Abstract
Background
Thromboembolic disease is common in coronavirus disease-2019 (COVID-19). There is limited evidence on the association of in-hospital anticoagulation (AC) with outcomes and postmortem findings.
Objectives
The purpose of this study was to examine association of AC with in-hospital outcomes and describe thromboembolic findings on autopsies.
Methods
This retrospective analysis examined the association of AC with mortality, intubation, and major bleeding. Subanalyses were also conducted on the association of therapeutic versus prophylactic AC initiated ≤48 h from admission. Thromboembolic disease was contextualized by premortem AC among consecutive autopsies.
Results
Among 4,389 patients, median age was 65 years with 44% women. Compared with no AC (n = 1,530; 34.9%), therapeutic AC (n = 900; 20.5%) and prophylactic AC (n = 1,959; 44.6%) were associated with lower in-hospital mortality (adjusted hazard ratio [aHR]: 0.53; 95% confidence interval [CI]: 0.45 to 0.62 and aHR: 0.50; 95% CI: 0.45 to 0.57, respectively), and intubation (aHR: 0.69; 95% CI: 0.51 to 0.94 and aHR: 0.72; 95% CI: 0.58 to 0.89, respectively). When initiated ≤48 h from admission, there was no statistically significant difference between therapeutic (n = 766) versus prophylactic AC (n = 1,860) (aHR: 0.86; 95% CI: 0.73 to 1.02; p = 0.08). Overall, 89 patients (2%) had major bleeding adjudicated by clinician review, with 27 of 900 (3.0%) on therapeutic, 33 of 1,959 (1.7%) on prophylactic, and 29 of 1,530 (1.9%) on no AC. Of 26 autopsies, 11 (42%) had thromboembolic disease not clinically suspected and 3 of 11 (27%) were on therapeutic AC.
Conclusions
AC was associated with lower mortality and intubation among hospitalized COVID-19 patients. Compared with prophylactic AC, therapeutic AC was associated with lower mortality, although not statistically significant. Autopsies revealed frequent thromboembolic disease. These data may inform trials to determine optimal AC regimens.
Key Words: anticoagulation, COVID-19, intubation, mortality
Abbreviations and Acronyms: AC, anticoagulation; CI, confidence interval; COVID-19, coronavirus disease-2019; DOAC, direct oral anticoagulant; HR, hazard ratio; IPTW, inverse probability treatment weighted; LMWH, low molecular weight heparin; PRBC, packed red blood cell; UFH, unfractionated heparin
Central Illustration
Coronavirus disease-2019 (COVID-19) has led to >22 million affected (1) and >784,000 deaths worldwide. Among hospitalized patients, new thromboembolism has emerged as an important disease manifestation (2, 3, 4, 5). Autopsy studies have corroborated these observations by demonstrating a high incidence of macrothrombi and microthrombi (6, 7, 8). Accordingly, it has been hypothesized that inflammation associated with SARS-CoV-2 infection leads to a “COVID-19–related coagulopathy” (5), resulting in increased thrombosis (6).
Observational analyses have suggested potential benefit for in-hospital use of anticoagulation (AC) in COVID-19 treatment (9,10). Yet, practice patterns vary significantly due to lack of rigorous evidence for optimal regimens. Specifically, anticoagulant choice, dosing, and treatment duration are not well understood. In a preliminary analysis of 2,700 patients admitted to the Mount Sinai Health System in New York, we found an association between in-hospital therapeutic AC and lower mortality compared with patients on no/prophylactic AC (9). The present analysis expands upon those results in a larger cohort to explore the impact of therapeutic and prophylactic AC, as well as choice of agent, on survival, intubation, and major bleeding compared with no AC. We also review the first consecutive autopsies performed at our institution and describe their pre-mortem management as related to AC.
Methods
Data sources
Data were retrieved from the electronic health record. Variables collected included demographics, laboratory measurements, vital signs, disease diagnoses, comorbidities, procedures, and outcomes (death, intubation, and hospital discharge). The Mount Sinai Institutional Review Board approved this study.
Study design and participants
We included all patients age >18 years admitted with laboratory-confirmed SARS-CoV-2 infection between March 1, 2020, and April 30, 2020, to 5 New York City hospitals. Patients who left the hospital within 24 h of admission as well as those patients treated with both therapeutic and prophylactic regimens of AC during their hospitalization were excluded. If treated for <48 h total with a therapeutic or prophylactic dose, they were conservatively categorized as “not treated with AC” unless AC was stopped due to major bleeding (Supplemental Figure 1). Details on how patients were categorized into therapeutic/prophylactic AC are in the Supplemental Appendix.
Exposures
The primary exposure of interest was therapeutic or prophylactic AC compared with no AC. We also conducted a subanalysis of patients initiated therapeutic or prophylactic anticoagulants within 48 h of admission.
