Abstract
Objective:
A relatively low level of lysosomal photodamage has been shown capable of promoting the efficacy of photodamage simultaneously or subsequently directed to mitochondrial/ER sites. The procedure has hitherto involved the use of two photosensitizing agents that require irradiation at two different wavelengths and different formulation techniques. This, together with different pharmacokinetic profiles of the photosensitizers, adds a layer of complexity to a protocol that we have sought to circumvent. In this study, liposomal formulations were used to direct photodamage created by benzoporphyrin derivative (BPD, Verteporfin) to lysosomes, mitochondria and the ER. This resulted in the development of an optimal targeting profile using a single agent and a single wavelength of activating irradiation.
Materials/Methods:
These studies were carried out in monolayer cultures of OVCAR5 tumor cells. BPD localization was modified by lipid anchoring and formulation in liposomes, and was assessed by fluorescence microscopy. Irradiation was carried out at 690±10 nm with photodamage assessed also using fluorescent probes and microscopy.
Results:
BPD normally localizes in a wide variety of sub-cellular loci that include both mitochondria and the ER, but lysosomes are spared from photodamage. Using a liposomal formulation containing BPD anchored to a lipid resulted in the targeting of lysosomes. A mixture of liposomes containing “free” and “anchored” BPD was shown to significantly promote photokilling. Eliminating cholesterol from the formulation of the anchored product enhanced lysosomal photodamage; prior studies had revealed that excess cholesterol can have a cytoprotective effect when lysosomes are the PDT target.
Discussion:
The ability of a liposomal formulation to change localization patterns permits directing photodynamic therapy toward specific sub-cellular loci, thereby promoting photokilling. Incorporating chemotherapeutic agents into such formulations could represent a logical next step in assessing the ability of directed photodamage to enhance tumor eradication. Lasers Surg. Med. 50:499–505, 2018. © 2018 Wiley Periodicals, Inc.
Keywords: photodynamic therapy, liposomes, benzoporphyrin derivative, photodamage, formulation
INTRODUCTION
A 1996 report described the ability of photodynamic therapy (PDT) involving a two-photosensitizer combination to eradicate transplanted tumors in a rat model at a 1 cm thickness [1]. This depth is greater than the predicted ability of light at 650–700 nm to penetrate tissues by a factor of 2 [2]. A later series of studies established that the observed enhanced PDT efficacy resulted from an effect of prior or simultaneous lysosomal photodamage that significantly enhanced photokilling by photodamage directed at mitochondria and ER [3–5]. This enhanced efficacy was traced to release of calcium ions from photodamaged lysosomes that initiated a calpain-catalyzed cleavage of the autophagy-related protein ATG5 to a truncated form that promoted pro-apoptotic effects after mitochondrial/ER photodamage [6].
While the enhanced degree of photokilling was impressive, several concerns need to be dealt with before this multi-targeting approach can be translated into clinical practice. Pharmacokinetic considerations led to a complex drug-administration protocol [1] and the requirement for light at two different wavelengths would add an element of complexity to clinical protocols.
BPD is usually administered in a liposomal formulation to solubilize a relatively hydrophobic product. We have prepared two liposomal formulations to deal with these problems. One contained “free” BPD and was tested for its ability to mimic the localization observed during studies in cell culture where BPD was dissolved in dimethylformamide. A second new liposomal formulation consisted of BPD anchored to a lipid. This is intended to redirect BPD targeting to lysosomes. The objective of this study was to enhance photokilling of tumor cells by simultaneously targeting lysosomes and ER/mitochondria using: (i) liposomal formulations to modulate the subcellular localization of BPD and (ii) simplified light dosimetry with a single activation wavelength to trigger photodamage in multiple subcellular loci. The current findings demonstrate that this approach significantly enhances the tumoricidal potency of BPD at significantly lower energy densities than either formulation alone.
MATERIALS AND METHODS
Chemicals and supplies
Reagents specified in the protocols were obtained from Sigma–Aldrich (St Louis, MO) and were of the highest available purity. The fluorescent probes used in this study were purchased from Life Technologies (Carlsbad, CA), Inc. Benzoporphyrin derivative (BPD) was provided by VWR and was either formulated in liposomes as described below or dissolved in dimethylformamide (DMF) to form a 250 μM stock solution. This was diluted 1:200 when added to cell cultures to provide a concentration of 0.5 μM. The extracellular BPD level was adjusted as indicated below.
Synthesis of BPD liposomes and lysophosphocholine (LPC)-BPD liposomes
A moderately cationic liposomal formulation that promotes cellular uptake was used to entrap free (BPDf) and anchored (BPDa) and was prepared as reported [7,8]. All lipids were obtained from Avanti® Polar Lipids (Alabaster, AL). A lipid mixture in chloroform was prepared consisting of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, 60 mol%), 1,2-dioleoyl-3-trimethylammonium-propane (chloride salt) (DOTAP, 7.9 mol%), cholesterol (28.9 mol%), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy-(polyethyleneglycol)-2000] (ammonium salt) (DSPE-mPEG-2000, 3.1 mol%). A similar formulation lacking cholesterol was also prepared. BPD or LPC-BPD (0.6 mol%) were then added to the mixture and the chloroform was evaporated by nitrogen gas flow and storage under vacuum to prepare a dry lipid film. The lipid film was hydrated using PBS and was subject to five freeze-thaw cycles consisting of 5 min incubation in ice, a 10 min incubation in a darkened water bath at 428C, and 30 seconds of vortexing. The resultant multilamellar vesiclemixturewas extruded 11 times through two 0.1 μm polycarbonate extrusion membranes (What-man®). The liposomes were dialyzed against PBS 48C for 24 hours and stored in the dark at 48C. BPD content in liposomes was quantified using absorption spectrophotometry (ε687 nm = 34,895 M-1.cm-1). Liposome hydrodynamic diameter and ζ-potential were measured using a Zetasizer Nano ZS Dynamic Light Scattering Instrument (Malvern Instruments, Ltd., Houston, TX).
