Abstract
Background and Objectives:
Patients with in-transit or limited cutaneous metastatic melanoma may benefit from intralesional injections with talimogene laherparepvec (TVEC), a modified oncolytic herpesvirus. However, its use in patients with adverse prognostic scores in a real-life clinical setting has not been studied.
Methods:
We performed a two-center retrospective analysis of 40 patients with metastatic melanoma treated with TVEC from 2015–2017. Demographics, overall response, and survival following therapy were noted.
Results:
Overall, there was a durable response rate (DRR) of 40%; median progression-free survival (PFS) was 10.5 months (m) and median overall survival (OS) was not reached. Bulky disease was associated with decreased OS (15.7m vs. not reached, P<0.05) and mPFS (2.3m vs. not reached, P<0.05) when compared with smaller tumors. Poor performance status (ECOG 2–3) was associated with worse OS (10.2m vs. not reached, P<0.05) and PFS (2.1m vs. not reached, P<0.05) compared to patients with ECOG 0–1. There was no difference in outcomes with age greater than 75 or with prior therapies. Adverse events were relatively tolerable.
Conclusions:
These findings demonstrate TVEC is an effective and safe treatment for metastatic melanoma in a real-life clinical setting, and suggest parameters to aid in appropriate therapy selection for optimal response.
Keywords: oncolytic virotherapy, talimogene laherparepvec, malignant melanoma
INTRODUCTION
Melanoma is a common and aggressive skin cancer that is increasing in incidence in the USA and worldwide [1]. Local or regional disease may be curable with surgical resection but relapse is common for patients with high risk disease. Prognosis for more advanced disease is poor with 5 year survival rates for stage IIID at <30% [2]. Patients with advanced disease limited to mostly cutaneous or subcutaneous disease, or have in-transit metastasis) present an unique challenge [3]. These may be amenable to surgical resection or systemic immune or targeted therapy [3,4]. However, injectable therapies could be ideal, given their low morbidity and clinically-accessible lesions.
Oncolytic viruses are naturally occurring or engineered viruses which selectively replicate and kill malignant cells and can induce systemic anti-tumor immune responses [5,6]. Although the therapeutic potential for oncolytic viruses has been recognized for decades, they were only recently introduced into the clinical setting [5]. Talimogene laherparepvec (TVEC) is a herpes simplex virus-1 based oncolytic virus demonstrated to have potent anti-tumor effects against melanoma [7]. It has several genetic modifications to enhance its clinical activity against tumor cells. TVEC has been developed as an intralesional therapy and is injected directly into accessible melanoma lesions. Several clinical trials in humans have demonstrated favorable anti-tumor responses with TVEC therapy in metastatic melanomas [8–11]. There is evidence not only for response of the primary tumor to TVEC treatment but also secondary regression of un-injected lesions and the presence of an anti-tumor immune response [8,12]. It is generally well tolerated; the most common toxicities were low-grade injection site reactions, fatigue, chills, and fevers [13].
Although approved by the FDA in 2015, the real-world use and efficacy of TVEC is unclear as the trials were performed in patients with generally excellent performance status with minimal co-morbidities. Here, we sought to characterize the clinical features, response to therapy and adverse events of patients receiving TVEC therapy through a retrospective analysis from two academic institutions.
METHODS
Patients
After receiving IRB approval from each site, we screened and included all patients with melanoma at Vanderbilt University Medical Center and the University of Alabama who received the FDA approved TVEC therapy between December 2015 to July 2017 into our study. Patients who were participating in concurrent combination immunotherapy clinical trials were excluded.
Study Design
We obtained baseline demographic data for each patient including age, gender, American Joint Committee on Cancer (AJCC, 7th edition. 2010) pathologic stage, performance status defined by the Eastern Cooperative Oncology Group (ECOG), and disease burden at the time of starting therapy based on lesion number and size. Additional information regarding prior treatments and responses were also recorded. To assess the efficacy of TVEC, we evaluated the objective response based on the Response Evaluation Criteria In Solid Tumors (RECIST) v1.1 criteria. Progression-free survival (PFS), overall survival (OS), and durable response rate (DRR) as previously published with other studies assessing TVEC [14]. Lesion injections were performed at the provider’s judgement based on therapy guidelines [8] Only injected lesion sizes were captured in this study due to the nature of documentation in real clinical practice. Adverse events were documented using clinic notes and graded based on Common Terminology Criteria for Adverse Events (CTCAE) guidelines.
Statistical analysis
OS and PFS were calculated on the basis of Kaplan-Meier curves and were compared using the logrank test. PFS was defined as the time from the start of treatment until disease progression. OS was defined as the time from the start of treatment until death for any reason. DRR was defined as complete response or partial response lasting for 6 months at any time after the best response date [8,14]. Tumor response was assessed from a combination of lesion measurement, pathology, and imaging. Patients were censored at their last follow-up. Analyses were performed using the statistical software GraphPad Prism 7 and R 3.5.1.
