Abstract
Background
Hydroxychloroquine or chloroquine with or without azithromycin have been widely promoted to treat coronavirus disease 2019 (COVID-19) following early in vitro antiviral effects against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Objective
The aim of this systematic review and meta-analysis was to assess whether chloroquine or hydroxychloroquine with or without azithromycin decreased COVID-19 mortality compared with the standard of care.
Data sources
PubMed, Web of Science, Embase Cochrane Library, Google Scholar and MedRxiv were searched up to 25 July 2020.
Study eligibility criteria
We included published and unpublished studies comparing the mortality rate between patients treated with chloroquine or hydroxychloroquine with or without azithromycin and patients managed with standard of care.
Participants
Patients ≥18 years old with confirmed COVID-19.
Interventions
Chloroquine or hydroxychloroquine with or without azithromycin.
Methods
Effect sizes were pooled using a random-effects model. Multiple subgroup analyses were conducted to assess drug safety.
Results
The initial search yielded 839 articles, of which 29 met our inclusion criteria. All studies except one were conducted on hospitalized patients and evaluated the effects of hydroxychloroquine with or without azithromycin. Among the 29 articles, three were randomized controlled trials, one was a non-randomized trial and 25 were observational studies, including 11 with a critical risk of bias and 14 with a serious or moderate risk of bias. After excluding studies with critical risk of bias, the meta-analysis included 11 932 participants for the hydroxychloroquine group, 8081 for the hydroxychloroquine with azithromycin group and 12 930 for the control group. Hydroxychloroquine was not significantly associated with mortality: pooled relative risk (RR) 0.83 (95% CI 0.65–1.06, n = 17 studies) for all studies and RR = 1.09 (95% CI 0.97–1.24, n = 3 studies) for randomized controlled trials. Hydroxychloroquine with azithromycin was associated with an increased mortality (RR = 1.27; 95% CI 1.04–1.54, n = 7 studies). We found similar results with a Bayesian meta-analysis.
Conclusion
Hydroxychloroquine alone was not associated with reduced mortality in hospitalized COVID-19 patients but the combination of hydroxychloroquine and azithromycin significantly increased mortality.
Keywords: Azithromycin, Chloroquine, Coronavirus, Coronavirus disease 2019, Hydroxychloroquine, Meta-analysis, Mortality, Severe acute respiratory syndrome coronavirus 2
Introduction
On 31 December 2019, the WHO identified an unknown pneumonia caused by a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in Wuhan, China. By 30 July 2020, WHO confirmed more than 17 million cases and 667 935 deaths [1]. Chloroquine (CQ) and its derivative hydroxychloroquine were rapidly identified as potential drug candidates because chloroquine had an antiviral activity against Middle East respiratory syndrome and severe acute respiratory syndrome in vitro [2]. In vitro antiviral activity of the aminoquinolines hydroxychloroquine and chloroquine was confirmed against SARS-CoV-2 and a study reported a synergistic effect of hydroxychloroquine with azithromycin against SARS-CoV-2 [3]. These drugs appeared as potential low-cost treatments for individuals with coronavirus disease 2019 (COVID-19) [[4], [5], [6], [7]] and received wide and speculative coverage by the international press and the US President [8].
Subsequently, hydroxychloroquine and azithromycin were tested in a study where macaques were infected by SARS-CoV-2 and received either a high dose of hydroxychloroquine (90 mg/kg on day 1 then 45 mg/kg) or a low hydroxychloroquine dose (30 mg/kg on day 1 then 15 mg/kg) [9]. Hydroxychloroquine with or without azithromycin did not improve the time to viral clearance regardless of the stage of disease: prophylaxis, early treatment or late treatment.
Among the ongoing trials, chloroquine or hydroxychloroquine are among the most studied drugs [10,11]. Until today, most of the published studies on hydroxychloroquine with a comparative group (standard care) were observational and non-randomized with inconsistent results [[12], [13], [14], [15], [16], [17], [18]]. Given the magnitude of the COVID-19 pandemic and the need for effective therapeutics, timely meta-analyses can play an important role in assessing the impacts of chloroquine and hydroxychloroquine compared with standard of care on reliable clinical outcomes such as mortality. Previous meta-analyses on COVID-19 included a limited number of studies and used unadjusted risk ratios [[19], [20], [21]].
The aim of this systematic review and meta-analysis was to assess whether chloroquine or hydroxychloroquine with or without azithromycin decreased the mortality of COVID-19 compared with standard of care.
Methods
The research question was: in individuals with confirmed COVID-19, is the addition of hydroxychloroquine or chloroquine with or without azithromycin to the standard of care effective in improving survival?
PICO question
Population patients with confirmed COVID-19.
Intervention hydroxychloroquine or chloroquine, with or without azithromycin.
Comparison a standard of care.
Outcomes the survival rate of COVID-19 patients.
Data sources, search strategy
A search was performed using PubMed, Web of Science, Embase and Cochrane Review up to 25 July 2020 with the following string search: (COVID-19 OR SARS-CoV-2) AND (MORTALITY OR DEATH) AND (HYDROXYCHLOROQUINE OR hydroxychloroquine) (see Supplementary material, Text S1). Given that the number of articles about hydroxychloroquine and COVID-19 is rapidly growing, we also manually searched for additional references on the MedRxiv preprint server and on Google Scholar with the same terms. An additional search on PubMed, Web of Science and Cochrane Review was conducted for CQ with the search terms described in the Supplementary materials (Text S1): (COVID-19 OR SARS-CoV-2) AND (MORTALITY OR DEATH) AND (CHLOROQUINE OR chloroquine). This meta-analysis was conducted following the PRISMA statements in the Supplementary material (Text S2). This study has been recorded on the international database of prospectively registered systematic reviews, PROSPERO (Registration number: CRD42020190801).