BPD was anchored to the phospholipid 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (16:0 LPC, Avanti® Polar Lipids, Inc.) through Steglich esterification in dichloromethane using a modified protocol previously reported for other porphyrin lipid conjugates [9]. BPD was mixed with a 10-fold molar excess of 1-ethyl-3-(3dimethylaminopropyl) carbodiimide, a fivefold molar excess of 4-(dimethylamino)pyridine and a 12-fold molar excess of N,N-diisopropylethylamine. The mixture was added to 16:0 LPC at a BPD-to-lipid ratio of 5-to-1 and was reacted for 48 hours at room temperature. The lipid anchored BPD (BPDa) was purified from the mixture using preparatory thin layer chromatography with a 10% DCM in methanol mobile phase and quantified using absorption spectrophotometry (ε687 nm = 34,895 M-1.cm-1).
In the context of this report, BPDf refers to free BPD solubilized using liposomes, BPDa+c indicates anchored BPD contained in liposomes containing cholesterol while BPDa−c = anchored BPD in cholesterol-free liposomes.
Fluorescence polarization
Polarization of solutions of BPD dissolved in ethanol, glycerol, or contained in the various liposomal preparations (diluted into PBS) was determined using a Beacon 2000 Fluorescence Polarization apparatus at 37°C. Appropriate interference filters were used to set excitation at 400 nm and fluorescence emission at 690 nm. The average ± standard deviation is reported for five determinations.
Cell culture and clonogenic assays
OVCAR5 cells were grown in RPMI1640 medium +10% fetal bovine serum and antibiotics. Methodology for carrying out clonogenic assays has been described [10]. Viability was determined by colony counting using an Oxford Optronix GelCount device. This device can identify groups of 30 cells or more as colonies. Although the GelCount can detect colonies without additional staining, crystal violet was used as a colony marker. All experiments were performed in triplicate.
PDT protocols
Cells were grown on 30 mm cover slips in sterile plastic dishes. Cultures were incubated at 37°C with 0.5 μM BPD for 3–24 hours in various formulations. The medium was replaced and the dishes irradiated with a 600-watt quartz-halogen source. The light beam was passed through a 10 cm layer of water to remove IR and the bandwidth further confined by interference filters (Oriel, Stratford CT) to 690 ± 10 nm. BPD concentrations are specified for each experiment. The effect of each formulation alone, and of mixtures of BPDf + BPDa were examined. For all such studies, the light dose was 200 mJ/sq cm unless otherwise indicated.
When lysosomes and mitochondria/ER are separately targeted, using different photosensitizing agents and different wavelengths of light, it is feasible to adjust the PDT doses (photosensitizer concentration and light dose) independently. With a mixture of liposomal formulations containing the same photosensitizer, the only variable is the relative proportions of each formulation. To produce appropriate levels of photodamage for some studies, we reduced the level of BPDf to 0.05 μM while maintaining the anchored preparations at 0.5 μM. In studies designed to assess sites of photodamage, the light dose was increased to 600 mJ/sq cm. This high light dose was needed to demonstrate sites of photodamage. Light doses used to reveal synergistic interactions produce barely detectable effects with individual formulations since photokilling is limited to 20–30% of the cell population.
Microscopy
Effects of photodamage on maintenance of the mitochondrial membrane potential (ΔΨm) were assessed by fluorescence microscopy using the probe mitotracker orange (MTO). Lysotracker green was used to track lysosomal localization patterns and to assess maintenance of the lysosomal pH gradient after photodamage. Co-localization studies were carried out 3 hours after loading incubations. Fluorescence probes were loaded during 10 min incubations at 378. Images showing BPD localization were obtained using 360–420 nM excitation and a 650 nm high-pass filter in the emission light path. MTO was detected with 450–490 nm excitation and 520–600 nm emission filters. LTG was detected with 440–500 nm excitation and a 520–600 nm emission filter. Images were acquired with a Nikon E-600 microscope using a Rolera EM-CCD camera and MetaMorph software (Molecular Devices, Sunnyvale, CA). Phase contrast images were also obtained. At least three images were acquired for each sample, with typical representations shown here.
RESULTS
A comparison of localization patterns of BPD formulated with DMF versus liposomal free BPD (BPDf) is shown in Figure 1. The extracellular BPD concentration was 0.5 μM for both formulations to provide a direct comparison. There was no obvious difference in the fluorescence patterns. Co-localization studies with LTG revealed that both BPDa−c and BPDaþc demonstrated a fluorescence pattern consistent with lysosomal localization while the non-anchored BPDf was broadly distributed (Fig. 2). The latter result is consistent with prior studies relating to the affinity of BPD for mitochondria and the ER [5].
Fig. 1.