RESULTS
Patient Characteristics and Demographics
A total of 40 patients were included from two medical centers that received TVEC intralesional injections. Patient characteristics are described in Table 1. Of these, 70% were male (n=28) and age ranged from 43 to 89 years with a median of 73. Performance status and medical comorbidities varied, with 32.5% of patients having an ECOG score of 2–3 and 32.5% having greater or equal to 5 independent comorbid medical conditions. Many patients received prior therapies, 35% with conventional therapies such as chemotherapy or targeted therapy and 45% with immune checkpoint inhibitors. Most patients had skin/subcutaneous/lymph node only involvement (≤Stage IV M1a: 85%) whereas the remainder had visceral disease (Stage ≥ IV M1b-d 15%), and approximately half of the patients (47.5%) had any tumor ≥2cm in greatest dimension.
Table 1.
Patient baseline demographics
| Characteristic | N=40 | % |
|---|---|---|
| Age | ||
| Median (range) | 73 (43–89) | |
| Sex | ||
| Male | 28 | 70 |
| Female | 12 | 30 |
| ECOG | ||
| 0–1 | 27 | 67.5 |
| 2–3 | 13 | 32.5 |
| Bulky disease | ||
| Max lesion size ≥2cm | 19 | 47.5 |
| Prior therapies | ||
| Any | 22 | 55 |
| Conventional therapy | 14 | 35 |
| Immunotherapy | 18 | 45 |
| Comorbidities | ||
| 0–2 comorbidities | 14 | 35 |
| 3–4 comorbidities | 13 | 32.5 |
| ≥5 comorbidities | 13 | 32.5 |