Outcomes
The primary endpoint was in-hospital mortality. Secondary endpoints were intubation and major bleeding. Consistency checks were performed to properly align these data tables and minimize missing data. If the amount of missing data was <1%, the patient was considered as not having the condition (e.g., for comorbidities). Missing values were mostly present for the vitals and the laboratory data, for which we used a “missing” category in the propensity score models to account for the missing data (Supplemental Appendix). Major bleeding was defined using International Classification of Diseases-10th Revision codes (Supplemental Table 1) or receiving ≥2 packed red blood cell (PRBC) transfusions within 48 h. Two physicians (G.N.N. and S.Z.) reviewed bleeding cases (n = 153) to adjudicate major bleeding. Disagreements were resolved by consensus discussion with an independent physician (V.F.). Criteria for confirmation of major bleeding included: 1) physician documentation of an active source of bleeding; 2) confirmatory imaging or other evidence (neuroimaging for intracranial bleed); 3) bleeding necessitating ≥2 PRBC transfusion within 48 h; or 4) suspected bleeding without confirmation of an active bleeding source. PRBCs transfused for other reasons included: 1) chronic anemia (dialysis or other reasons like cancer); 2) maintenance of hemoglobin over 7 g/dl; and 3) other reasons (peri-operative or symptom improvement). We also ascertained the bleeding site.
Autopsy data
Autopsies were performed at the Mount Sinai Hospital after obtaining appropriate consent and verifying SARS-CoV-2 infection status by nasopharyngeal swab unless already appropriately documented. Examinations were carried out in a negative pressure room with enhanced airborne precautions. Histological processing of tissue blocks was performed in standard fashion after extended formalin-fixation. Slides were reviewed by a team of pathology subspecialists.
Statistical analysis
General characteristics of the sample were summarized using appropriate descriptive statistics for continuous and categorical variables. Some continuous variables (e.g., body mass index, age, D-dimer, respiratory rate, and oxygen saturation) were categorized using clinically meaningful cutpoints to improve interpretability. Patients were divided into 3 groups according to whether they were treated with a therapeutic regimen, prophylactic regimen, or no anticoagulant. Patients receiving both therapeutic and prophylactic anticoagulants were excluded.
Inverse probability treatment weighted (IPTW) models, were used to correct for the potential bias brought about by AC indication. A multinomial logistic model was fit with therapeutic, prophylactic, or no use of AC during the hospitalization as the dependent variable, and age, sex, race and ethnicity, body mass index, history of hypertension, atrial fibrillation, heart failure, chronic kidney disease or renal failure, use of anticoagulants or antiplatelet agents prior to hospitalization, month of admission, intubation during hospitalization, time of implementation of institutional guidelines for AC at Mount Sinai, respiratory rate, oxygen saturation, and D-dimer at admission as the predictors. These predictors were chosen based on clinical judgment and model fit. We derived stabilized inverse IPTW by multiplying the inverse of the predicted probability of treatment from the propensity score model by the observed probability of treatment. The IPTW approach was used in all analyses. A robust variance was estimated in all models to account for the clustering effect resulting from IPTW. Standardized differences were calculated to determine the level of adjustment induced by the IPTW. To account for residual confounding, all models were adjusted for variables with absolute standardized differences >0.2 (Supplemental Figure 1). Regarding missing data, if the amount of missingness was <1%, a patient was considered as not having the condition (e.g., for comorbidities). Missing values were mostly in vitals and laboratory data (e.g., D-dimer), for which we used a “missing” category in the propensity score models to account for the missing data.
The primary analysis used IPTW Fine and Gray's subdistribution hazard models to determine AC association with in-hospital mortality (11). Survival in days was calculated as time from hospital admission to in-hospital death, discharge, or the date of dataset lock (May 7, 2020). Patients who were still hospitalized at the time of the data lock were censored. Discharge alive was considered a competing risk. To minimize immortal time bias, therapeutic and prophylactic AC use were entered in the model as time-dependent variables and similarly for intubation status. The multivariable model also accounted for admission respiratory rate and oxygen saturation.
For the time to intubation analysis, the time between hospital admission and intubation was considered in IPTW competing risk models using the method of Fine and Gray. Death and hospital discharge were considered competing events, and patients who were in hospital but not intubated at the time of data lock were censored. AC use was entered as time-dependent variables with the same covariate adjustment made previously. The hazard ratios (HRs) and their respective 95% confidence intervals (CIs) are reported for all time-to-event models. Frequency tables were used to describe the association between AC use and bleeding events. A similar approach was used for the subgroup of patients treated with therapeutic or prophylactic anticoagulants within 48 h of admission.
Landmark analyses were considered at 3 different time points: days 2, 3, and 4 after hospital admission (Supplemental Appendix). All analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
Patient and hospital presentation characteristics
A total of 4,389 patients met inclusion criteria for analysis (Supplemental Figure 2). The median age was 65 years (interquartile range: 53 to 77 years), 44% were women, 26% self-identified as African American, and 27% as Hispanic/Latino. Table 1 shows baseline characteristics and laboratory values stratified by therapeutic AC (n = 900), prophylactic AC (n = 1,959), and no AC (n = 1,530). Pre-hospital medications of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, prior AC, and antiplatelet therapy by group are also shown in Table 1. Approximately one-tenth of the total cohort were on AC or antiplatelet medications prior to admission (1.8% and 8.5%, respectively). On hospital presentation, patients in the therapeutic AC group had higher blood pressures, faster heart and respiratory rates, and lower oxygen saturation (Table 1). D-dimer concentrations were highest in the patients who received therapeutic AC (median 2.3 μg/ml; interquartile range: 1.2 to 5.8 μg/ml). Elevated inflammatory markers including ferritin, lactate dehydrogenase, and c-reactive protein increased progressively from the no AC to prophylactic AC and then therapeutic AC patient groups.
Table 1.