Study selection
Study selection was conducted by two investigators (TF and YM) who screened the titles and the abstracts. Discrepancies were resolved by a third investigator (AG). Inclusion criteria were (a) reports containing original data with available risk estimates (hazard ratios (HR), odds ratios (OR), relative risk (RR) and/or with data on the number of deaths in hydroxychloroquine/chloroquine and control groups; (b) any publication dates; (c) comparative studies with a control group with no hydroxychloroquine nor chloroquine; and (d) PCR-confirmed cases of COVID-19. Studies reporting no deaths, reviews and meta-analyses, commentaries, editorials and in vitro and in vivo animal studies were excluded.
Data extraction
Two investigators (TF and YM) extracted the following data for each study: study design, publication date, journal, location, number of participants and deaths (in treatment and control groups), hydroxychloroquine or chloroquine doses when available, effect size (HR, OR or RR) and 95% CI for reported risk estimates. The estimates from the model, adjusted for the maximum number of covariates, were used to control potential confounders, according to Cochrane Methodology [22]. For each study, risk factors associated with higher mortality were taken into account through the reported adjusted effect sizes.
When studies did not report an effect size for mortality risk [17,23,24], we used the number of deaths per group to calculate an unadjusted relative risk using metabin function in meta package in R Software [25].
For all the other studies, reported adjusted OR, RR or HR were used.
Individual risk of bias
The quality of each study was assessed with the ROBIN-I tool following Cochrane guidelines for non-randomized studies and with Rob2 for randomized studies [26,27].
Outcome
The outcome was the mortality of COVID-19 patients.
Statistical analysis
Effect of chloroquine/hydroxychloroquine alone and hydroxychloroquine + azithromycin
A primary meta-analysis was performed to compare the survival rate (or mortality) between patients treated with chloroquine or hydroxychloroquine and standard of care. Then, the relationship between hydroxychloroquine associated with azithromycin and mortality was assessed. HR, OR and RR were treated as equivalent measures of mortality risk. Pooled RR were determined by using a random effect model with inverse variance weighting (DerSimonian–Laird method) [28]. Significance was checked using a Z-test, where p < 0.05 is considered as significant. The absolute risk difference (RD) was calculated from the UK baseline hospital mortality risk (BR) of 26% (according to ISARIC WHO CCP-UK cohort based on 20 133 patients) using the formula RD = BR × (RR – 1) [29].
Heterogeneity was assessed by the Cochrane Q test and I 2 test [30]. 30% < I 2 < 60% was interpreted as moderate heterogeneity and I 2 > 60 as substantial heterogeneity. A funnel plot was constructed to assess the publication bias. Begg's and Egger's tests were conducted to assess the publication bias [31,32]. RR or HR were used to assess mortality risk within a 95% CI. In the main analysis, studies with critical bias were excluded. A sensitivity analysis including these studies was conducted. A Bayesian meta-analysis was performed to test the robustness of our results, allowing incorporation of full uncertainty in all parameters [33]. The traditional random-effect model has fixed parameters for the distribution of the true treatment effect RR with an unknown mean θ, within-study variance σ2 and between-study variance τ2. The Bayesian random-effect model assumes that these parameters are random with a probability distribution. Two prior distributions were tested μ~Normal (1,100) with a large variance and τ~Half-Cauchy (0,0.5) and a second scenario with μ~Normal (1,1) and τ~Half-Cauchy (0,0.5). The Bayesian analysis was conducted with the R package brms [34].
Subgroup analysis
Subgroup analyses were conducted according to the quality assessment to explore the source of heterogeneity among observational studies. We performed stratified analyses by type of article (peer-reviewed versus unpublished), use of an adjustment on confounding factors (studies with RRunadjusted versus RRadjusted), mean daily dose of hydroxychloroquine or chloroquine (continuous), median population age across the studies, level of bias risk identified with ROBIN-I (moderate/serious/critical) [26] and when we excluded studies with cancer and dialysis patients. Mean daily dose of hydroxychloroquine or chloroquine was the daily average between the loading dose and the maintenance doses. Additionally, influence analysis was conducted by omitting each study to find potential outliers [34]. Influence analysis is used to detect studies that influence the overall estimate of a meta-analysis the most, omitting one study at a time (leave-one-out method).
A two-sided p-value <0.05 was considered statistically significant. All analyses were conducted using R version 3.6.1 with meta package and robvis package [35].