| Disease substage | ||
| ≥IV M1a | 34 | 85 |
| >IV M1b | 6 | 15 |
Patient Outcomes
Of the patients evaluated, 42.5% had CR (n=17), 5% had PR (n=2), and the remainder were either non-responders or had progressive disease (n=21) (Fig 1). Median follow-up for this cohort was 14 months (range 1.9–26.7 months). Median overall survival for all patients was not reached at the time of last follow-up (Fig 2a). Progression free survival was similar to the prior studies [8] with a median of 10.5 months (Fig 2b). Durable response rate overall at 40% was higher than previously reported [8] (Fig 2c). Half of the patients in this cohort experienced disease progression during follow-up (50%, n=20). Subsequent treatment, if indicated, included immunotherapy, targeted therapies, clinical trials, additional TVEC injections, or resection/radiation therapy (Supplemental table 1). Lesion-specific response (either injected or non-injected) was not able to be captured due to lack of documentation in standard clinical practice.
Figure 1.
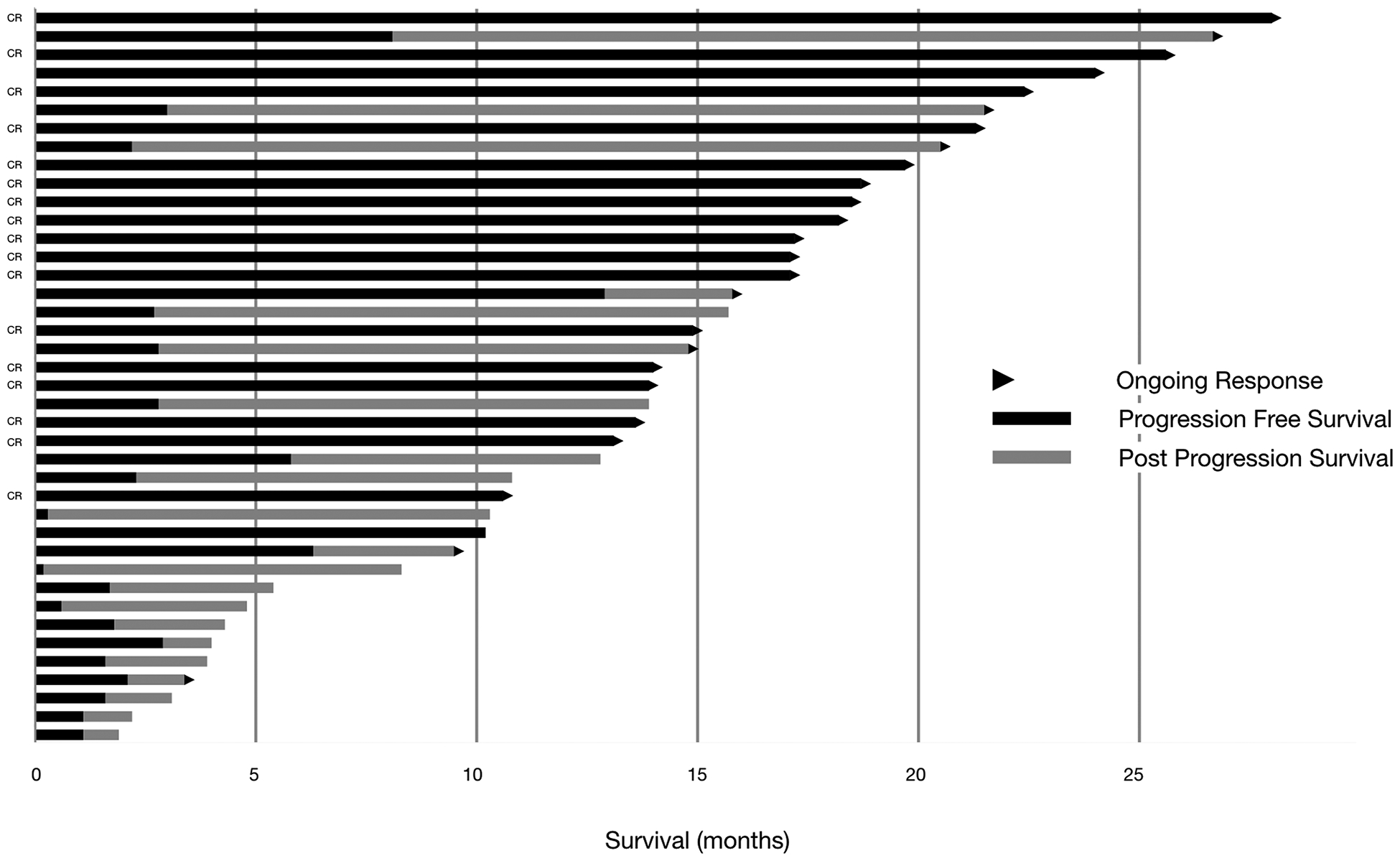
Swimmer’s plot for progression free and overall survival in patients treated with TVEC. Arrow indicates ongoing response.
Figure 2.
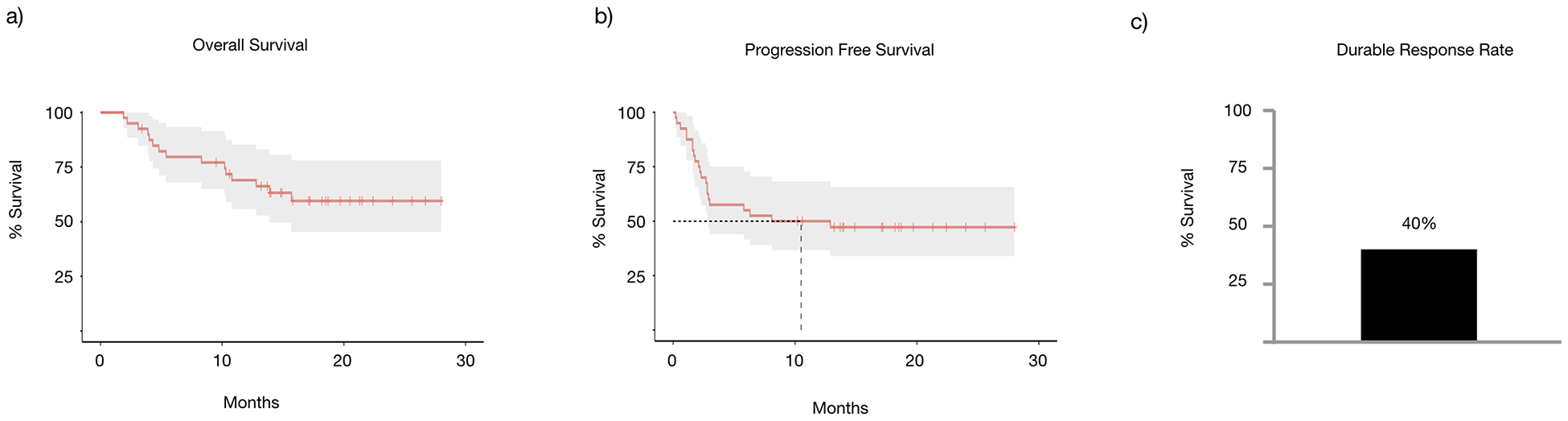
(a) Overall survival and (b) Progression-free survival in patients treated with TVEC over time. (c) Overall durable response rate of patients treated with TVEC.