Baseline Characteristics of Patients Stratified by Therapeutic, Prophylactic, and No Anticoagulation (n = 4,389)
| n | Total (N = 4,389) | Therapeutic Anticoagulation (n = 900) | Prophylactic Anticoagulation (n = 1,959) | No Anticoagulation (n = 1,530) | p Value∗ | |
|---|---|---|---|---|---|---|
| Age, yrs | 4,389 | 65 (53–77) | 70 (59–80) | 65 (54–76) | 61 (45–75) | <0.001 |
| Female | 4,389 | 1,932 (44.0) | 353 (39.2) | 851 (43.4) | 728 (47.6) | <0.001 |
| Race/ethnicity | 4,389 | 0.01 | ||||
| Black | 1,152 (26.2) | 228 (25.3) | 567 (28.9) | 357 (23.3) | ||
| Hispanic | 1,172 (26.7) | 222 (24.7) | 523 (26.7) | 427 (27.9) | ||
| White | 1,060 (24.2) | 234 (26.0) | 432 (22.1) | 394 (25.8) | ||
| Asian | 201 (4.6) | 38 (4.2) | 94 (4.8) | 69 (4.5) | ||
| Other | 804 (18.3) | 178 (19.8) | 343 (17.5) | 283 (18.5) | ||
| Body mass index, kg/m2 | 3,940 | 28 (25–33) | 29 (25–34) | 28 (24–32) | 28 (24–33) | <0.001 |
| Current smoking | 3,405 | 184 (5.4) | 29/687 (4.2) | 92/1,533 (6.0) | 63/1,185 (5.3) | 0.23 |
| Comorbid conditions | ||||||
| Asthma | 4,377 | 274 (6.3) | 59/896 (6.6) | 137/1,958 (7.0) | 78/1,523 (5.1) | 0.07 |
| Chronic obstructive pulmonary disease | 4,377 | 216 (4.9) | 61/896 (6.8) | 102/1,958 (5.2) | 53/1,523 (3.5) | <0.001 |
| Type 2 diabetes | 4,377 | 991 (22.6) | 243/896 (27.1) | 462/1,958 (23.6) | 286/1,523 (18.8) | <0.001 |
| Hypertension | 4,380 | 1,526 (34.8) | 362/898 (40.3) | 706/1,959 (36.0) | 458/1,523 (30.1) | <0.001 |
| Coronary artery disease | 4,352 | 541 (12.4) | 152/895 (17.0) | 224/1,950 (11.5) | 165/1,507 (10.9) | <0.001 |
| Atrial fibrillation | 4,352 | 298 (6.8) | 158/895 (17.7) | 49/1,950 (2.5) | 91/1,507 (6.0) | <0.001 |
| Heart failure | 4,380 | 362 (8.3) | 104/898 (11.6) | 139/1,959 (7.1) | 119/1,523 (7.8) | <0.001 |
| Chronic kidney disease | 4,352 | 493 (11.3) | 105/895 (11.7) | 239/1,950 (12.3) | 149/1,507 (9.9) | 0.08 |
| End-stage kidney disease | 4,286 | 291 (6.8) | 56/835 (6.7) | 144/1,938 (7.4) | 91/1,513 (6.0) | 0.26 |
| Liver disease | 4,286 | 69 (1.6) | 9/835 (1.1) | 38/1,938 (2.0) | 22/1,513 (1.5) | 0.2 |
| Cancer | 4,377 | 340 (7.8) | 78/896 (8.7) | 160/1,958 (8.2) | 102/1,523 (6.7) | 0.14 |
| HIV/AIDS | 4,377 | 73 (1.7) | 9/896 (1.0) | 39/1,958 (2.0) | 25/1,523 (1.6) | 0.56 |
| Medications at baseline | ||||||
| ACE inhibitor or ARB | 4,389 | 331 (7.5) | 69 (7.7) | 134 (6.8) | 128 (8.4) | 0.24 |
| Anticoagulant | 4,389 | 79 (1.8) | 43 (4.8) | 7 (0.36) | 29 (1.9) | <0.001 |
| Antiplatelet agents | 4,389 | 374 (8.5) | 69 (7.7) | 174 (8.9) | 131 (8.6) | <0.001 |
| Initial vital signs | ||||||
| Systolic blood pressure, mm Hg | 4,347 | 138 (125–155) | 143 (128–158) | 140 (125–156) | 136 (122–151) | <0.001 |
| Diastolic blood pressure, mm Hg | 4,347 | 80 (72–89) | 83 (75–91) | 80 (72–89) | 79 (72–87.5) | <0.001 |
| Heart rate, beats/min | 4,354 | 99 (88–113) | 102 (89–119) | 99 (88–112) | 98 (87–111) | <0.001 |
| Oxygen saturation, % | 4,275 | 94 (90–96) | 92 (88–95) | 94 (91–96) | 95 (92–97) | <0.001 |
| Respiration, breaths/min | 4,354 | 20 (18–24) | 22 (20–30) | 20 (18–24) | 20 (18–20) | <0.001 |
| Initial laboratory tests | ||||||
| Hemoglobin, g/dl | 3,557 | 12.7 (11.2–14.0) | 12.6 (11.0–13.9) | 12.8 (11.4–14.1) | 12.6 (11.0–13.9) | <0.001 |
| White blood cell count, cells/mm3 | 4,206 | 7.6 (5.5–10.6) | 8.5 (6.0–11.9) | 7.3 (5.3–10.0) | 7.5 (5.6–10.5) | <0.001 |
| Lymphocyte, % | 3,831 | 9.8 (6.0–15.5) | 8.2 (5.2–13.0) | 9.8 (6.1–15.2) | 11.0 (6.6–17.8) | <0.001 |
| Neutrophil, % | 3,831 | 66 (44.2–80.7) | 75.6 (47.5–85.1) | 56.9 (42.6–78.9) | 67 (44.9–79.8) | <0.001 |
| D-dimer, μg/ml | 3,259 | 1.7 (0.9–3.6) | 2.3 (1.2–5.8) | 1.5 (0.8–2.9) | 1.7 (0.8–3.7) | <0.001 |
| Ferritin, ng/ml | 3,389 | 706 (317–1,617) | 830 (417–1,969) | 710 (316–1,594) | 601 (272–1,437) | <0.001 |