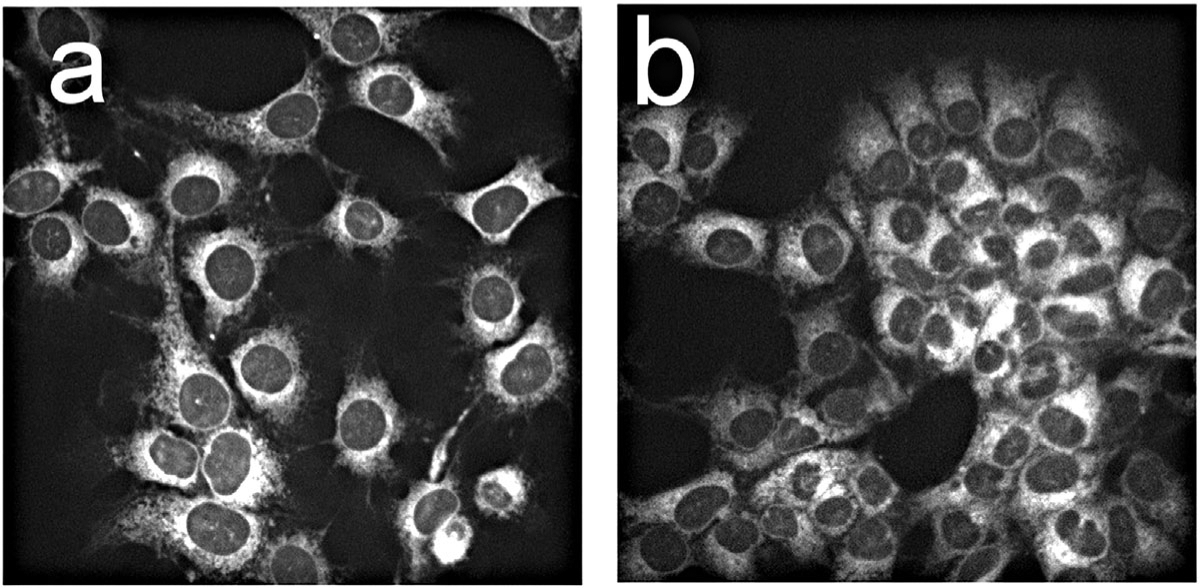
Fluorescence localization in OVCAR5 cells of BPD dissolved in DMF (a) or delivered as the lipo-BPD formulation termed BPDf (b). The extracellular concentration of BPD was 0.5 μM with images acquired after 1 hour incubations.
Fig. 2.
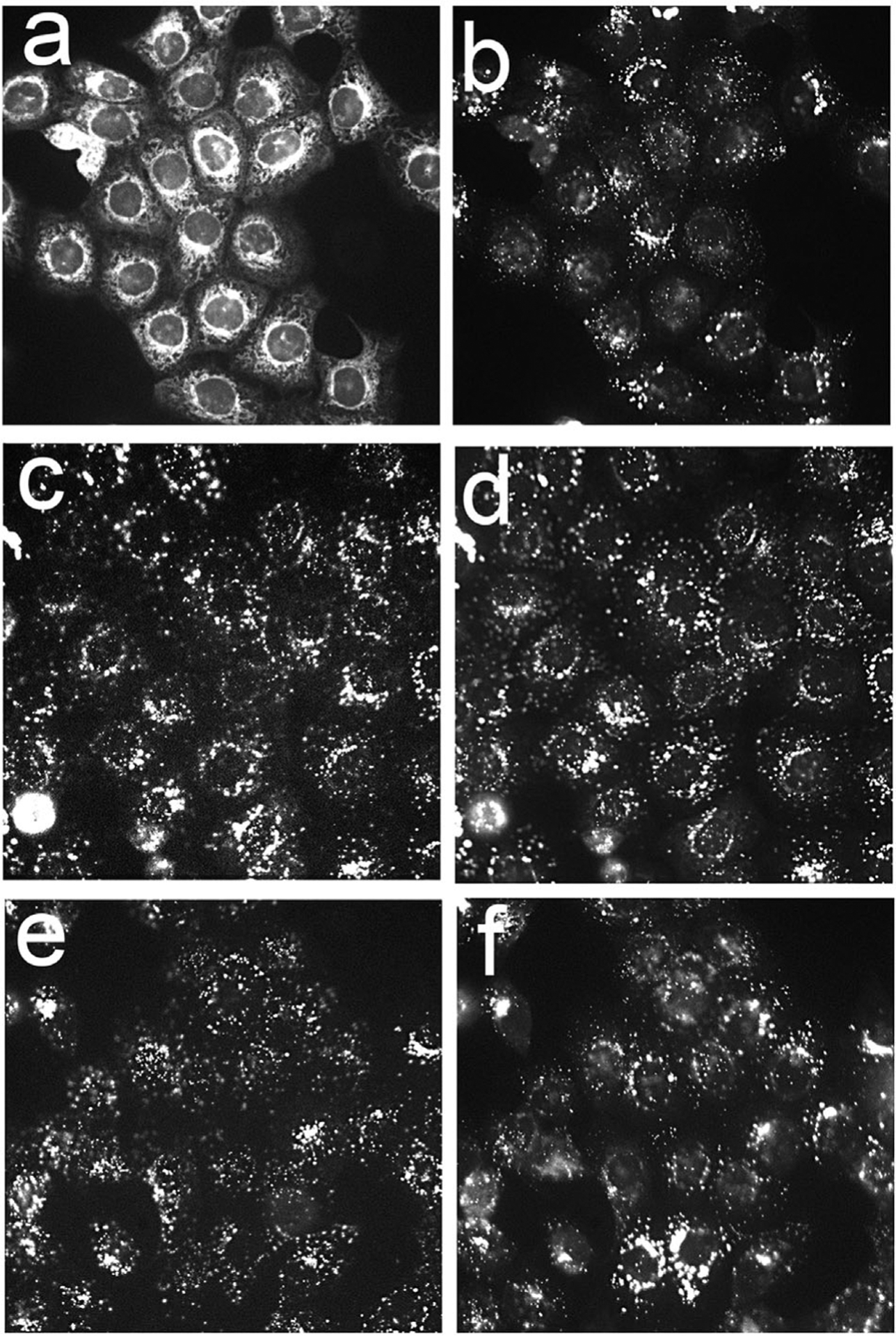
Fluorescence localization patterns in OVACR5 cells of BPDf (a), BPDa+c (c) and BPDa−c (e) compared with localization of Lysotracker Green (b,d,f). Cells were incubated for 3 hours with liposomal preparations with extracellular BPD concentrations of 0.5 μM.