Results
Literature search
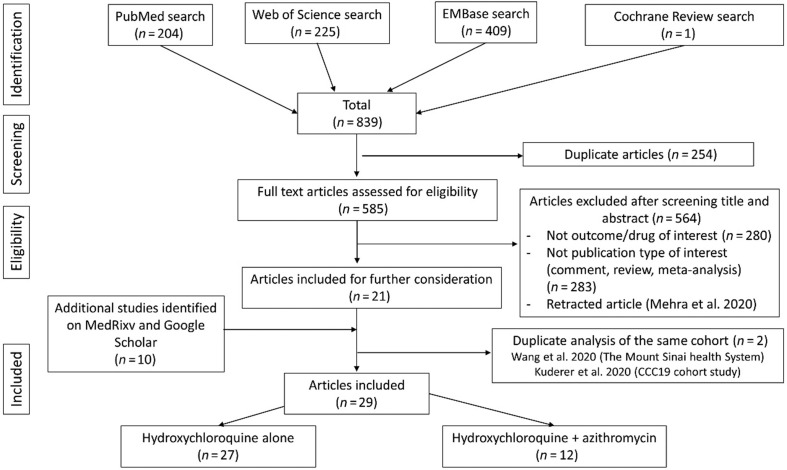
A flow chart is presented in Fig. 1 . After searching PubMed, Cochrane Review and Web of Science, 839 articles were identified. After screening the title and the abstract, only 21 articles about hydroxychloroquine and COVID-19 were included for further consideration. We excluded 564 articles that did not meet the inclusion criteria. We did not find any non-English articles meeting our inclusion criteria. Two duplicate studies on the same cohort were excluded [12,36]. Two Chinese randomized controlled trials (RCT) on hydroxychloroquine reported zero deaths in both treatment and control groups [37,38] and so their results were not included in our meta-analysis. Ten articles from Medrxiv/Google Scholar were added, so 29 articles were included, of which 25 were observational studies, one was an interventional non-randomized study and three were RCT. These studies included 27 articles for hydroxychloroquine [[14], [15], [16], [17], [18], [19],23,24,36,[39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56]] and 12 articles for hydroxychloroquine + azithromycin [18,36,41,42,47,48,50,51,[57], [58], [59], [60]]. For chloroquine, after searching PubMed, Cochrane Review, Embase and Web of Science, 449 articles were identified. After screening the title and the abstract, only one Brazilian RCT and three observational studies described chloroquine and COVID-19. However, among these studies, those by Borba et al. and Saleh et al. did not have a standard of care comparative group [61,62]. Khamis et al. did not report death data related to CQ and Huang et al. did not report any death [63,64]. Consequently, no study on chloroquine met our inclusion criteria.
Fig. 1.
Flow diagram of study selection process.
Study characteristics
This meta-analysis included 15 190 patients in the hydroxychloroquine group, 8081 patients in the hydroxychloroquine with azithromycin group and 14 060 patients in the standard of care group with 3152 deaths, 1063 deaths and 2857 deaths, respectively. Individual studies are described in the Supplementary material (Tables S1 and S2). All included studies were carried out on hospitalized patients except for one [39]. Mean (±SD) age of participants was 62.1 ± 8.5 years. Ten studies were conducted in the USA [15,18,23,41,42,49,50,53,56,58], four in Spain [16,17,44,57], seven in France [13,24,46,48,54,59,60], one in the UK [40], two in Italy [43,65], one in China [14], one in Brazil [51] and three in several other countries (USA, Canada, Italy and Spain) [39,47,52]. Twenty-two articles were published [[13], [14], [15],17,18,24,39,41,43,44,46,47,[49], [50], [51], [52], [53], [54],56,57,59,60,65] and six articles were preprints [16,23,40,42,48,58]. Mean daily dose of hydroxychloroquine ranged from 333 mg/day to 945 mg/day. Few studies precisely described concomitant use of corticosteroids (see Supplementary material, Table S3) [[15], [16], [17],44,48,[50], [51], [52],65]. Only the RECOVERY trial precisely reported the use of dexamethasone (8% versus 9% in both arms) [40].
Study quality
Risk of bias was assessed with ROBIN-I for non-randomized studies (n = 26) and Rob2 for RCT (n = 3) (see Supplementary material, Figs S1 and S2). Three RCT had some concerns [39,40,51] and one interventional non-randomized study had critical risk of bias [24]. Among the observational studies, fourteen articles had a moderate or serious risk of bias [[13], [14], [15], [16], [17], [18],41,42,44,[46], [47], [48],56,58] and eleven studies had a critical risk of bias [23,43,49,50,[52], [53], [54],57,59,60,65]. Eleven observational studies did not report adjusted effect sizes to control confusion and selection bias [23,24,43,44,49,53,54,57,59,60,65]. Quality of studies was lowered by the lack of information about the assignment of treatment, the time between start of follow up and start of intervention, some unbalanced co-intervention with other antiviral and antibiotic drugs and imbalance between groups for confounders such as co-morbidities and age.