| Lactate dehydrogenase, U/l | 3,268 | 414 (311–564) | 484 (366–670.5) | 402 (310–534) | 380 (279–512) | <0.001 |
| C-reactive protein, mg/l | 3,524 | 108 (51–195) | 141 (65–234) | 106 (54–186) | 90 (34–168) | <0.001 |
| Procalcitonin, ng/ml | 3,124 | 0.2 (0.1–0.6) | 0.2 (0.1–0.7) | 0.2 (0.1–0.6) | 0.1 (0.1–0.6) | <0.001 |
| Albumin, g/dl | 4,033 | 3.1 (2.8–3.5) | 3.0 (2.7–3.4) | 3.2 (2.8–3.6) | 3.1 (2.7–3.6) | <0.001 |
| Total bilirubin, mg/dl | 2,240 | 0.6 (0.4–0.8) | 0.7 (0.5–1.0) | 0.6 (0.4–0.8) | 0.6 (0.4–0.8) | <0.001 |
| Sodium, MeQ/l | 4,057 | 137 (134–140) | 137 (134–140.5) | 137 (134–140) | 138 (135–141) | <0.001 |
| Creatinine, mg/dl | 4,156 | 1.0 (0.8–1.6) | 1.0 (0.8–1.6) | 1.0 (0.8–1.5) | 1.0 (0.7–1.6) | 0.004 |
| Prothrombin time, s | 2,604 | 13.7 (12.0–15.3) | 14.7 (13.6–16.6) | 13.4 (8.2–14.5) | 13.7 (11.5–15.7) | <0.001 |
| Partial thromboplastin time, s | 2,501 | 16.6 (13.8–31.3) | 16.6 (14.3–31.0) | 17.9 (13.7–32.0) | 15.8 (13.5–30.5) | 0.02 |
| International normalized ratio | 2,743 | 1.1 (1.0–1.3) | 1.2 (1.1–1.4) | 1.1 (1.0–1.2) | 1.1 (1.0–1.3) | <0.001 |
| Platelet count, cells/mm3 | 4,129 | 211 (161–280) | 227 (167–303) | 207 (160–270) | 210.5 (156–276) | <0.001 |
Values are median (interquartile range), n (%), or n/N (%), unless otherwise indicated. Values at baseline are within 48 h of admission.
ACE = angiotensin-converting enzyme, ARB = angiotensin receptor blocker.
Chi-square test used for categorical variables. Kruskal-Wallis test used for continuous variables.
Mortality, intubation, and outcomes
Overall, 1,073 (24.4%) patients died during the study period, 2,892 (65.9%) were discharged alive, and 424 (9.7%) were still hospitalized by dataset freeze date. Among the no AC group, 931 (60.8%) patients were discharged alive, 392 (25.6%) expired in the hospital, and 207 (13.5%) were still hospitalized. In the prophylactic AC group, 1,472 (75.1%) patients were discharged alive, 424 (21.6%) expired in the hospital, and 63 (3.2%) were still hospitalized. Finally, in the therapeutic AC group, 89 (54.3%) patients were discharged alive, 257 (28.6%) expired in the hospital, and 154 (17.1%) were still hospitalized. Therapeutic AC was associated with a 47% reduction in the hazard of in-hospital mortality (aHR: 0.53; 95% CI: 0.45 to 0.62; p < 0.001) (Figure 1A ) compared with no AC. Similarly, prophylactic AC was associated with a lower hazard of mortality (aHR: 0.50; 95% CI: 0.45 to 0.57; p < 0.001) compared with no AC. Overall, 467 (10.6%) patients required intubation and mechanical ventilation during hospitalization. Therapeutic AC was associated with a 31% reduction in the hazard of intubation (aHR: 0.69; 95% CI: 0.51 to 0.94; p = 0.02) (Figure 1B) compared with no AC. Prophylactic AC was also associated with similarly reduced incidence of intubation (adjusted HR: 0.72; 95% CI: 0.58 to 0.89; p = 0.003) compared with no AC. Landmark analyses showed similar associations (Supplemental Tables 2 and 3).
Figure 1.
Association of Prophylactic/Therapeutic Versus No Anticoagulation for In-Hospital Mortality and Intubation
Stabilized weight-adjusted cumulative incidence curves for the effect of anticoagulation on (A) in-hospital mortality with discharge as a competing risk and (B) intubation with death and discharge as competing risks. The estimates are adjusted for the inverse probability of treatment weighting (IPTW) using propensity scores. Hazard ratio (HR) and 95% confidence interval (CI) are based on stabilized IPTW Fine and Gray’s subdistribution hazard models with robust variance and (A) discharge and (B) death and discharge as competing events. The multivariable model includes therapeutic and prophylactic anticoagulation as time-dependent variables and controls for the effect of time-varying intubation status and respiratory rate and oxygen saturation at admission.