A further comparison of the effects of irradiation on maintenance of ΔΨm and of the lysosomal pH gradient by the different liposomal formulations is shown in Figure 3. In this study, BPD concentrations were 0.05 μM for BPDf and 0.5 μM for the other formulations. To assess photodynamic effects, the same high light dose was employed (600 mJ/sq cm). Under these conditions, photodamage catalyzed by BPDf abolished ΔΨm but there was no detectable effect on ΔΨm by either anchored formulation. BPDf did not cause any detectable lysosomal photodamage as indicated by the unchanged LTG localization pattern. Irradiation of cells photosensitized by BPDa−c or BPDa+c had no effect on ΔΨm. There was, however, a loss of the lysosomal pH gradient that was more substantial with the BPDa−c preparation. These results are consistent with localization studies shown in Figure 2.
Fig. 3.
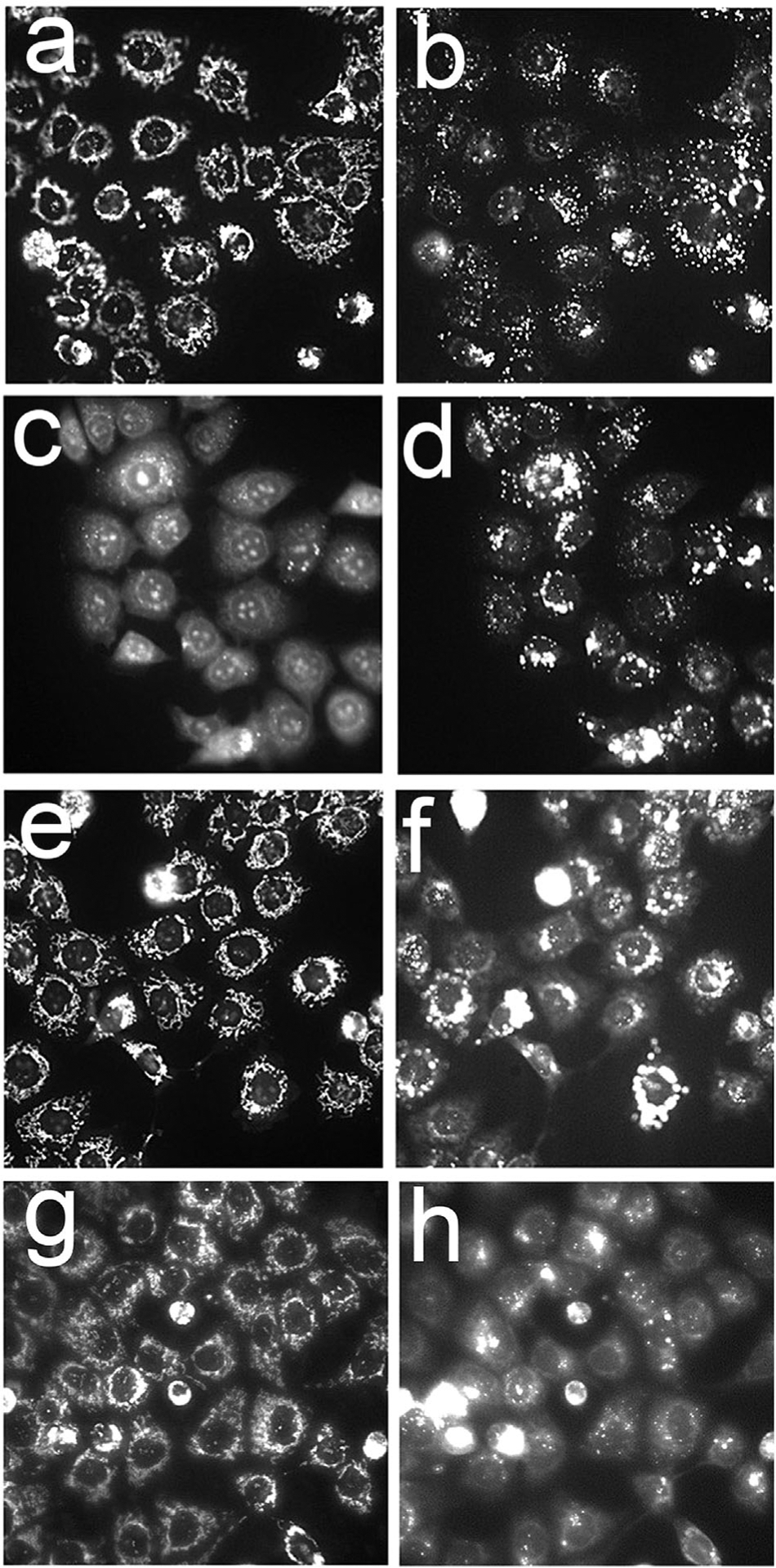
Effects of different BPD formulations on the mitochondrial membrane potential probed with MTO (a,c,e,g) and the lysosomal pH gradient as detected by LTG (b,d,f,h) directly after irradiation (690 ± 10 nm, 600 mJ/sq cm). a,b = untreated cells; c,d = BPDf; e,f = BPDa+c; g,h = BPDa−c The extracellular BPD concentration was 0.05 μM (BPDf) or 0.5 μM (BPDa). Cells were incubated with the liposomal formulations for 3 hours before irradiation.