Subgroup Analysis - Survival
We then assessed what patient or tumor characteristics would affect response and potentially guide therapy selection. In particular, the response of TVEC in patients with reduced performance status, prior therapies, and bulky disease is not well characterized. In our study, patients with poorer performance status at ECOG 2–3, when compared to patients with ECOG 0–1, had significantly decreased OS (median 10.2 months vs. not reached, P=0.0049) (Fig 3a), PFS (median 2.1 months vs. not reached, P=0.03) (Fig 3b), and DRR (23% vs 44%, P=0.19) (Fig 5a). Similarly, patients with larger tumors (≥2cm), when compared to those with smaller tumors, had significantly decreased OS (15.7 months vs. median not reached P=0.046) (Fig 3c), PFS (2.3 months vs. median not reached, P=0.00085) (Fig 3d), and DRR (16% vs. 62%, P=0.029) (Fig 5b). Age ≥75 years, when compared to those < 75, did not affect OS (not reached in either group, P=0.94) (Fig 3e), PFS (median 2.8 months vs. not reached, P=0.24) (Fig 3f), or DRR (39% vs. 41%, P=0.89) (Fig 5c). Additionally, prior therapies had no statistically significant effect on OS (median not reached in either group, P=0.24) (Fig 4a), PFS (median 2.9 months vs 10.5 months, P=0.76) (Fig 4b), or DRR (33% vs 42%, P=0.56) (Fig 5d). This held true for both prior immunotherapies (OS: median not reached, P=0.94; PFS: median 6.05 months vs. not reached, P=0.32) (Fig 4c&d) and prior other therapies (OS: median not reached, P=0.47; PFS median 4.65 months vs 12.9 months, P=0.5) (Fig 4e&f).
Figure 3.
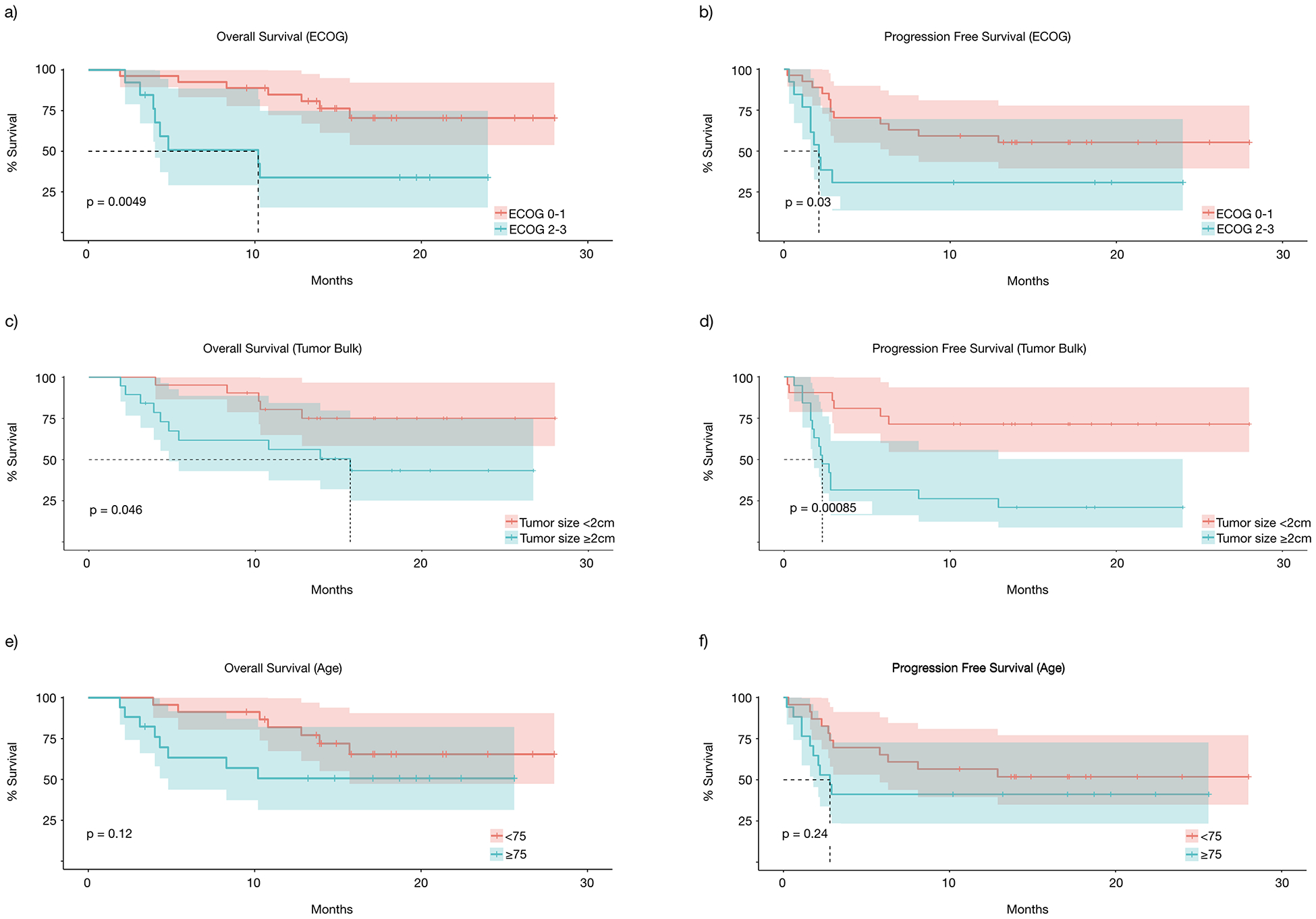
Subgroup survival analysis, overall survival and progression free survival based on (a&b) ECOG, (c&d) tumor bulk, and (e&f) age.