Therapeutic and prophylactic dose AC
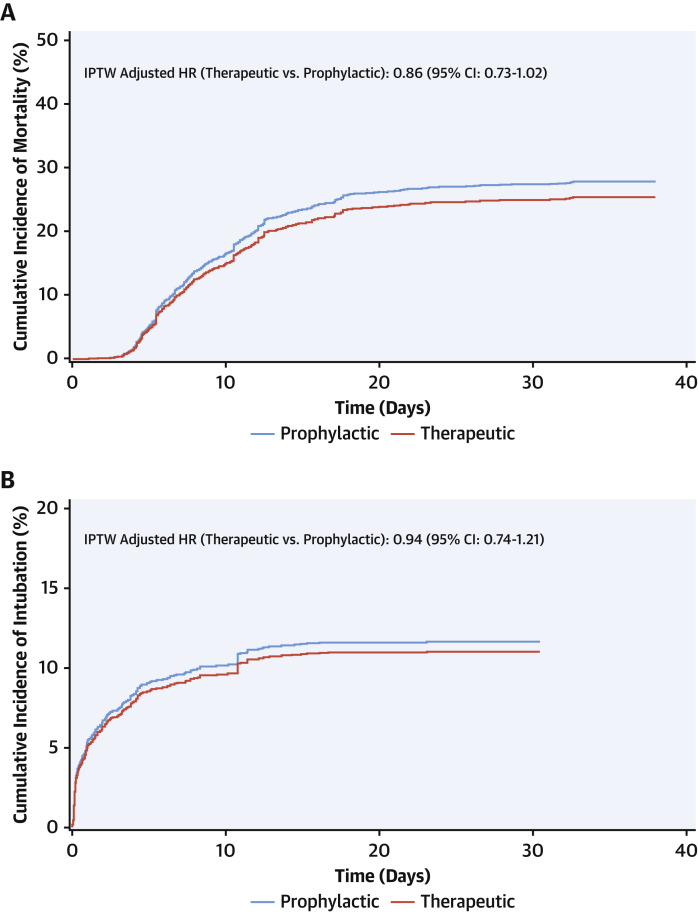
We conducted a subanalysis for patients initiated on therapeutic (n = 766) or prophylactic doses (n = 1,860) of AC ≤48 h of admission. Baseline characteristics are presented in Supplemental Table 4. Patients who received therapeutic AC were older, had more comorbid conditions, and were more likely to be on an anticoagulant prior to admission compared with those receiving prophylactic AC. Patients on therapeutic AC also presented with more altered vital signs and inflammatory markers, in particular D-dimer (2.4 μg/ml vs. 1.4 μg/ml) compared with those receiving prophylactic AC. In adjusted analyses, therapeutic AC was associated with lower in-hospital mortality (aHR: 0.86; 95% CI: 0.73 to 1.02; p = 0.08) (Figure 2A ), although not statistically significant. There was no difference in incidence of intubation (aHR: 0.94; 95% CI: 0.74 to 1.21; p = 0.63) (Figure 2B).
Figure 2.
Association of Prophylactic Versus Therapeutic Anticoagulation Started Within 48 h of Hospital Admission on In-Hospital Mortality and Intubation
Stabilized weight adjusted cumulative incidence curves comparing the effect of therapeutic versus prophylactic anticoagulation (within 48 h of hospital admission) on (A) in-hospital mortality with discharge as a competing risk and (B) intubation with deaths and discharge as competing risks. The estimates are adjusted for the IPTW using propensity scores. HR and 95% CI are based on stabilized IPTW Fine and Gray’s sub-distribution hazard models with robust variance and (A) discharge and (B) death and discharge as competing events. The multivariable model includes therapeutic and prophylactic anticoagulation as time-dependent variables and controls for the effect of time-varying intubation status. Abbreviations as in Figure 1.
Bleeding outcomes
A total of 153 patients met the pre-specified definition of major bleeding. Of these, 89 either had a confirmed or suspected bleed (Supplemental Figure 3). For patients on AC, bleeding was counted only if it occurred after initiation of treatment. The proportion of patients with bleeding events after initiation of AC treatment was highest in patients on therapeutic AC (27 of 900; 3.0%) compared with patients on prophylactic AC (33 of 1,959; 1.7%) and no AC (29 of 1,530; 1.9%) (Supplemental Table 5). Among patients on a single therapeutic agent, bleeding rates were higher in those on low molecular weight heparin (LMWH) compared with direct oral anticoagulants (DOACs) (2.6% vs. 1.3%, respectively), and among those on a single prophylactic agent, bleeding rates were higher in those on unfractionated heparin (UFH) compared with LMWH (1.7% vs. 0.7%, respectively). The site of bleeding was determined in 67 of 89 (75%), with gastrointestinal being most common (50.7%), followed by mucocutaneous (19.4%), bronchopulmonary (14.9%), and then intracranial (6%).