A hallmark of mitochondrial photodamage is an immediate loss of the membrane potential ΔΨm, an effect associated with migration of cytochrome c into the cytoplasm and initiation of apoptosis [11]. In prior studies, we had shown that sequential photodamage to lysosomes, then mitochondria, orsimultaneous photodamage at both sitespotentiated the efficacy of mitochondrial targeting, leading to a loss of ΔΨm [3–5]. When a mixture of BPDf and BPDa−c was used, irradiation at 690 nm (200 mJ/cm sq) resulted in a signifi-cant loss of ΔΨm (Fig. 4f). The anchored liposomal preparation containing cholesterol (BPDa+c) was less effective in this regard (compare panels e and f). These “combination PDT” studies were carried out at a lower light dose than was employed in Figure 3 to demonstrate the efficacy of the combination protocols. At the lower light dose, the individual components of the combination had almost no detectable effect on the mitochondrial membrane potential.
Fig. 4.
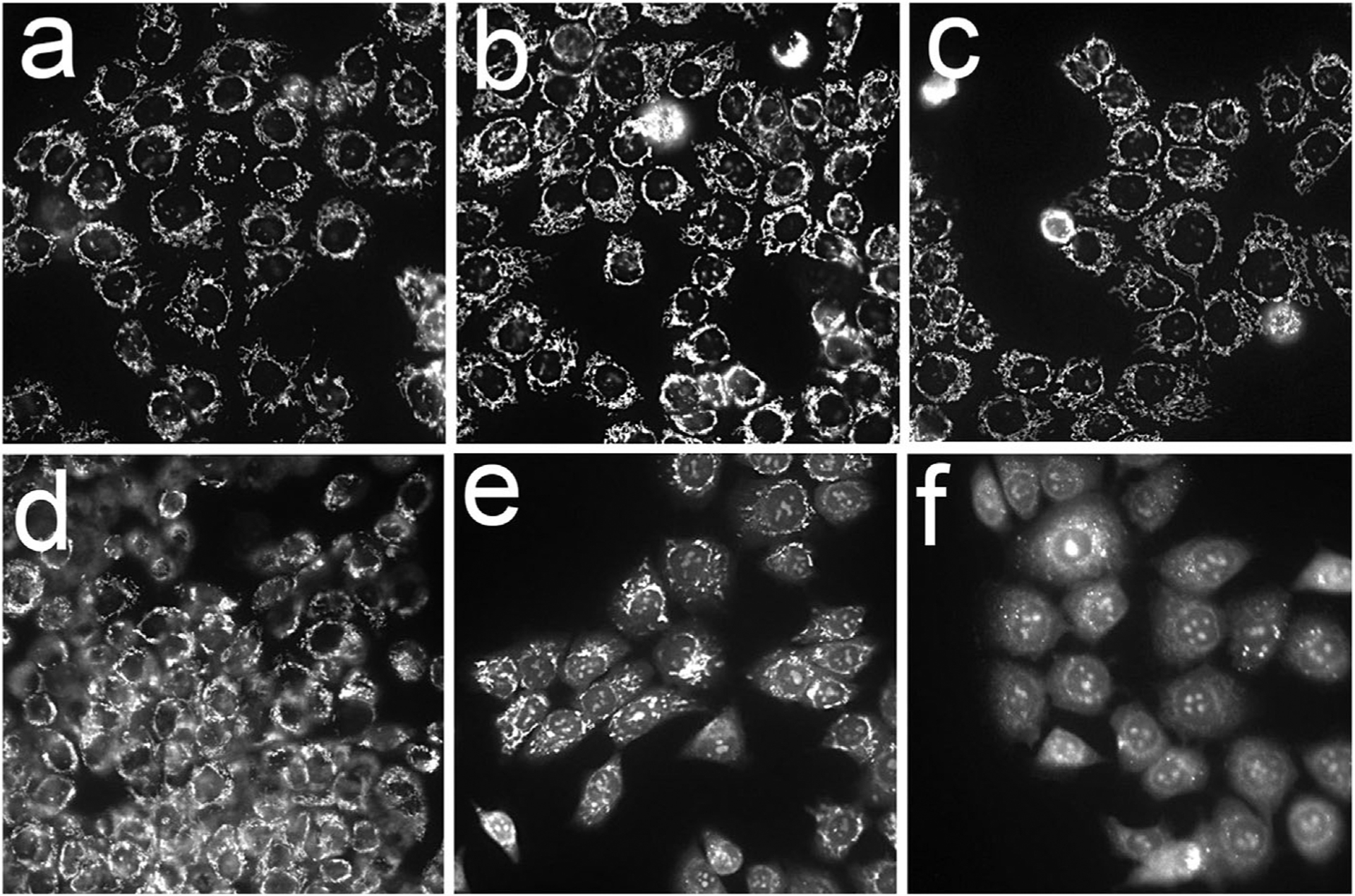
Photodynamic effects of different BPD formulations on the mitochondrial membrane potential. After 3 hours loading incubations, OVCAR5 cells were irradiated at 690 nm (200 mJ/ sq cm) with the MTO fluorescence labeling pattern determined directly after irradiation. a = untreated cells, b –BPDf; c, BPDa+c; d, BPDa−c; e, BPDf/BPDa+c; f, BPDf/BPDa-c. Extracellular levels of the photosensitizers are indicated in the legend to Figure 2.
To utilize a combination of the two liposomal preparations (free vs. anchored BPD) with a single irradiation interval, it was therefore necessary to reduce the concentration of BPDf to 0.05 μM while keeping the BPD concentration in the BPDa formulations at 0.5 μM. Clonogenic studies using a 200 mJ/sq cm light dose (Fig. 5) indicate that the BPDa−c formulation provided superior results. While efficacy may be improved by altering the ratio, the conditions chosen (BPDa at a 10-fold excess over BPDf and cholesterol absent from the anchored liposomal structure) lead to a ~90% cell kill. The BPDf formulation alone resulted in only a ~35% loss of viability while the anchored preparations were less effective.