Figure 5.
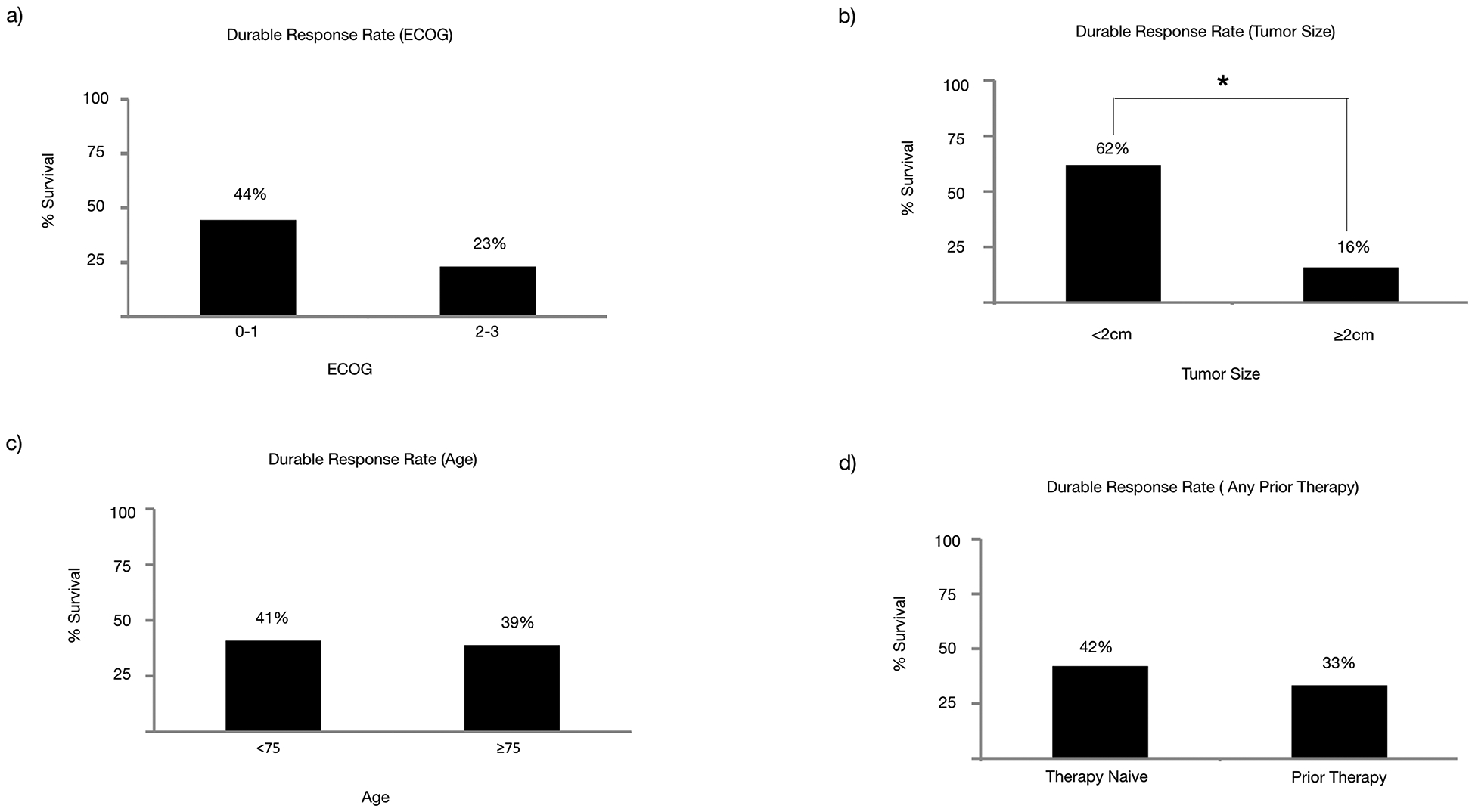
Durable response rate analysis based on (a) ECOG, (b) Tumor bulk, (c) Age, or (d) Any Prior Therapies. Statistically significant differences are denoted with *.
Figure 4.