Anticoagulation agents
A sizable proportion of patients were on more than 1 AC agent over the course of their hospitalization, preventing direct comparisons between anticoagulants. In a descriptive analysis, we present differences in cumulative incidence of mortality and intubation among individuals who were on a single anticoagulant received within 48 h of admission. Among patients on therapeutic AC, differences in mortality and intubation between DOACs (n = 178) versus LMWH (n = 211) are shown in Supplemental Figures 4A and 4B, respectively, and suggest that DOACs may be associated with better survival and lower intubation rates compared with LMWH. Patients on UFH were not included due to the relatively small sample size of this group (n = 35). Similarly, among patients on prophylactic dose AC, cumulative incidence of mortality and intubation for patients on UFH (n = 941) and LMWH (n = 445) are shown in Supplemental Figures 4C and 4D, respectively. Patients on prophylactic DOACs are not shown due to limited sample size (n = 34).
Autopsy findings
Autopsies were performed on COVID-19 positive patients at Mount Sinai Health System starting on March 20, 2020, with 72 completed by May 7, 2020 (8). Of these, the first 26 sequential cases were evaluated microscopically by a team of subspecialty pathologists across organ systems. These cases are presented with a focus on thromboembolism and contextualized by premortem AC regimens (Table 2 ). Among 26 patients, 4 were on AC prior to admission due to atrial fibrillation (n = 3) or prior DVT (n = 1) (3 on DOACs, 1 on warfarin). Of the remaining 22 patients, 4 died within 24 h of presentation without ever receiving AC, 14 were placed on AC upon admission (13 prophylactic, 1 therapeutic), and 4 received AC later during their hospital course (mean number of days post-admission: 2.3).
Table 2.
Clinical and Pathological Features of Thromboembolic Disease in Sequential Autopsies (n = 26)
| Age Range, yrs | Sex | Prior Indication | Type of Anticoagulation | Time From Admission to Death, days | Duration of Anticoagulation | Type (Therapeutic/Prophylactic/None) | Bleeding | Pulmonary Embolism | Microthrombi∗ | Suspicion of Thrombosis Before Autopsy |
|---|---|---|---|---|---|---|---|---|---|---|
| 50–59 | M | NA | UFH | 9 | Whole admission | Prophylactic | × | × | No | |
| 80–89 | F | NA | UFH | 11 | Whole admission | Prophylactic | × | No | ||
| 60–69 | M | Atrial fibrillation | DOACs | 4 | Whole admission | Therapeutic | × | No | ||
| <50 | M | NA | LMWH | 6 | Whole admission | Prophylactic | × | × | No | |
| 60–69 | F | NA | None | 0 | NA | None | No | |||
| 30–39 | M | NA | LMWH | 7 | Whole admission | Prophylactic | No | |||
| 80–89 | F | NA | UFH | 10 | Whole admission | Prophylactic | × | No | ||
| 70–79 | M | NA | LMWH | 10 | Whole admission | Prophylactic | No | |||
| <50 | M | NA | None | 0 | NA | None | No | |||
| 80–89 | M | NA | None | 0 | NA | None | No | |||
| 70–79 | M | Atrial fibrillation | Warfarin | 1 | Whole admission | Therapeutic | Retro-peritoneal | No | ||
| <50 | F | NA | UFH | 3 | Whole admission | Prophylactic | No | |||
| 80–89 | F | NA | UFH | 1 | Whole admission | Prophylactic | No | |||
| 70–79 | M | Deep venous thrombosis | DOACs | 1 | Whole admission | Prophylactic | × | No | ||
| 50–59 | M | NA | UFH | 4 | 1 day | Subtherapeutic† | × | No | ||
| 50–59 | M | NA | UFH, LMWH | 5 | Whole admission | Prophylactic | No | |||
| 60–69 | M | NA | None | 1 | — | None | × | No | ||
| 50–59 | M | NA | UFH, LMWH | 5 | Whole admission | Prophylactic | No | |||
| 70–79 | F | NA | LMWH | 6 | Whole admission | Prophylactic | No | |||
| 50–59 | F | NA | UFH | 4 | Whole admission | Prophylactic | × | No | ||
| 70–79 | F | Atrial fibrillation | DOACs | 5 | Whole admission | Therapeutic | No | |||
| 50–59 | F | NA | UFH, LMWH | 15 | 2 days | Therapeutic | No | |||
| 80–89 | F | NA | LMWH | 10 | Whole admission | Prophylactic | No | |||
| 70–79 | M | NA | UFH | 9 | Whole admission | Therapeutic | No | |||
| 60–69 | M | NA | UFH | 22 | 5 days | Therapeutic | × | No | ||
| <50 | M | NA | UFH | 11 | 1 day | Subtherapeutic† | × | Yes |
DOAC = direct oral anticoagulant; LMWH = low molecular weight heparin; NA = not applicable; UFH= unfractionated heparin.
Organs assessed for microthrombi in hematoxylin and eosin include heart (found in 4 of 26), kidneys (found in 2 of 26), liver (found in 1 of 26), lymph nodes (found in 2 of 26) and brain (found in 2 of 26). Microthrombi in the lungs are normally seen as part of diffuse alveolar damage and are discussed separately (see Results and Discussion sections).
Anticoagulation in this case was intended to be therapeutic; however, PTT never reached the therapeutic range.
