Orally delivered double-headed nanoparticle curcumin has potential to treat intraocular inflammation.
Abstract
Novel approaches circumventing blood-ocular barriers in systemic drug delivery are lacking. We hypothesize receptor-mediated delivery of curcumin (CUR) across intestinal and ocular barriers leads to decreased inflammation in a model of lens-induced uveitis. CUR was encapsulated in double-headed polyester nanoparticles using gambogic acid (GA)–coupled polylactide-co-glycolide (PLGA). Orally administered PLGA-GA2-CUR led to notable aqueous humor CUR levels and was dosed (10 mg/kg twice daily) to adult male beagles (n = 8 eyes) with induced ocular inflammation. Eyes were evaluated using a semiquantitative preclinical ocular toxicology scoring (SPOTS) and compared to commercial anti-inflammatory treatment (oral carprofen 2.2 mg/kg twice daily) (n = 8) and untreated controls (n = 8). PLGA-GA2-CUR offered improved protection compared with untreated controls and similar protection compared with carprofen, with reduced aqueous flare, miosis, and chemosis in the acute phase (<4 hours). This study highlights the potential of PLGA-GA2 nanoparticles for systemic drug delivery across ocular barriers.
INTRODUCTION
Uveitis is a common ocular inflammation that can lead to reduced vision or, in certain cases, result in severe vision loss if left untreated (1). On the basis of the anatomical involvement of the eye, uveitis can be classified into anterior, intermediate, posterior, and panuveitis (1). Anterior uveitis, the most common form of uveitis, is caused by diverse etiologies including cataract surgeries (2). Cataract surgery results in the release of inflammatory mediators, leading to some degree of postoperative inflammation in all patients, of which patients with diabetes are at an increased risk. One of the most common postoperative complications is uveitis that can cause severe corneal edema, posterior synechia, and progression of diabetic retinopathy or neovascular glaucoma (3–5).
While there is no clear consensus on the therapeutic management of postsurgical intraocular inflammation, topical steroids and nonsteroidal anti-inflammatory drugs (NSAIDs) are currently used in clinics. However, these are associated with side effects, such as increased intraocular pressure (IOP), occurrence of adverse systemic events, indolent corneal ulceration, and full-thickness corneal melts (6–8). Topical delivery of ophthalmic medication (eye drops) is a widely used route for treating the anterior segment of the eye. However, this route is associated with poor drug bioavailability because of transient contact time, limited absorption, and rapid washout by tears. Moreover, there is much literature available on patient compliance and the difficulty in instilling eye drops (9, 10). On the other hand, the use of intravitreal implants such as dexamethasone implant is also associated with severe side effects, such as blurred vision, uncontrolled inflammation, increased IOP, risk of infections, and retinal detachments (11). It is also recognized that steroids are often a last resort in pre- or diabetic patients to treat postoperative inflammation because they significantly increase glucose levels as well as cause insulin resistance in healthy individuals (12–14).
In this context, there is significant interest in curcumin (CUR), a natural anti-inflammatory that has shown potential as an aldose reductase (AR) inhibitor, along with other promising properties to minimize endoplasmic reticulum stress, avoid β cell dysfunction, and suppress lipid abnormalities, endothelial dysfunction, and inflammation associated with the onset and progression of diabetic cataracts and other complications (15–19). Our recent studies using noncompetitive active delivery strategies targeting the transferrin receptor (TfR) (20, 21) significantly improved oral bioavailability of CUR, which otherwise is poorly bioavailable (22). While oral delivery to the eye is very attractive and compliant, lack of target tissue bioavailability and systemic toxicity hinder progress, and such a route is limited to very few agents (23). The literature suggests that TfR is widely expressed in the blood-ocular barriers and can potentially be useful for active delivery across these barriers (24). In addition, our ex vivo and in vivo data support the role of TfR in transport across the blood-ocular barriers (22, 25). Here, we report the efficacy of oral CUR aided by double-headed nanoparticles in a model of lens-induced uveitis that mimics the intraocular inflammation seen after cataract surgery. This approach provides a potential treatment option for postsurgical intraocular inflammation as well as prevent/delay cataracts in diabetes and aging populations.
