, similar titrations were conducted for NAS-2 using the same experimental conditions used for NAS-1. As shown in the Figure 6, after incubating NAS-2(1.0 × 10 −5 M) with different amount of HSO3− (0-200 μM) in the aqueous media (PBS buffer (pH 7.4)/DMSO (4:6)) at 25 °C for 15 min, neither absorption nor emission spectra displayed a significant change, indicating the conjugation system of NAS-2 was not interrupted by HSO3− and no proof was collected for a nucleophilic addition reaction between NAS-2 and HSO3−. To test the selectivity, NAS-2(1.0 × 10 −5 M) was individually incubated with C2O42−, ascorbic acid, GSH, Cys, and S2O22− (150 μM) for 10 min in a PBS buffer (pH 7.4)/DMSO (4:6) at 25 °C. No significant emission spectra change was observed. All these findings clearly suggested that NAS-2 was not able to react with nucleophiles to give a spectroscopic signal as NAS-1.
Figure 6.
NAS-2 (1.0 × 10−5 M) did not show a significant change in absorption and emission spectra after incubating with HSO3−, C2O42−, ascorbic acid, GSH, Cys, and S2O32− (150 μM) for 10 min in PBS buffer (pH 7.4)/DMSO (4:6) at 25 °C (λex=412 nm).
Conclusion
In summary, a reaction-based sensor (NAS-1) was reported to quantitatively measure HSO3− by using a nucleophilic addition reaction in the aqueous media. NAS-1 showed a high affinity to HSO3− over other bio-abundant nucleophiles, which was reflected by a rapid fluorescence quenching and a ratiometric change in the absorption spectra, indicating a nucleophilic addition reaction occurred. NAS-1 also was applied to RK13 epithelial cells for cell imaging, which supported that NAS-1 could be used as a reliable approach to detect HSO3− in a cellular environment.
Figure 2:

The rational mechanism of HSO3− triggered a nucleophilic addition reaction for NAS-1.
Acknowledgments
Authors thank the financial support from the National Center for Research Resources (NCRR; 5P20RR016469) the National Institute for General Medical Science (NIGMS; INBRE-8P20GM103427), a component of the National Institutes of Health (NIH), and the Nebraska Research Initiative for equipment used in this project.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Zhang Y, Guan L, Yu H, Yan Y, Du L, Liu Y, Sun M, Huang D, Wang S (2016) Reversible fluorescent probe for selective detection and cell imaging of oxidative stress indicator bisulfite. Anal Chem 88: 4426–4431 [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Yang Q, Chen W, Mo L, Chen S, Kang J, Song X (2015) A ratiometric fluorescent probe for rapid, sensitive and selective detection of sulfur dioxide with large Stokes shifts by single wavelength excitation. Org Biomol Chem 13: 8663–8668 [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Song L, Sun Q, Chen Z, Ge Y, Zhang W, Qian J (2015) BODIPY-based colorimetric/ratiometric fluorescence probes for sulfite in aqueous solution and in living cells. RSC Adv 5: 91863–91868 [Google Scholar]
- 4.Samanta S Haider S, Dey P, Manna U, Ramesh A, Das G (2018) A ratiometric fluorogenic probe for the real-time detection of SO32− in aqueous medium: application in a cellulose paper based device and potential to sense SO32− in mitochondria. Analyst 143: 250–257 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Meng Q, Zhang R, Jia H, Zhang X, Zhang Z (2017) A ratiometric fluorescence probe for imaging sulfur dioxide derivatives in the mitochondria of living cells. Org Biomol Chem 15: 2734–2739 [DOI] [PubMed] [Google Scholar]
- 6.Sun YQ, Wang P, Liu J, Zhang J, Guo W (2012) A fluorescent turn-on probe for bisulfite based on hydrogen bond-inhibited C[double bond, length as m-dash]N isomerization mechanism. Analyst 137: 3430–3433 [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Liu L, Li X, Shao C, Huang X, Zhu B, Zhang X (2014) A highly selective colorimetric and far-red fluorescent probe for imaging bisulfite in living cells. RSC Adv 4: 33507–33513 [Google Scholar]
- 8.Wu MY, Li K, Li Y, Hou JT, Yu XQ (2014) A water-soluble near-infrared probe for colorimetric and ratiometric sensing of SO2 derivatives in living cells. Chem Commun 50: 183–185 [DOI] [PubMed] [Google Scholar]
- 9.Li J, Li R, Meng Z (2010) Sulfur dioxide upregulates the aortic nitric oxide pathway in rats. Eur J Pharmacol 645: 143–150 [DOI] [PubMed] [Google Scholar]