Fig. 5.
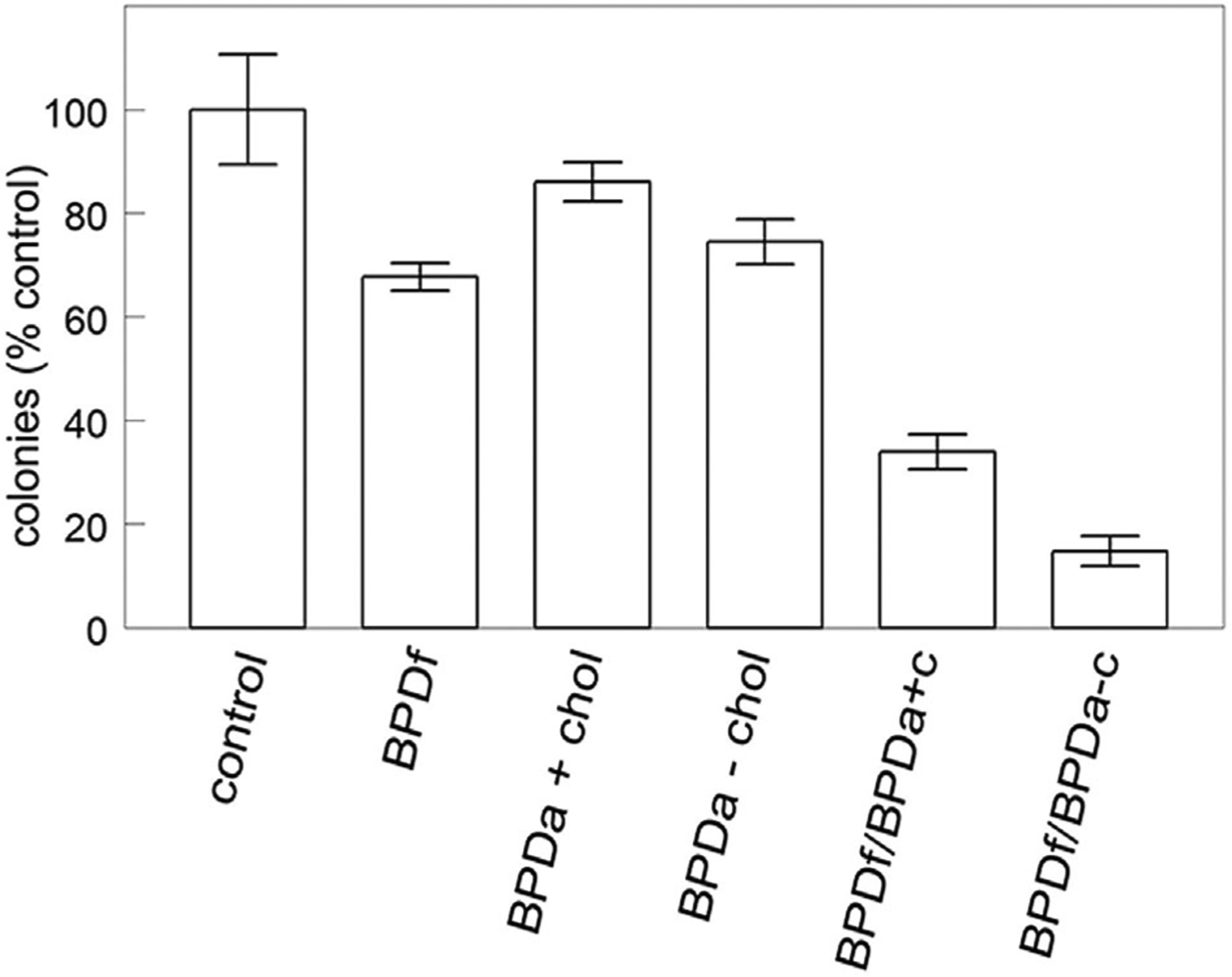
Clonogenic assays of OVCAR cells. Cultures were incubated with liposomal formulations as specified in the legend to Figure 2 for 3 hours, then irradiated at 690 nm (200 mJ/sq cm). Colonies were counted 7 days later. Data represent average ± SD for three determinations.
Polarization studies summarized in Table 1 indicate the expected loss of rotational freedom when a fluorophore is contained in a liposome. Rotational freedom is further impaired in the lipid-anchored preparation. The presence of cholesterol further decreases the fluidity of the phospholipid bilayer from a liquid-crystal state to a gel-like state as the temperature of the system nears the phase transition temperature of the lipids.
TABLE 1.
Fluorescence Polarization of BPD Preparations
| BPD formulation | mP |
|---|---|
| EtOH | 5.65 ± 0.5 |
| Glycerol | 118 ± 6.4 |
| BPDf | 274 ± 15 |
| BPDa+c | 344 ± 17 |
| BPDa−c | 394 ± 10 |
The BPD concentration was uniformly set at 1 μM. Liposomal formulations were diluted into phosphate-buffered saline, pH 7.0.
In a prior study, we described a death pathway termed paraptosis associated with extensive cytoplasmic vacuolization after photodamage to sites that included the ER [5]. Such an effect was also observed in OVCAR5 cells 1 day after irradiation with the combination PDT protocol (Fig. 6). Eliminating cholesterol from the anchored formulation promoted paraptosis. There was no evidence of an apoptotic morphology in the cell population but ~50% of the cells exhibited the vacuolated morphology consistent with paraptosis (panel d).
Fig. 6.