RESULTS
PLGA-GA2-CUR nanoparticles
We have selected PLGA-GA2 nanoparticles as a vehicle for the delivery of CUR because of its ability to navigate across the intestinal barriers via the TfRs in a noncompetitive manner (independent of transferrin-binding sites), resulting in improved oral bioavailability of CUR (21, 22). The optimized synthesis protocols (Fig, 1A) led to scalable quantities of PLGA-GA2 in which the GA coupling was confirmed by nuclear magnetic resonance (NMR), characterized by amide bond formation [8.01 parts per million (ppm)] and doublets at 5.7 and 6.7 ppm for GA. The conjugation was further confirmed by Fourier transform infrared (FTIR), characterized by amide bond formation (1690 to 1640 cm−1 for C=O stretching; 1550 to 1640 cm−1 for N─H bending in amides; 1560 to 1530 cm−1 for C─N stretching) (22). The PLGA-GA2 can efficiently encapsulate CUR upon emulsification, resulting in nanoparticles with good size distribution [250 nm; 0.17 pDI (polydispersity index)], and these structures are in spherical shape (Fig. 1, B and C). The freeze-drying process led to esthetically pleasing cake formation that is easily reconstituted by simple shaking upon addition of water. The final freeze-dried preparations used in the subsequent studies contained 0.15 mg of CUR per milligram PLGA-GA2. The atomic force microscopy (AFM) images further reveal that CUR loading did not cause any change to particle morphology (fig. S1). These preparations are free of microbial burden (fig. S1).
Fig. 1. Synthesis and characterization of PLGA-GA2 and their CUR encapsulated nanoparticles (PLGA-GA2-CUR).
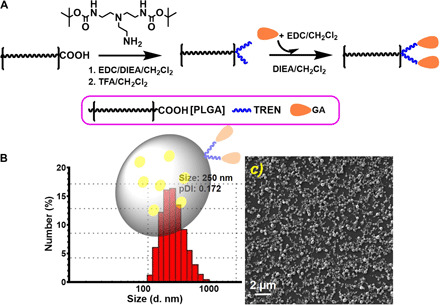
(A) Schematic illustration of synthesis of PLGA-GA2. (B) Dynamic light scattering size distribution of PLGA-GA2-CUR with an insert depicting model particle. (C) Scanning electron microscopy photomicrographs of PLGA-GA2-CUR. TFA, trifluoroacetic acid; DIEA, N,N-diisopropylethylamine; TREN, Tris(2-aminoethyl)amine; EDC, 1-ethyl-3- (dimethylaminopropyl) carbodiimide.
Single-dose preliminary safety assessment of PLGA-GA2-CUR, oral and topical administrations
This study was conducted to provide basic data on physiological and hematological characteristics of dogs upon single-dose oral administration of PLGA-GA2-CUR at 10 mg/kg (CUR equivalent), the dose used in the subsequent efficacy study. Because of individual dog, breed, and age variations with regard to normal hematological and serum biochemical values, baseline blood tests were obtained before dosing the dogs to serve as reference standards. At large, we did not observe any adverse events. There were no clinically meaningful differences for any of the clinical pathology tests (Tables 1 and 2). All findings were considered incidental and characteristic of normal dogs of that age.
Table 1. Summary of hematology values in healthy dogs before and after oral PLGA-GA2-CUR administration.