Hydroxychloroquine and mortality
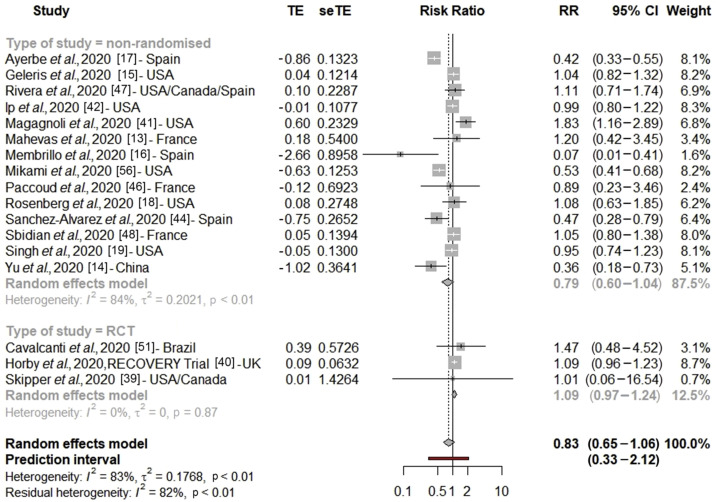
After excluding studies with critical bias, the pooled RR for COVID-19 mortality was 0.83 (95% CI 0.65–1.06, n = 17 studies) indicating no significant association between hydroxychloroquine and COVID-19 mortality (Fig. 2 ). Under the hypothesis of having a baseline mortality risk of 26% (based on ISARIC WHO CCP-UK cohort [29]), these pooled relative risk values would correspond to a non-significant risk difference of –4.4% [29] (Table 1 ). There was a significant subgroup difference between RCT and non-randomized studies (Pheterogeneity between = 0.03) with respectively RRRCT = 1.09 (95% CI 0.97–1.24) and RRnon-randomized = 0.79 (95% CI 0.60–1.04) (Fig. 2). Among observational studies with a moderate risk of bias, we found no association between hydroxychloroquine and mortality RRmoderate bias = 1.03 (95% CI 0.91–1.17, I 2 = 0%, n = 7 studies) with no subgroup heterogeneity (see Supplementary material, Table S4, Fig. S3). Results remained non-significant with influence analysis (see Supplementary material, Fig. S4). The Bayesian meta-analysis led to similar results with a pooled RR for mortality of 0.93 (95% CI 0.72–1.14, n = 17 studies) (see Supplementary material, Table S5, Fig. S5). In sensitivity analysis, after inclusion of studies with critical risk of bias, the global RR was marginally not significant 0.80 (95% CI 0.65–1.00) (see Supplementary material, Table S6).
Fig. 2.
Forest plot of the association between hydroxychloroquine alone and COVID-19 mortality (excluding studies with critical risk of bias). RR, risk ratio.
Table 1.
Relative risk and risk difference for mortality associated with hydroxychloroquine with or without azithromycin, assuming a UK mortality rate in hospital of 26% according to the ISARIC WHO CCP-UK cohort
| Outcome: All-cause mortality | Number of studies | Pooled relative risk (95% CI) | Risk difference (95% CI) |
|---|---|---|---|
| Hydroxychloroquine alone | |||
| All studies | 17 | 0.83 (0.65–1.06) | -4.4% (–9% to +1.5%) |
| Non-randomized studies | 14 | 0.79 (0.60–1.04) | -5.5% (–10% to +1%) |
| Randomized studies | 3 | 1.09 (0.97–1.24) | +2.3% (–0.8% to +6.2%) |
| Hydroxychloroquine with azithromycin | |||
| All studies | 7 | 1.27 (1.04–1.54) | +7% (+1% to +14%) |
| Non-randomized studies | 6 | 1.29 (1.06–1.58) | +7.5% (+1.6% to +15%) |
| Randomized studies | 1 | 0.64 (0.18–2.24) | -9% (–21% to +32%) |
There was a significant higher heterogeneity among non-randomized studies compared with RCT (I 2 = 84%, Pheterogeneity within < 0.01). In fact, heterogeneity was null for RCT. Egger's test (p 0.68) and Begg's test (p 0.13) were not significant for asymmetry of the funnel plot, indicating that there was no major publication bias for non-randomized studies (see Supplementary material, Fig. S6).
Hydroxychloroquine with azithromycin and mortality
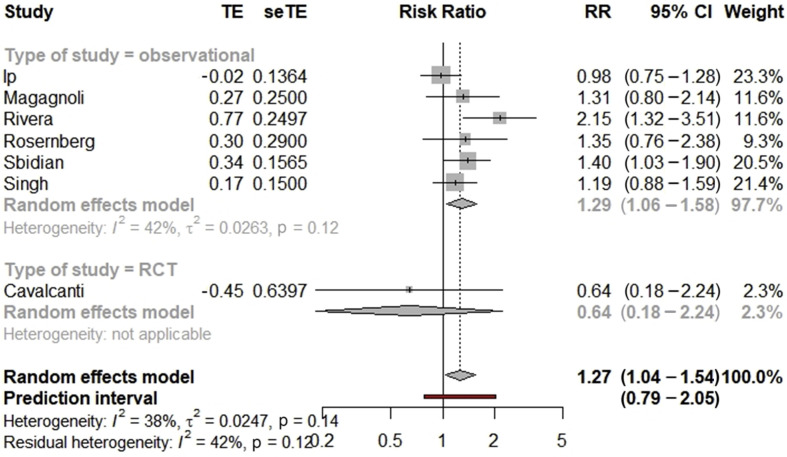
After exclusion of studies with critical bias, the pooled RR for COVID-19 mortality was 1.27 (95% CI 1.04–1.54, n = 7), indicating an increased mortality linked to the use of hydroxychloroquine with azithromycin. With a baseline hospital mortality of 26%, we identified a significant absolute risk difference of +7%. We found an increased risk of mortality in patients treated with hydroxychloroquine and azithromycin compared with standard of care (RR 1.29, 95% CI 1.06–1.58, n = 6) among non-randomized studies, but this relationship was not found in the single Brazilian RCT, with no heterogeneity observed across the study design (Pheterogeneity between = 0.28) (Fig. 3 ). There was a low heterogeneity across the included studies (I 2 = 38%, p 0.14). Egger's test (p 0.70) and Begg's test (p 0.65) were not significant but the asymmetry in the funnel plot indicates that a publication bias could be present (see Supplementary material, Fig. S7). However, the number of included studies was small. Subgroup analyses are described in the Supplementary material (Table S4, Fig. S8). The Bayesian meta-analysis led to similar results with a pooled RR for mortality of 1.32 (95% CI 0.97–1.68, n = 7 studies) (see Supplementary material, Table S5, Fig. S9). The increase in mortality was also significant with influence analysis (see Supplementary material, Fig. S10).