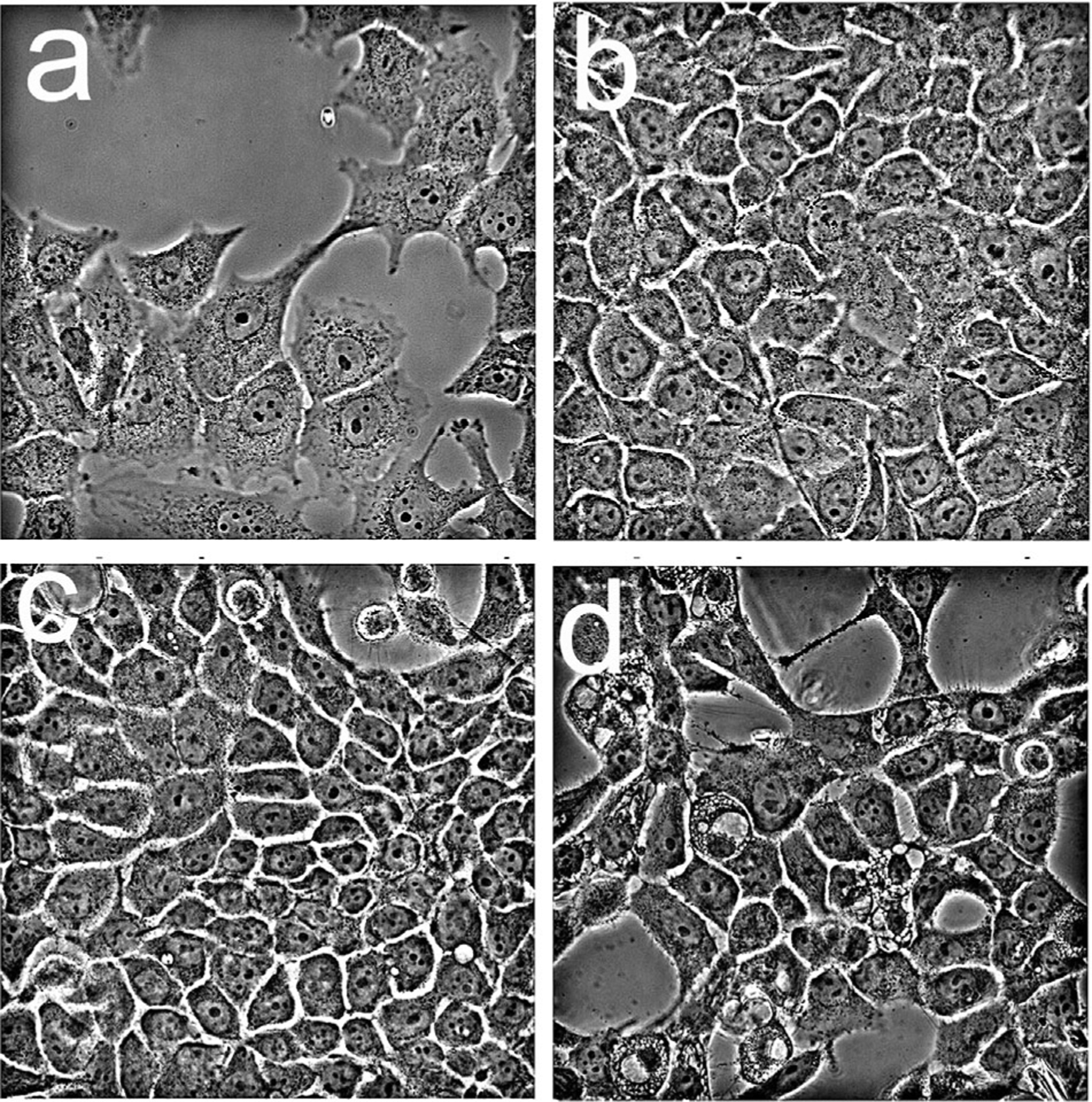
Morphology of OVCAR5 cells one day after irradiation as detected by phase-contrast microscopy. Cells were incubated with liposomal formulations of lipo-BPD (0.05 μM) and anchored formulations (0.5 μM) prior to irradiation (200 mJ/sq cm): a, untreated cells; b, BPDf; c, BPDf/BPDa+c; d, BPDf/BPDa−c.
DISCUSSION
A sequential PDT protocol involving agents that target lysosomal and mitochondrial loci has been shown to promote PDT efficacy in vitro [3–5] and in vivo [1]. A problem with this approach becomes apparent when in vivo studies are planned. The pharmacokinetics and biodistribution of the pertinent photosensitizers can be significantly different. The approach adopted in the 1996 study involving two different routes of drug administration is clearly not ideal. Moreover, the need for two different wavelengths of activating light complicates the procedure. Since BPD in the clinically-approved liposomal formulation preferentially localizes in ER and mitochondria, the approach utilized here uses a lipid-anchored derivative of BPD in a liposomal formulation to direct the photosensitizer to endosomes and lysosomes. The interaction between these liposomes and cells results in BPD accumulation via the endosomal route ultimately leading to lysosomal localization. Simultaneous treatment of cell cultures with the two formulations of BPD (free vs. anchored) changes the localization of the photosensitizer so as to include both ER/mitochondrial and lysosomal sites, respectively. Figure 1 demonstrates that the BPDf preparation yields results similar to the pattern observed where a DMF solvent was employed [3–5].
Studies involving a comparison of the localization of the liposomal BPD preparations and Lysotracker Green (Fig. 2) reveal that the anchored formulations lead to the deposition of BPD in lysosomal loci while BPDf is localized elsewhere. Irradiation of photosensitized cells caused mitochondrial photodamage occurs only with the BPDf formulation (Fig. 3). The anchored preparations selectively photosensitized lysosomes with a greater effect observed with the BPDa−c formulation. It is interesting to note, in this regard, a prior report indicating that excess cholesterol can protect lysosomes from photodamage [12]. If exogenous cholesterol from the liposomal formulation is being sequestered in lysosomes, this could account for the superior photosensitizing behavior of the BPDa−c formulation. Since the ratio of BPDa to BPDf was 10:1, the level of cholesterol in the “free” BPD formulation (BPDf) may be insufficient to affect the level of photokilling.