WBC, white blood cells; Abs, absolute; Rel, relative; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; PLT, platelet; MPV, mean platelet volume; PCT, procalcitonin; Retic, reticulocyte; Retic abs count, absolute reticulocyte count.
| CBC whole blood | Before dosing | 24 hours after dosing PLGA-GA2-CUR | 7 days after dosing PLGA-GA2-CUR | Reference range |
| Total WBC (K/μl) | 13.46 ± 3 | 10.79 ± 2.35 | 11.5 ± 1.36 | 6.0–17.0 |
| Abs neutrophils (K/μl) | 8.93 ± 2.94 | 6.00 ± 1.4 | 6.98 ± 1.53 | 3.0–11.5 |
| Abs lymphocytes (K/μl) | 3.7 ± 0.46 | 3.95 ± 1.04 | 3.33 ± 0.81 | 1.0–4.8 |
| Abs monocytes (K/μl) | 0.57 ± 0.42 | 0.6 ± 0.29 | 0.9 ± 0.22 | 0.15–1.35 |
| Abs eosinophils (K/μl) | 0.27 ± 0.06 | 0.3 ± 0.1 | 0.25 ± 0.17 | 0.10–1.25 |
| Rel neutrophils (%) | 65.33 ± 6.81 | 55.5 ± 1.29 | 60.5 ± 8.58 | – |
| Rel lymphocytes (%) | 29 ± 9.54 | 36.25 ± 2.87 | 29.25 ± 7.63 | – |
| Rel monocytes (%) | 4.00 ± 3.00 | 6.25 ± 3.77 | 8.00 ± 2.00 | – |
| Rel eosinophils (%) | 3.67 ± 0.58 | 3.46 ± 1.65 | 2.25 ± 1.26 | – |
| RBC (M/μl) | 5.94 ± 0.27 | 8.08 ± 3.76 | 6.54 ± 0.27 | 5.5–8.5 |
| HGB (g/dl) | 10.6 ± 4.81 | 20.35 ± 12.77 | 15.13 ± 0.93 | 12.0–18.0 |
| HCT (%) | 28.1 ± 13.9 | 46.9 ± 13.97 | 43.55 ± 0.86 | 32–55 |
| MCV (fl) | 51.17 ± 16.69 | 54.23 ± 20.69 | 66.68 ± 3.36 | 60–77 |
| MCH (pg) | 35.8 ± 23.13 | 25.6 ± 6.17 | 23.15 ± 0.93 | 19.5–24.5 |
| MCHC (g/dl) | 32.53 ± 7.68 | 29.73 ± 10.49 | 34.73 ± 0.38 | 32–36 |
| RDW (%) | 22.63 ± 13.22 | 15.15 ± 1.5 | 14.63 ± 01.59 | 11.9–14.9 |
| PLT (K/μl) | 445.4 ± 66.72 | 444.33 ± 21.95 | 521.25 ± 79.28 | 200–500 |
| MPV (fl) | 9.00 ± 3.89 | 7.46 ± 5.13 | 9.9 ± 1.75 | 7.9–16.2 |
| PCT (%) | 3.27 ± 4.7 | 0.53 ± 0.2 | 0.52 ± 0.15 | 0.12–0.43 |
| Retic (%) | 0.66 ± 0.34 | 0.94 ± 0.61 | 1.39 ± 0.84 | – |
| Retic abs count (K/μl) | 43.46 ± 14.30 | 41.4 ± 22.7 | 50.66 ± 11.42 | 10–80 |
Table 2. Summary of serum biochemistry values in healthy dogs before and after oral PLGA-GA2-CUR administration.
GA2-CUR administration. A/G ratio, albumin-to-globulin ratio; BUN, blood urea nitrogen; ALKP, alkaline phosphatase; AST, aspartate aminotransaminase; ALT, alanine aminotransaminase; GGT, gamma-glutamyl transferase; GLDH, glutamate dehydrogenase.