Fig. 3.
Forest plot of the association between hydroxychloroquine with azithromycin and COVID-19 mortality (excluding studies with critical risk of bias). RR, risk ratio.
Discussion
This meta-analysis summarized the results of 25 observational studies, three RCT and one interventional non-randomized study on the effect of hydroxychloroquine with or without azithromycin on the mortality of COVID-19 patients (Table 1). Despite our inclusion criteria that did not specify the stage of the disease, all the studies were conducted with hospitalized patients except the RCT by Skipper et al. [39]. Our results show that hydroxychloroquine alone was not associated with reduced mortality in COVID-19 patients, but the combination of hydroxychloroquine and azithromycin significantly increased mortality. We found similar results with a Bayesian analysis.
Our meta-analysis reported a high heterogeneity for hydroxychloroquine alone, but this heterogeneity was lowered among RCT, studies with moderate risk of bias and for the association of hydroxychloroquine + azithromycin. The variable quality of the studies (not reporting hydroxychloroquine dose, the lack of adjustment in reported estimates) may explain one part of the heterogeneity observed according to our subgroup analysis (see Supplementary material, Table S4).
A previous systematic review only included eight studies on all-cause mortality in COVID-19 patients [[13], [14], [15], [16],23,38,41,66] and concluded that the level of evidence for a hydroxychloroquine effect was very weak [67]. A preprint meta-analysis, using routinely collected records from clinical practice in Germany, Spain, the UK, Japan and the USA compared the use of hydroxychloroquine with sulfasalazine [68]. This study observed an increased risk of 30-day cardiovascular mortality (HR = 2.19, 95% CI 1.22–3.94), although the study lacked a standard of care comparative group. Some previous meta-analyses were also conducted on hydroxychloroquine and various health end points including mortality. However, these studies did not report all the published and unpublished literature, including a very limited number of studies: from three articles [19,20] to six articles [21]. These previous meta-analyses did not perform subgroup and sensitivity analyses to test the effect of pooling RCT and observational studies, nor did they study the source of heterogeneity. They used unadjusted risk ratios (calculated with the number of events in each group) whereas in our meta-analysis, we used adjusted relative risk [69] and we ran sensitivity analyses on the adjustment of effect size. Statistical adjustments for key prognostic variables limit confusion bias, especially in observational studies, which are not randomized. This meta-analysis confirmed the partial preliminary results of these other meta-analyses about the absence of effect for hydroxychloroquine on survival and found an increased mortality with the use of the combination of hydroxychloroquine with azithromycin in COVID-19 patients. These results confirm the preliminary findings of several observational studies, which have shown that the combination of hydroxychloroquine and azithromycin might increase the risk of acute, life-threatening cardiovascular events [70]. A first study found that, among individuals treated with this combination, 6 out of 18 (33%) developed a significant increase in the QTc interval [70]. Another work found that in 84 patients treated with hydroxychloroquine + azithromycin, nine had a severe prolongation of QTc [71]. The combination of hydroxychloroquine + azithromycin was associated with a greater variation in the QTc interval compared with hydroxychloroquine alone in a study with 90 patients [72]. In a study conducted in New York on 1438 patients, cardiac arrest was significantly more likely in patients receiving hydroxychloroquine with azithromycin compared to patients receiving neither of the two drugs (adjusted OR 2.13, 95% CI 1.12–4.05) [18]. Finally, a study conducted on the WHO database bringing together more than 167 000 patients found an increased risk of potentially fatal acute cardiac events in patients treated with azithromycin alone or with hydroxychloroquine alone [73]. The combination of the two drugs posed an even greater risk of life-threatening acute cardiac effects [18,72,73].
Several national health organizations (US Food and Drug Administration [74], French Agency for the Safety of Health Products [75], European Medicine Agency [76]) raised concerns about using unapproved drugs for COVID-19. The French Agency for the Safety of Health Products and the US Food and Drug Administration removed the authorization for the use of hydroxychloroquine outside clinical trials. The Indian Council of Medical Research took the opposite position and recommended chemoprophylaxis with hydroxychloroquine for asymptomatic individuals [77]. Finally, in the comparative peer-reviewed studies, a clear conclusion on hydroxychloroquine is not possible because of the small sample size, the lack of well-performed RCT (mainly non-randomized and retrospective studies) and inconsistent results. Many preprints without a comparative group and without randomization added to confusion surrounding this highly politicized topic [78]. There is a gap between the speed of clinical research and the expectation of a clear solution to treat people with COVID-19. Indeed, producing robust clinical trials is necessarily time-consuming. In a press communication, on 20 June 2020, the US National Institutes of Health stopped the clinical trial of hydroxychloroquine because this drug was very unlikely to be efficient for treatment of individuals with COVID-19 [79]. Based on SOLIDARITY trial results, the WHO previously took the same decision [80].
A Bayesian meta-analysis confirmed our findings from classical random-effect meta-analysis. We included several unpublished papers to minimize the publication bias. Our subgroup analysis by published studies (versus unpublished studies) found that the inclusion of preprints did not change the results. Exclusion of grey literature (unpublished studies, with limited distribution) could lead to an exaggeration of the intervention effect by 15% [81]. There is limited evidence to identify whether grey studies have a poorer methodological quality than published studies [82].