Use of a combination of these liposomal BPD formulations could facilitate the implementation of protocols using a single agent and a single wavelength of light for PDT, eliminating the need for multiple wavelengths while simultaneously targeting multiple subcellular compartments. Preliminary photokilling studies in cell culture suggest that this protocol may provide a superior PDT model. In this regard, it is noteworthy that solubilization of BPD in clinical practice also involves a liposomal formulation [13]. Use of such formulations in this report is therefore important with a view toward developing procedures for a multi-targeting approach into a clinical setting. Studies in animal models are expected to reveal whether the pharmacokinetics of BPD distribution would permit use of a mixture of BPDf +BPDa liposomes or whether it would be necessary to put both free and anchored BPD into a single liposomal structure.
An additional factor uncovered in earlier reports relates to the ability of ER photodamage to evoke a death pathway termed paraptosis [5,14]. This process is associated with an extensive network of cytoplasmic vacuoles that leads to loss of viability but has otherwise not been fully characterized. Paraptosis appears to be a response to misfolded proteins within the ER [15]. The route whereby lysosomal photodamage can potentiate apoptotic death has been described [16]. Data shown in Figure 6 indicate that lysosomal photodamage can also enhance paraptosis initiated by targeting mitochondria and ER. Lockshin has proposed that a lethally damaged cell will take any available pathway to death [17]. Since combinations are involved in most protocols involving chemotherapy, it is not surprising that a multi-targeting approach can also enhance photokilling. Use of liposomal formulations appears to be a useful method for directing photodamage to sub-cellular sites.
ACKNOWLEDGMENTS
Excellent technical assistance was provided by Ann Marie Santiago.
Contract grant sponsor:
NIH; Contract grant numbers: K99CA175292, P01 CA 084203, R00CA175292, R01 23378, R01 CA 156177, R01 CA 160998, R01CA160098.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and the authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Cincotta L, Szeto D, Lampros E, Hasan T, Cincotta AH. Benzophenothiazine and benzoporphyrin derivative combination phototherapy effectively eradicates large murine sarcomas. Photochem Photobiol 1996;63:229–237. [DOI] [PubMed] [Google Scholar]
- 2.Wilson BC. Photodynamic therapy: Light delivery and dosage for second-generation photosensitizers. (Photosensitizing compounds: Their chemistry, biology and clinical use) Ciba foundation symposium. John Wiley & Sons 1989;146:60–77. [DOI] [PubMed] [Google Scholar]
- 3.Kessel D, Reiners JJ. Enhanced efficacy of photodynamic therapy via a sequential targeting protocol. Photochem Photobiol 2014;90:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessel D, Evans CL. Promotion of pro-apoptotic signals by lysosomal photodamage: Mechanistic aspects and influence of autophagy. Photochem Photobiol 92:620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessel D, Reiners JJ. Effects of combined lysosomal and mitochondrial photodamage in a non-small-cell lung cancer cell line: The role of paraptosis. Photochem Photobiol 2017;93:1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yousefi IS, Perozzo R, Schmid I, et al. Calpain-mediated cleavage of ATG5 switches autophagy to apoptosis. Nat Cell Biol 2006;8:1124–1132. [DOI] [PubMed] [Google Scholar]
- 7.Tangutoori S, Spring BQ, Mai Z, Palanisami A, Mensah LB, Hasan T. Simultaneous delivery of cytotoxic and biologic therapeutics using nanophotoactivatable liposomes enhances treatment efficacy in a mouse model of pancreatic cancer. Nanomedicine 2016;12:223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spring BQ, Bryan Sears R, Zheng LZ, et al. A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways. Nat Nanotechnol 2016;11:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovell JF, Jin CS, Huynh E, MacDonald TD, Cao C, Zheng G. Enzymatic regioselection for the synthesis and biodegradation of porphysome nanovesicles. Angew Chem Int Ed 2012;51:2429–2433. [DOI] [PubMed] [Google Scholar]
- 10.Andrzejak M, Price M, Kessel DH. Apoptotic and autophagic responses to photodynamic therapy in 1c1c7 murine hepatoma cells. Autophagy 2011;7:979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997;14:479–489. [DOI] [PubMed] [Google Scholar]
- 12.Reiners JJ, Kleinman M, Kessel D, Mathieu PA, Caruso JA. Nonesterified cholesterol content of lysosomes modulates susceptibility to oxidant-induced permeabilization. Free Radic Biol Med 2011;50:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang H-I, Yek M-K. Clinical development of liposome-based drugs: Formulation, characterization and therapeutic efficacy. Int J Nanomedicine 2012;7:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperandio S, Poksay K, de Belle I, Lafuente MJ, Liu B, Nasir J, Bredesen DE. Paraptosis: Mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ 2004;11:1066–1075. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Gundelach JH, Bram RJ. Cycloheximide promotes paraptosis induced by inhibition of cyclophilins in glioblastima multiforme. Cell Death Dis 2017;8:e2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiners JJ, Jr, Caruso JA, Mathieu P, Chelladurai B, Yin XM, Kessel D. Release of cytochrome c and activation of procaspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ 2002;9:934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockshin RA, Zakeri Z. Apoptosis, autophagy and more. Int J Biochem Cell Biol 2004;35:2405–2419. [DOI] [PubMed] [Google Scholar]