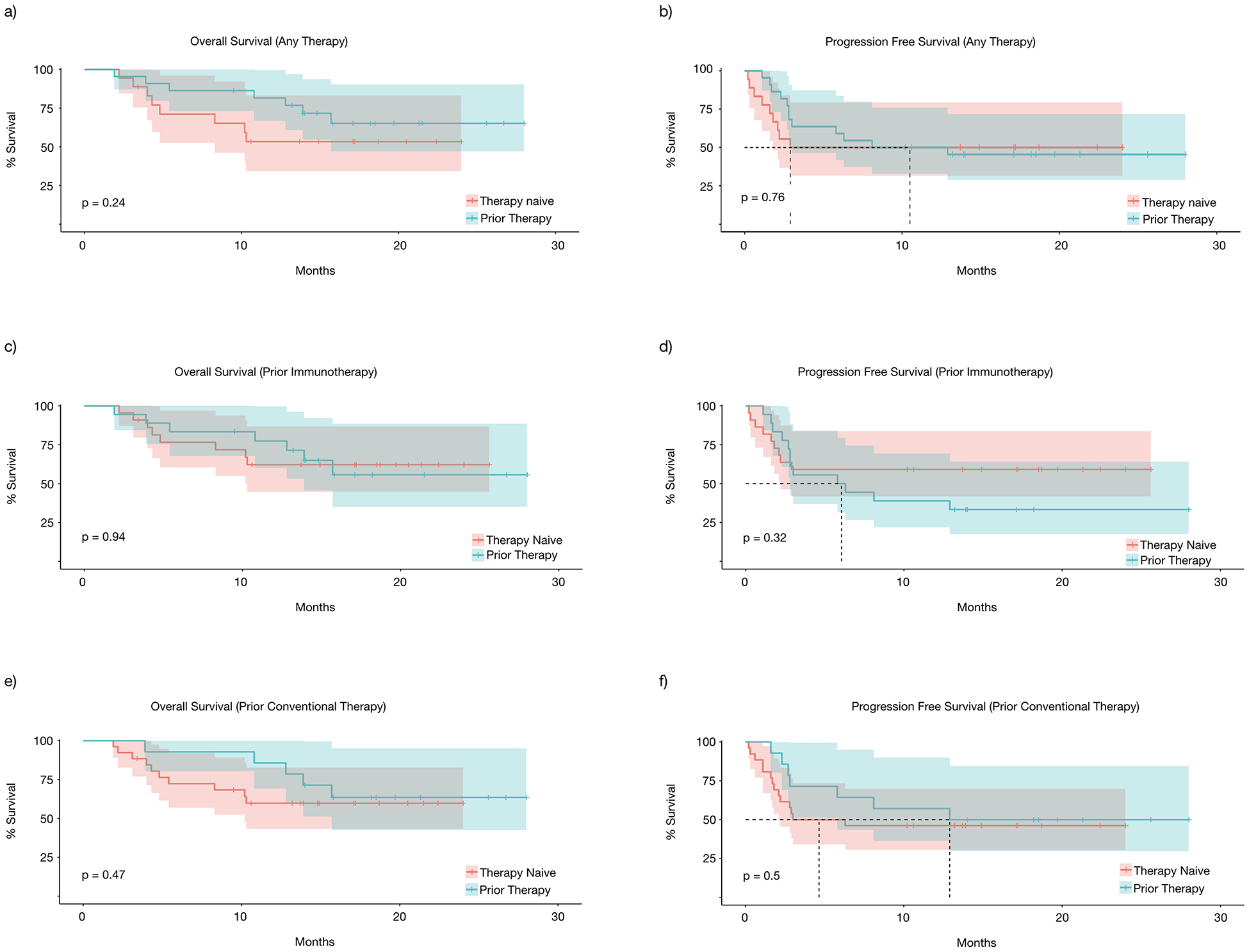
Subgroup survival analysis, both OS and PFS based on (a&b) any prior therapies, (c&d) prior immunotherapies, (e&f) prior conventional therapies.
Adverse Events
Adverse events in patients receiving TVEC treatments were relatively mild (7.5% AE were grade 3 or higher), which was similar or lower than previously reported [8,15] with 3 patients (7.5%) discontinuing therapy due to treatment side effects. Most adverse effects were fevers and fatigue (37.5% and 35%, respectively), followed by local reactions such as cellulitis and pain (12.5% and 7.5%, respectively) (Table 2). One patient experienced grade 3 periorbital edema. No deaths were attributed to treatment.
Table 2.