A major limitation is the inclusion of individuals with different levels of COVID-19 severity. However, we could not conduct subgroup analysis for severity because most study reports do not use the same definition of severity and do not report the same biological and clinical outcomes. We also noted a high level of heterogeneity in the administration of hydroxychloroquine (dosing, timing between hospital administration and intervention, duration). In some studies, these data were not reported at all. Another limitation comes from the studies that did not report adjusted effect size when mortality was not the primary end point, leading to a high risk of confounding bias. As is usually done, this meta-analysis was based on aggregated data, without access to original patient data. Most of the included studies were observational studies, which are not adapted to identify a causal association. Indeed, some of the included studies had very low quality of evidence (missing data, small sample size, confusion bias, bias in classification of intervention and selection bias), although our supplementary analyses and the exclusion of these articles did not change the results. Finally, this meta-analysis did not include results from the European DisCoVeRy trial and the WHO Solidarity trial, which are not yet published or communicated [80].
In conclusion, this meta-analysis clearly shows that hydroxychloroquine alone is not effective for the treatment of people with COVID-19 and that the combination of hydroxychloroquine and azithromycin increases the risk of mortality. These data support current clinical recommendations such as those of the National Institutes of Health [83], which do not recommend the use of hydroxychloroquine alone or in combination with azithromycin for COVID-19. There is already a great number of studies that have evaluated hydroxychloroquine alone or in combination [10] and it seems unlikely at this stage that any efficacy will emerge. Our results suggest that there is no need for further studies evaluating these molecules, and the European DisCoveRy clinical trial or the WHO international Solidarity clinical trial have already discontinued treatment arms using hydroxychloroquine [80,84].
Transparency declaration
All authors declare no support from any organization for the submitted work other than that described above; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Funding
There was no specific funding for this meta-analysis.
Authors' contributions
TF designed the research. TF, MR, AG, MM, NPS and YMS conducted the research. TF performed the statistical analysis and wrote the first draft of the paper. MR, AG, MM, NPS and YMS contributed to the writing of the paper. All authors contributed to the data interpretation, revised each draft for important intellectual content, and read and approved the final manuscript.
Acknowledgements
The authors would like to thank Dominique Meroux for proofreading the manuscript. We also thank Drifa Belhadi for her helpful comments on the manuscript.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.08.022.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization (WHO) 21 June 2020. Coronavirus disease (COVID-19). Situation Report - 153.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available from: [Google Scholar]
- 2.Dyall J., Gross R., Kindrachuk J., Johnson R.F., Olinger G.G., Hensley L.E. Middle East respiratory syndrome and severe acute respiratory syndrome: current therapeutic options and potential targets for novel therapies. Drugs. 2017;77:1935–1966. doi: 10.1007/s40265-017-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020;145:104228. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill A., Wang J., Levi J., Heath K., Fortunak J. Minimum costs to manufacture new treatments for COVID-19. J Virus Erad. 2020;6:61–69. doi: 10.1016/S2055-6640(20)30018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis n.d. 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed]
- 6.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:1–4. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R. Hydroxychloroquine in the treatment and prophylaxis of SARS-CoV-2 infection in non-human primates. Nature. 2020 doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 8.DeJong C., Wachter R.M. The risks of prescribing hydroxychloroquine for treatment of COVID-19—first, do no harm. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.1853. [DOI] [PubMed] [Google Scholar]
- 9.Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020:1–8. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 10.Fragkou P.C., Belhadi D., Peiffer-Smadja N., Moschopoulos C.D., Lescure F.-X., Janocha H. Review of trials currently testing treatment and prevention of COVID-19. Clin Microbiol Infect. 2020;26:988–998. doi: 10.1016/j.cmi.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peiffer-Smadja N, Lescure F-X, Sallard E, Ravaud P, Vegreville B, Zeitoun J-D. Anticovid, a comprehensive open-access real-time platform of registered clinical studies for COVID-19. J Antimicrob Chemother n.d. 10.1093/jac/dkaa223. [DOI] [PMC free article] [PubMed]
- 12.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahévas M., Tran V.-T., Roumier M., Chabrol A., Paule R., Guillaud C. Clinical efficacy of hydroxychloroquine in patients with COVID-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369 doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu B., Li C., Chen P., Zhou N., Wang L., Li J. Low dose of hydroxychloroquine reduces fatality of critically ill patients with. Sci China Life Sci. 2020:1–7. doi: 10.1007/s11427-020-1732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Membrillo F.J., Ramírez-Olivencia G., Estébanez M., Dios B de, Herrero M.D., Mata T. 2020. Early hydroxychloroquine is associated with an increase of survival in COVID-19 patients: an observational study. Preprints. [DOI] [Google Scholar]