- 10.Migliore L, Copperdè F, (2009) Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res 674:73–84 [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Li K, Hou J, Li L, Lu C, Xie Y, Wang X, Yu X (2016) Novel tumor-specific and mitochondria-targeted near-infrared-emission fluorescent probe for SO2 derivatives in living cells. ACS Sens 1: 166–172 [Google Scholar]
- 12.Chen W, Liu X, Chen S, Song X, Kang J (2015) A real-time colorimetric and ratiometric fluorescent probe for rapid detection of SO2 derivatives in living cells based on a near-infrared benzopyrylium dye. RSC Adv 5: 25409–25415 [Google Scholar]
- 13.Tan L, Lin W, Zhu S, Yuan L, Zheng K (2014) A coumarin-quinolinium-based fluorescent probe for ratiometric sensing of sulfite in living cells. Org Biomol Chem 12: 4637–4643 [DOI] [PubMed] [Google Scholar]
- 14.Gu X, Liu C, Zhu Y, Zhu Y (2011) A boron-dipyrromethene-based fluorescent probe for colorimetric and ratiometric detection of sulfite. A boron-dipyrromethene-based fluorescent probe for colorimetric and ratiometric detection of sulfite. J Agric Food Chem 23: 11935–11939 [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Huo F, Zhang J, Xie Z, Chao J, Yin C, Tong H, Liu D, Jin S, Cheng F, Yan X (2012) A novel coumarin-based fluorescent probe for selective detection of bissulfite anions in water and sugar samples. Sens Actuators B 2012, 166 665–670 [Google Scholar]
- 16.Santos-Figueroa LE, Giménez C, Agostini A, Aznar E, Marcos MD, Sancenón F, Martínze-Máñez R (2013) Selective and sensitive chromofluorogenic detection of the sulfite anion in water using hydrophobic hybrid organic-inorganic silica nanoparticles. Angew Chem Int Ed 52: 13712–13716 [DOI] [PubMed] [Google Scholar]
- 17.Li H (2015) Rapidly responsive and highly selective fluorescent probe for sulfite detection in real samples and living cells. Anal Chim Acta 897:102–108 [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Pan J, Jiang X, Qin C, Zeng L, Zhang H, Zhang JF (2016) A mitochondria-targeted ratiometric fluorescent probe for rapid, sensitive and specific detection of biological SO2 derivatives in living cells. Biosens Bioelectron 77:725–732 [DOI] [PubMed] [Google Scholar]
- 19.Ye Z, Duan C, Sheng R, Xu J, Wang H, Zeng L (2018) A novel colorimetric and ratiometric fluorescent probe for visualizing SO2 derivatives in environment and living cells. Talanta 176: 389–396 [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Peng A, Lv Y, Zhang Y, Wang X, Zhang G, Tian Z (2017) A colorimetric fluorescent probe for SO2 derivatives-bisulfite and sulfite at nanomolar level. J Fluoresc 27:1767–1775 [DOI] [PubMed] [Google Scholar]
- 21.Jiang X, Wang L, Carroll SL, Chen J, Wang MC, Wang J (2018) Challenges and opportunities for small-molecule fluorescent probes in redox biology applications. Antioxid Redox Signal 29: 518–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li DP, Wang ZY, Cui J, Wang X, Miao JY, Zhao BX (2017) A new fluorescent probe for colorimetric and ratiometric detection of sulfur dioxide derivatives in liver cancer cells. Nature 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen R, Qian Y (2019) A mitochondria-oriented fluorescent probe for ultrafast and ratiometric detection of HSO3− based on naphthalimide-hemicyanine. New J Chem 43: 7606–7612 [Google Scholar]
- 24.Niu T, Yu T, Yin G, Chen H, Yin P, Li H (2019) A novel colorimetric and ratiometric fluorescent probe for sensing SO2 derivatives and their bio-imaging in living cells. Analyst 144:1546–1554 [DOI] [PubMed] [Google Scholar]
- 25.Yu F, Han X, Chen L (2014) Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem Commun 50:12234–12249 [DOI] [PubMed] [Google Scholar]
- 26.Houtwed H, Xie M, Ahmad A, Masters CD, Davison MM, Ding T, Kounovsky-Shafer K, Cao H (2019) Analysis of bisulfite via a nitro derivative of Cyanine-3 (NCy3) in the microfluidic channel, J. Fluorescence 29: 523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang E, Ding T, Xie M, Cao H (2018) Investigation of photophysical properties of 1,8-naphthalimides with an extended conjugation on naphthalene moiety via Suzuki coupling reaction. J Photochem Photobiol A 364: 145–150 [Google Scholar]
- 28.Rémy C, Allain C, Leray I (2017) Synthesis and photophysical properties of extended π conjugated naphthalimides. Photochem Photobiol Sci 16: 539–546 [DOI] [PubMed] [Google Scholar]
- 29.Paul S, Ghoshal K, Bhattacharyya M, and Maiti DK (2017) Detection of HSO3−: A rapid colorimetric and fluorimetric selective sensor for detecting biological SO2 in food and living cells. ACS Omega 2: 8633–8639 [DOI] [PMC free article] [PubMed] [Google Scholar]