Adverse event reporting
| AE | Any Grade (%) | Grade 3–4 (%) |
|---|---|---|
| All | 32 (80) | 3 (7.5) |
| Fatigue | 15 (37.5) | 0 |
| Fever | 14 (35) | 0 |
| Rash/cellulitis | 5 (12.5) | 1 (2.5) |
| Pain | 3 (7.5) | 0 |
| Neuro | 3 (7.5) | 1 (2.5) |
| GI | 2 (5) | 0 |
| Cough | 1 (2.5) | 0 |
| Peri-orbital edema | 1 (2.5) | 1 (2.5) |
DISCUSSION
The intralesional oncolytic treatment TVEC was approved by the FDA for use in 2015. To our knowledge, this study is the first report of its use in a “real-world” clinical setting. In this population, we found that TVEC was well-tolerated and had a higher than expected durable response rate of 40%. Patients with bulky tumors or poor functional status had decreased survival and response. In contrast, higher age and prior therapies had no significant effect on survival or response rates.
The durable response rate, used to describe response to TVEC therapy in the Optim III trial [8] takes into account the response pattern including pseudo-progression seen with immunotherapies [14]. Using the definition described by prior studies, we find that our patient population had a durable response rate of 40%, significantly higher than previously described at 16% [8]. This is surprising as most therapies perform worse in clinical settings as compared to a trial setting. Several factors may contribute to this finding. Patients were selected based on the physician’s discretion and not limited to trial guidelines. While patients in our study were older with worse functional status than patients in the trial setting, they had less visceral disease, as noted by 15% stage IV M1b-d disease. Importantly, TVEC is known to perform superiorly in stage III and IVM1a disease as compared to stage IVM1b or greater disease (33% response rate versus 16% versus 2%, respectively in the Optim study) [8]. Our study corroborates this finding and shows that with judicious patient selection, a high rate of clinical response can be observed. Given our limited sample size and the retrospective nature of this study, we were unable to assess the response of non-injected sites as well as visceral disease, and future studies to gauge the magnitude of those responses in a clinical setting would be helpful.
Contrary to prior reports [8], our study finds that prior treatments, whether immune-related or not, did not significantly affect response to TVEC therapy. This may relate to the smaller sample size and subtle differences may not be apparent at this scale. Additional factors which may contribute are concurrent comorbidities or immunosuppressive medications that may interfere with response to immunotherapies. Notably, this suggests TVEC therapy is appropriate for patients who has received and failed prior lines of therapies. More research, however, is warranted to fully understand the relationship between prior therapy and response to TVEC.
Many of the patients in our cohort are elderly patients with poorer functional status who are not ideal candidates for systemic therapies or surgery. One could hypothesize that immunosenescence, or immune changes caused by advancing age, could compromise responses to immunotherapy [16,17]. Our study finds that patients older than 75 responded similarly to TVEC therapy as those younger than 75. This recapitulates other studies showing that response to immunotherapies is not hampered by advanced age [18,19] and may even be enhanced [20] in the setting of decreased T regulatory cells. Impaired functional status, however, had a greater impact of response rates, consistent with prior experience with ipilimumab [21,22] and nivolumab [23].
In theory, TVEC therapy is a suitable treatment for larger tumors. Our results, however, indicate that patients with greater tumor burden have inferior responses with TVEC treatment. Prior studies have demonstrated baseline tumor size and location-specific tumor burden as independent prognostic factors for response to immunotherapy [24,25]. Notably, the degree of immune activation indicated by the ratio of reinvigorated T cells relative to tumor burden is associated with PD-1 response [26]. As TVEC causes a vigorous local immune response, it would be interesting to determine the relationship between immune activation and tumor burden with response rates. Combining T-VEC with immune checkpoint inhibitors could potentially overcome this limitation, as combination therapies have demonstrated early evidence of excellent clinical outcomes [27,28].
Similar to prior studies, TVEC has a tolerable safety profile. Frequently occurring adverse events were either local reactions or flu-like symptoms. There were three (7.5%) grade 3 events and no treatment related death. In relation to systemic immunotherapies, TVEC injections incurred lower rates of adverse events [29], although the assessment is skewed by different patient populations and would benefit from a head to head comparison.
In summary, this retrospective study of patients who received TVEC dissects clinical factors that affect therapy response. In an era where the paradigm for advanced melanoma is rapidly changing, the findings of this study offers guidance in therapy selection and for predicting response for patients with advanced melanoma.
Supplementary Material
Supplemental Table 1. Clinical Progression
Synopsis for Table of Contents:
Study of TVEC use in a real-life clinical population leads to identification of clinical factors associated with response to TVEC therapy to aid in patient selection.
Funding:
Douglas B Johnson K23 CA204726
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Conflict of Interest: DBJ serves on advisory boards for Array Biopharma, Bristol-Meyers Squibb, Incyte, Merck, Novartis, and receives research funding from Bristol-Meyers Squibb and Incyte.