- 17.Ayerbe L., Risco C., Ayis S. The association between treatment with heparin and survival in patients with Covid-19. J Thromb Thrombolysis. 2020:1–4. doi: 10.1007/s11239-020-02162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020 doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh A.K., Singh A., Singh R., Misra A. Hydroxychloroquine in patients with COVID-19: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:589–596. doi: 10.1016/j.dsx.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarma P., Kaur H., Kumar H., Mahendru D., Avti P., Bhattacharyya A. Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine: a systematic review and meta-analysis. J Med Virol. 2020;92:776–785. doi: 10.1002/jmv.25898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel T.K., Barvaliya M., Kevadiya B.D., Patel P.B., Bhalla H.L. Does adding of hydroxychloroquine to the standard care provide any benefit in reducing the mortality among COVID-19 patients?: a systematic review. J Neuroimmune Pharmacol. 2020 doi: 10.1007/s11481-020-09930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochrane Training. 2019. Cochrane handbook for systematic reviews of interventions. Part 3: special topics. 13.6.2.2.https://handbook-5-1.cochrane.org/chapter_13/13_6_2_2_combining_studies.htm Combining studies (version 6) Available from: [Google Scholar]
- 23.Barbosa J., Kaitis D., Freedman R., Le K., Lin X. Clinical outcomes of hydroxychloroquine in hospitalized patients with COVID-19: a quasi-randomized comparative study. Dropbox. 2020 https://www.dropbox.com/s/urzapkyij542qx5/NEJM_Clinical%20Outcomes%20of%20Hydroxychlorquine%20in%20Patients%20with%20COVID19.pdf.pdf.pdf.pdf.pdf.pdf.pdf.pdf?dl=0 Available from: [Google Scholar]
- 24.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 28.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trial. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020:369. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 32.Al-Bari MdAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5 doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins J.P.T., Thompson S.G., Spiegelhalter D.J. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viechtbauer W., Cheung M.W.-L. Outlier and influence diagnostics for meta-analysis. Res Synth Method. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 35.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2020 doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 36.Wang A.-L., Zhong X., Hurd Y. Comorbidity and sociodemographic determinants in COVID-19 mortality in a US urban healthcare system. Infectious Diseases (except HIV/AIDS) 2020 doi: 10.1101/2020.06.11.20128926. [DOI] [Google Scholar]
- 37.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369 doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Jun L.D., Chen Jun L.D. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. J Zhejiang Univ (Med Sci. 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skipper C.P., Pastick K.A., Engen N.W., Bangdiwala A.S., Abassi M., Lofgren S.M. Hydroxychloroquine in nonhospitalized adults with early COVID-19. Ann Intern Med. 2020 doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. MedRxiv. 2020 doi: 10.1101/2020.07.15.20151852. [DOI] [Google Scholar]
- 41.Magagnoli J., Narendran S., Pereira F., Cummings T.H., Hardin J.W., Sutton S.S. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Med. 2020 doi: 10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ip A., Berry D.A., Hansen E., Goy A.H., Pecora A.L., Sinclaire B.A. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients—an observational study. Infectious Diseases (except HIV/AIDS) 2020 doi: 10.1101/2020.05.21.20109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020 doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sánchez-Álvarez J.E., Fontán M.P., Martín C.J., Pelícano M.B., Reina C.J.C., Prieto Á.M.S. Report of the COVID-19 registry of the Spanish society of nephrology (SEN). Nefrología. English Edition; 2020. Status of SARS-CoV-2 infection in patients on renal replacement therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkinson E. RECOVERY trial: the UK COVID-19 study resetting expectations for clinical trials. BMJ. 2020;369 doi: 10.1136/bmj.m1626. [DOI] [PubMed] [Google Scholar]
- 46.Paccoud O, Tubach F, Baptiste A, Bleibtreu A, Hajage D, Monsel G, et al. Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe COVID-19 in a French university hospital. Clin Infect Dis n.d. 10.1093/cid/ciaa791. [DOI] [PMC free article] [PubMed]
- 47.Rivera D.R., Peters S., Panagiotou O.A., Shah D.P., Kuderer N.M., Hsu C.-Y. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: a COVID-19 and Cancer Consortium (CCC19) cohort study. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sbidian E., Josse J., Lemaitre G., Mayer I., Bernaux M., Gramfort A. Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France. MedRxiv. 2020 doi: 10.1101/2020.06.16.20132597. [DOI] [Google Scholar]
- 49.Luo J., Rizvi H., Preeshagul I.R., Egger J.V., Hoyos D., Bandlamudi C. COVID-19 in patients with lung cancer. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A. Hydroxychloroquine with or without azithromycin in mild-to-moderate COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cravedi P, Suraj SM, Azzi Y, Haverly M, Farouk S, Pérez-Sáez MJ, et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant n.D;n/a. 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed]
- 53.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L. Factors associated with death in critically ill patients with coronavirus disease 2019 in the USA. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lecronier M., Beurton A., Burrel S., Haudebourg L., Deleris R., Le Marec J. Comparison of hydroxychloroquine, lopinavir/ritonavir, and standard of care in critically ill patients with SARS-CoV-2 pneumonia: an opportunistic retrospective analysis. Crit Care. 2020;24:418. doi: 10.1186/s13054-020-03117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fontana F., Giaroni F., Frisina M., Alfano G., Mori G., Lucchi L. SARS-CoV-2 infection in dialysis patients in northern Italy: a single-centre experience. Clin Kidney J. 2020;13:334–339. doi: 10.1093/ckj/sfaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mikami T., Miyashita H., Yamada T., Harrington M., Steinberg D., Dunn A. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med. 2020:1–10. doi: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogado J., Obispo B., Pangua C., Serrano-Montero G., Martín Marino A., Pérez-Pérez M. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. 2020:1–5. doi: 10.1007/s12094-020-02381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh S., Khan A., Chowdhry M., Chatterjee A. Outcomes of hydroxychloroquine treatment among hospitalized COVID-19 patients in the United States—real-world evidence from a federated electronic medical record network. Infect Dis (except HIV/AIDS) 2020 doi: 10.1101/2020.05.12.20099028. [DOI] [Google Scholar]