In total, 11 of 26 (42%) had evidence of thromboembolic disease, including 4 patients with pulmonary emboli (15%) (Figures 3A and 3B ); 2 patients with cerebral infarctions (8%) (Figures 3C and 3D); and 5 patients with microthrombi in multiple organs including the heart (n = 4) (Figure 3E), liver (n = 1) (Figure 3F), kidneys (n = 2, not shown), and lymph nodes (n = 2, not shown). The lungs were examined and revealed an extensive burden of fibrin thrombi visible on hematoxylin and eosin stain (15 of 26); however, this was not counted toward the thrombotic burden as it is an expected and frequently encountered finding in diffuse alveolar damage. Of the 4 patients with pulmonary emboli, 2 were on prophylactic AC throughout, 1 was not on AC, and 1 was given AC using UFH to treat disseminated intravascular coagulation but at subtherapeutic levels. More generally, 8 of 11 (73%) patients with thromboemboli were not on therapeutic AC. There was no premortem suspicion of thromboemboli in 25 of 26 patients. There was only 1 major bleeding complication, which was a retroperitoneal bleed on presentation in a patient taking warfarin for atrial fibrillation prior to admission.
Figure 3.
Thromboembolic Disease in Autopsy Specimens From 26 Consecutive Autopsies
(A) Pulmonary embolus with lines of Zahn and adherence to the pulmonary vasculature (hematoxylin and eosin, 0.5×). (B) Pulmonary embolus near an intraparenchymal pulmonary lymph node, with lines of Zahn and adherence to the pulmonary vasculature (hematoxylin and eosin, whole slide image). (C) Sequential gross sections of the right frontal lobe of the brain with peripheral infarcts (arrows) and surrounding hemorrhage (ruler shows dimensions in centimeters). (D) Microthrombus in an intraparenchymal brain vessel (hematoxylin and eosin, 20×). (E) Microthrombus within the myocardium with lines of Zahn and adherence to the vascular wall (hematoxylin and eosin, 4×). (F) Microthrombus in a portal venule in the liver (hematoxylin and eosin, 20×).
Discussion
Thromboembolic disease has emerged as an important complication among hospitalized patients with COVID-19. In the present report of nearly 4,400 patients, we demonstrate the following findings (Central Illustration ). First, AC is associated with lower hazards of in-hospital mortality and intubation compared with no AC after controlling for relevant clinical factors. Second, after restricting analysis to those in whom AC was initiated within 48 h of admission, no statistically significant difference in in-hospital mortality or intubation for therapeutic versus prophylactic AC was observed. Third, overall rates of major bleeding were low. Finally, these observations were corroborated by autopsy findings, wherein 11 of 26 of patients had thromboembolic disease not otherwise suspected premortem. The majority of these patients were not treated with therapeutic AC.
Central Illustration.
In-Hospital Anticoagulation and Outcomes in Coronavirus Disease-2019
Thromboembolic disease is a complication of coronavirus disease-2019 (COVID-19). Prophylactic and therapeutic anticoagulation are associated with better outcomes in hospitalized patients with COVID-19. Randomized controlled trials evaluating different anticoagulation regimens in COVID-19 are needed. CI = confidence interval; DOAC = direct oral anticoagulant; HR = hazard ratio; LMWH = low molecular weight heparin.
The mechanisms by which thrombotic disease may occur in the setting of COVID-19 infection include inflammation, hypoxia, and potentially pharmacotherapeutic interactions (2,4,12,13). As such, the potential benefit of AC in the treatment of COVID-19 is based on the prevention and treatment of microvascular and macrovascular thrombosis. In addition, AC agents may exert antiviral and anti-inflammatory properties affording further benefit (14,15).
In our cohort of patients hospitalized with COVID-19, a strong association of AC with approximately 50% reduced hazard of in-hospital mortality was observed (Figure 1A). Both therapeutic and prophylactic doses of AC were associated with better in-hospital survival compared with no AC. As mortality rates for patients with COVID-19 who undergo intubation for respiratory failure range from 30% to 80% (16, 17, 18), we analyzed the association between AC and intubation. Both therapeutic and prophylactic AC were associated with an approximately 30% reduced hazard of intubation compared with patients on no AC (Figure 1B). Landmark analyses were performed to minimize immortal time bias and revealed similar associations (Supplemental Tables 2 and 3).
Therapeutic compared with prophylactic dose AC
Due to variation in timing of initiation and administration of AC across patients, a subanalysis of patients who received either therapeutic or prophylactic AC within 48 h of admission showed that therapeutic AC was associated with a 14% reduction in hazard of mortality compared with prophylactic AC that did not reach statistical significance (p = 0.08). There was no difference in intubation risk between the 2 doses (Figures 2A and 2B).
In entirely descriptive analyses examining individual agents, potential benefit with prophylactic LMWH compared with UFH may exist for mortality, but differences in intubation appear minimal. Therapeutic DOACs visually may be associated with lower mortality and intubation risk compared with LMWH (Supplemental Figure 4). No conclusions can be drawn from these purely descriptive comparisons, however, and randomized trials comparing specific agents are needed to inform whether comparative benefit exists.
Bleeding
Bleeding rates were low overall, but as expected, were slightly higher in the therapeutic AC group compared with the prophylactic and no AC groups (Table 2). In patients on a single therapeutic agent, the bleeding rates were higher in patients on LMWH versus DOACs. Further studies and trials are required, however, to better understand this observation. As always, the benefit-risk tradeoff, here between AC and bleeding, needs to be evaluated on an individual basis and discussed as part of shared-decision making.