REFERENCES
- 1.N H AM N MK, et al. : SEER Cancer Statistics Review, 1975–2014. Natl Cancer Institute Bethesda, MD 2017. https://seer.cancer.gov/csr/1975_2014/. [Google Scholar]
- 2.Gershenwald JE, Scolyer RA, Hess KR, et al. : Melanoma Staging: Evidence-Based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA A Cancer J Clin 2017; 67:472–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Read RL, Haydu L, Saw RPM, et al. : In-transit Melanoma Metastases: Incidence, Prognosis, and the Role of Lymphadenectomy. Ann Surg Oncol 2014; 22:475–81. [DOI] [PubMed] [Google Scholar]
- 4.Speicher PJ, Meriwether CH, Tyler DS.: Regional therapies for in-transit disease. Surg Oncol Clin N Am 2015; 24:309–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawler SE, Speranza M-C, Cho C-F, Chiocca EA.: Oncolytic Viruses in Cancer Treatment. JAMA Oncol 2017; 3:841. [DOI] [PubMed] [Google Scholar]
- 6.Hamid O, Hoffner B, Gasal E, et al. : Oncolytic immunotherapy: unlocking the potential of viruses to help target cancer. Cancer Immunol Immunother 2017; 66:1249–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohlhapp FJ, Kaufman HL.: Molecular pathways: Mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res 2016; 22:1048–54. [DOI] [PubMed] [Google Scholar]
- 8.Andtbacka RHI, Kaufman HL, Collichio F, et al. : Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015; 33:2780–8. [DOI] [PubMed] [Google Scholar]
- 9.Rehman H, Silk AW, Kane MP, Kaufman HL.: Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer 2016; 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson DB, Puzanov I, Kelley MC.: Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy 2015; 7:611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senzer NN, Kaufman HL, Amatruda T, et al. : Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 2009; 27:5763–71. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman HL, Amatruda T, Reid T, et al. : Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study. J Immunother Cancer 2016; 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long GV, Grob J-J, Nathan P, et al. : Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol 2016; 0:949–54. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman HL, Andtbacka RHI, Collichio FA, et al. : Durable response rate as an endpoint in cancer immunotherapy: Insights from oncolytic virus clinical trials. J Immunother Cancer 2017; 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chesney J, Awasthi S, Curti B, et al. : Phase IIIb safety results from an expanded-access protocol of talimogene laherparepvec for patients with unresected, stage IIIB–IVM1c melanoma. Melanoma Res 2018; 28:44–51. [DOI] [PubMed] [Google Scholar]
- 16.Tomihara K, Curiel TJ, Zhang B.: Optimization of Immunotherapy in Elderly Cancer Patients. Crit Rev Oncog 2013; 18:573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joudi F, Smith B, O’Donnell M, Konety B.: The Impact of Age on the Response of Patients With Superficial Bladder Cancer to Intravesical Immunotherapy. J Urol 2006; 175:1634–40. [DOI] [PubMed] [Google Scholar]
- 18.Betof AS, Nipp RD, Giobbie‐Hurder A, et al. : Impact of Age on Outcomes with Immunotherapy for Patients with Melanoma. Oncologist 2017; 22:963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnpulle RAN, Conry RM, Sosman JA, et al. : Responses to immune checkpoint inhibitors in nonagenarians. Oncoimmunology 2016; 5:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kugel CH, Douglass SM, Webster MR, et al. : Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin Cancer Res 2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad SS, Qian W, Ellis S, et al. : Ipilimumab in the real world: The UK expanded access programme experience in previously treated advanced melanoma patients. Melanoma Res 2015; 25:432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diem S, Kasenda B, Martin-Liberal J, et al. : Prognostic score for patients with advanced melanoma treated with ipilimumab. Eur J Cancer 2015; 51:2785–91. [DOI] [PubMed] [Google Scholar]
- 23.Heidelberger V, Goldwasser F, Kramkimel N, et al. : Clinical parameters associated with anti-programmed death-1 (PD-1) inhibitors-induced tumor response in melanoma patients. Invest New Drugs 2017; 35:842–7. [DOI] [PubMed] [Google Scholar]
- 24.Massa R, Sparrow M, Lin H, et al. : Relationship between pre-treatment organ-specific tumor burden and response to immunotherapy in advanced melanoma. J Clin Oncol 2018; 36. [Google Scholar]
- 25.Joseph RW, Elassaiss-schaap J, Kefford R, et al. : Baseline Tumor Size Is an Independent Prognostic Factor for Overall Survival in Patients with Melanoma Treated with Pembrolizumab. Clin Cancer Res 2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang A, Postow M, Orlowski R, et al. : T-cell invigoration to tumour burden ratio associated with anti- PD-1 response. Nature 2017; 545:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesney J, Puzanov I, Collichio F, et al. : Randomized , Open-Label Phase II Study Evaluating the Ef fi cacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced , Unresectable Melanoma. J Clin Oncol 2018; 36:1658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribas A, Dummer R, Puzanov I, et al. : Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017; 170:1109–1119.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015; 373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Clinical Progression