- 59.Lagier J.-C., Million M., Gautret P., Colson P., Cortaredona S., Giraud-Gatineau A. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Travel Med Infect Dis. 2020:101791. doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bousquet G., Falgarone G., Deutsch D., Derolez S., Lopez-Sublet M., Goudot F.-X. ADL-dependency, D-Dimers, LDH and absence of anticoagulation are independently associated with one-month mortality in older inpatients with COVID-19. Aging (Albany NY) 2020;12:11306–11313. doi: 10.18632/aging.103583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saleh M., Gabriels J., Chang D., Kim B.S., Mansoor A., Mahmood E. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol. 2020 doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang M, Li M, Xiao F, Pang P, Liang J, Tang T, et al. Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. Natl Sci Rev n.d. 10.1093/nsr/nwaa113. [DOI] [PMC free article] [PubMed]
- 64.Khamis F., Al-Zakwani I., Al Naamani H., Al Lawati S., Pandak N., Omar M.B. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health. 2020 doi: 10.1016/j.jiph.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fontana F., Alfano G., Mori G., Amurri A., Tei L., Ballestri M. COVID-19 pneumonia in a kidney transplant recipient successfully treated with tocilizumab and hydroxychloroquine. Am J Transplant. 2020;20:1902–1906. doi: 10.1111/ajt.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mallat J., Hamed F., Balkis M., Mohamed M.A., Mooty M., Malik A. Hydroxychloroquine is associated with slower viral clearance in clinical COVID-19 patients with mild to moderate disease: a retrospective study. MedRxiv. 2020:2020. doi: 10.1101/2020.04.27.20082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hernandez A.V., Roman Y.M., Pasupuleti V., Barboza J.J., White C.M. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 2020 doi: 10.7326/M20-2496. [DOI] [PubMed] [Google Scholar]
- 68.Lane J.C.E., Weaver J., Kostka K., Duarte-Salles T., Abrahao M.T.F., Alghoul H. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. Rheumatology. 2020 doi: 10.1101/2020.04.08.20054551. [DOI] [Google Scholar]
- 69.Guyatt G.H., Oxman A.D., Vist G., Kunz R., Brozek J., Alonso-Coello P. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 70.Bessière F., Roccia H., Delinière A., Charrière R., Chevalier P., Argaud L. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chorin E., Wadhwani L., Magnani S., Dai M., Shulman E., Nadeau-Routhier C. QT interval prolongation and Torsade De Pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J. Risk of QT Interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nguyen L.S., Dolladille C., Drici M.-D., Fenioux C., Alexandre J., Mira J.-P. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin: an analysis of the world health organization pharmacovigilance database. Circulation. 2020;142:303–305. doi: 10.1161/CIRCULATIONAHA.120.048238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Commissioner O of the. Coronavirus (COVID-19) Update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine. FDA. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and Available from: [Google Scholar]
- 75.Agence Nationale de Sécurité du médicament et des produits de santé (Ansm). COVID-19 : l’ANSM souhaite suspendre par précaution les essais cliniques évaluant l’hydroxychloroquine dans la prise en charge des patients—point d’Information—ANSM: agence nationale de sécurité du médicament et des produits de santé n.d. Available from: https://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/COVID-19-l-ANSM-souhaite-suspendre-par-precaution-les-essais-cliniques-evaluant-l-hydroxychloroquine-dans-la-prise-en-charge-des-patients-Point-d-Information (accessed 15 June 2020).
- 76.Dimitrova E.K. European Medicines Agency; 2020. COVID-19: reminder of risk serious side effects with chloroquine and hydroxychloroquine.https://www.ema.europa.eu/en/news/covid-19-reminder-risk-serious-side-effects-chloroquine-hydroxychloroquine Available from: [Google Scholar]
- 77.Rathi S., Ish P., Kalantri A., Kalantri S. Hydroxychloroquine prophylaxis for COVID-19 contacts in India. Lancet Infect Dis. 2020;0 doi: 10.1016/S1473-3099(20)30313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alexander P.E., Debono V.B., Mammen M.J., Iorio A., Aryal K., Deng D. COVID-19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J Clin Epidemiol. 2020 doi: 10.1016/j.jclinepi.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.NIH halts clinical trial of hydroxychloroquine. National Institutes of Health (NIH); 2020. https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine Available from [Google Scholar]
- 80.WHO (World Health Organization). “Solidarity” clinical trial for COVID-19 treatments n.d. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments (accessed 8 June 2020).
- 81.McAuley L., Pham B., Tugwell P., Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet. 2000;356:1228–1231. doi: 10.1016/S0140-6736(00)02786-0. [DOI] [PubMed] [Google Scholar]
- 82.Hopewell S., McDonald S., Clarke M.J., Egger M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.MR000010.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Us NIH . 2020. Hydroxychloroquine plus azithromycin | coronavirus disease COVID-19. COVID-19 treatment guidelines.https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/hydroxychloroquine-plus-azithromycin/ Available from: [Google Scholar]
- 84.INSERM . 2020. Discovery stopping inclusions in two treatment groups.https://presse.inserm.fr/en/discovery-stopping-inclusions-in-two-treatment-groups/40087/ Available from: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.