Autopsy findings
We show a high prevalence of thrombotic complications mostly occurring in patients receiving prophylactic/no AC, consistent with a recent autopsy study demonstrating thrombotic burden in 58% (6,19). Although lung microthrombi were not counted toward overall burden but rather as a feature of diffuse alveolar damage, it is worth noting that this finding emphasizes the endothelial dysfunction at play. Finally, in all except for 1 case of stroke, there was no clinical suspicion of thromboembolic disease prior to autopsy, suggesting that clinical estimates of thromboembolic disease may be underestimating the actual burden.
Study limitations
As an observational study, there may have been confounders leading to differences in the outcomes for the treatment groups. Although we minimized their potential impact through IPTW modeling, unmeasured confounders and residual bias may have been present. Despite a 2-physician manual review of different AC regimens for the purposes of categorizing patients, there may have been discrepancies between regimens of DOACs and LMWH wherein doses may not have accurately represented therapeutic and prophylactic AC. Patients who were on both therapeutic and prophylactic doses of AC were excluded due to an inability to definitively categorize them. Patients with hospital stay <24 h were also excluded. Nonetheless, we adopted a conservative approach wherein individuals receiving <48 h of AC were considered in the “no AC” group. To minimize immortal time bias, we analyzed AC as a time-dependent variable and conducted landmark sensitivity analyses. However, we cannot rule out residual bias even after using IPTW. We included UFH infusion in the therapeutic group, but patients may not be in the therapeutic activated partial thromboplastin time range. Because manual validation of each outcome was not feasible in the whole sample size, there exists the possibility of misclassification of outcomes. We did not conduct analysis on novel antiviral treatments (remdesivir, interleukin-1 antagonists) because these were still under investigation and administered in the context of clinical trials at our institution. The generalizability of the autopsy data may be limited due to small sample size and the fact that these were not consecutive deaths. Finally, we may have encountered higher proportions of patients on AC due to the fact that Mount Sinai initiated a system-wide protocol, wherein at least prophylactic AC was strongly encouraged with guidance provided for consideration of therapeutic AC based on various factors (Supplemental Figure 5).
Conclusions
Among patients hospitalized with COVID-19, AC was associated with a lower adjusted risk of mortality and intubation versus no AC. Rates of major bleeding were low. Consecutive autopsies revealed frequent thromboembolism, with most patients not on therapeutic AC. The results of randomized controlled trials evaluating different AC regimens for treatment for hospitalized patients with COVID-19 are needed.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In an observational study of patients hospitalized with COVID-19, intubation and mortality were less frequent among those managed with anticoagulation at prophylactic or therapeutic doses than those not anticoagulated. Bleeding rates were generally low. Consecutive autopsy samples revealed a high incidence of thromboembolism.
TRANSLATIONAL OUTLOOK: Clinical trials are needed to identify predictors of thromboembolism and bleeding and establish optimum antithrombotic strategies for patients with COVID-19 at various stages of illness and hospitalization.
Acknowledgments
The authors thank all of the nurses, physicians, and providers who contributed to the care of these patients, as well as the patients and their family members who were affected by this pandemic.
Footnotes
This study is funded by U54 TR001433-05 from the National Institutes of Health. The funding source had no role in the writing of the manuscript or the decision to submit it for publication. Dr. Nadkarni has received grants, personal fees, and nonfinancial support from Renalytix AI; has received nonfinancial support from Pensieve Health; and has received personal fees from AstraZeneca, BioVie, and GLG Consulting outside of the submitted work. Dr. Lala has received personal fees from Zoll outside of the submitted work. Dr. Dunn has received grants from Pfizer; and has received personal fees from Bristol Myers Squibb outside of the submitted work. Dr. Farkouh has received grants from Amgen, Novo Nordisk, and Novartis outside of the submitted work. Dr. Fayad has received grants from Daiichi-Sankyo, Amgen, Bristol Myers Squibb, and Siemens Healthineers; has received personal fees from Alexion, GlaxoSmithKline, and Trained Therapeutix Discovery outside of the submitted work; and has patents licensed to Trained Therapeutix Discovery. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Deepak L. Bhatt, MD, MPH, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
Appendix
For an expanded Methods section as well as supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Johns Hopkins University of Medicine Global map: COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) https://coronavirus.jhu.edu/map.html Available at:
- 2.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wichmann D., Sperhake J.-P., Lütgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Heide R.S.V. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryce C., Grimes Z., Pujadas E. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv. 2020 May 21 [E-pub ahead of print] [Google Scholar]
- 9.Paranjpe I., Fuster V., Lala A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 12.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilli V.S., Datta A., Afreen S., Catalano D., Szabo G., Majumder R. Hypoxia downregulates protein S expression. Blood. 2018;132:452–455. doi: 10.1182/blood-2018-04-841585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Haan C.A.M., Li Z., te Lintelo E., Bosch B.J., Haijema B.J., Rottier P.J.M. Murine coronavirus with an extended host range uses heparan sulfate as an entry receptor. J Virol. 2005;79:14451–14456. doi: 10.1128/JVI.79.22.14451-14456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S A. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers L.C., Parodi S.M., Escobar G.J., Liu V.X. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323:2195–2198. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lax S.F., Skok K., Zechner P. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020 May 14 doi: 10.7326/M20-2566. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.