| Chemistry profile | Before dosing |
24 hours after dosing PLGA-GA2-CUR |
7 days after dosing PLGA-GA2-CUR |
Reference range |
| Total protein (g/dl) | 5.33 ± 0.15 | 5.03 ± 0.10 | 5.13 ± 0.10 | 5.6–7.9 |
| Albumin (g/dl) | 2.98 ± 0.17 | 2.93 ± 0.05 | 3.05 ± 0.06 | 2.8–4.3 |
| Globulins (g/dl) | 2.35 ± 0.19 | 2.10 ± 0.08 | 2.08 ± 0.05 | 1.8–4.2 |
| A/G ratio | 1.30 ± 0.18 | 1.40 ± 0.08 | 1.48 ± 0.05 | 0.7–2.5 |
| Calcium (mg/dl) | 10.75 ± 0.34 | 10.48 ± 0.26 | 10.63 ± 0.13 | 7.2–12.8 |
| Phosphorus (mg/dl) | 7.45 ± 0.70 | 6.93 ± ±0.54 | 7.03 ± 0.21 | 2.3–6.5 |
| Glucose (mg/dl) | 73.00 ± 10.30 | 100.00 ± 6.88 | 106.25 ± 6.70 | 60–120 |
| BUN (mg/dl) | 14.25 ± 1.26 | 12.75 ± 1.05 | 10.25 ± 1.50 | 8–30 |
| Creatinine (mg/dl) | 0.53 ± 0.05 | 0.58 ± 0.10 | 0.63 ± 0.05 | 0.5–1.4 |
| Bilirubin–total (mg/dl) | 0.15 ± 0.10 | 0.20 ± 0.08 | 0.15 ± 0.06 | 0.1–0.4 |
| ALKP (U/liter) | 108.50 ± 25.72 | 107.25 ± 16.66 | 103.50 ± 16.60 | 12–122 |
| AST (U/liter) | 46.25 ± 4.79 | 35.25 ± 1.50 | 40.50 ± 2.65 | 13–52 |
| ALT (U/liter) | 34.50 ± 3.70 | 32.50 ± 5.32 | 37.75v7.41 | 13–79 |
| GGT (U/liter) | 4.25 ± 1.26 | 2.50 ± 0.71 | 3.25 ± 0.50 | 0–10 |
| GLDH (U/liter) | 8.50 ± 1.91 | 7.75 ± 0.96 | 6.50 ± 1.29 | 0–14 |
| Amylase (U/liter) | 592.00 ± 93.03 | 590.00 ± 115.86 | 605.50 ± 47.76 | 200–953 |
| Cholesterol (mg/dl) | 172.75 ± 18.52 | 164.50 ± 20.86 | 160.50 ± 17.71 | 124–335 |
| Sodium (mEq/liter) | 146.75 ± 1.71 | 145.75 ± 0.50 | 146.00 ± 1.63 | 141–156 |
| Potassium (mEq/liter) | 5.18 ± 0.38 | 4.75 ± 0.13 | 1.93 ± 0.13 | 3.8–5.5 |
| Chloride (mEq/liter) | 107.50v0.58 | 109.50 ± 0.58 | 109.50 ± 0.58 | 109–124 |
| Sodium-to-potassium ratio | 28.45 ± 1.83 | 30.70 ± 0.86 | 29.65 ± 0.82 | – |
In addition, topical administration was studied for ocular irritation parameters. Overall, topical PLGA-GA2-CUR was well tolerated by the dogs with no observable signs of ocular discomfort with regard to blepharospasm or rubbing following drug instillation. There was no evidence of conjunctival hyperemia or swelling following drug installation. The ophthalmic suspension of PLGA-GA2-CUR, when instilled into the eye (fig. S2), left residual bright orange particulates on the ocular surface and within the medial canthus of the dogs (fig. S3).
Single oral/topical dose target levels: Aqueous humor
Systemic delivery to the eye is challenging; therefore, we did preliminary evaluation of PLGA-GA2-CUR for its ability to deliver CUR to target tissue, in this case aqueous humor. A single dose of 10 mg/kg (CUR equivalent) administered orally led to quantifiable CUR levels in aqueous humor within 30 min (Fig. 2A). On the other hand, topical application of a single drop of PLGA-GA2-CUR led to qualitative CUR concentration based on the mass spectrometry peak, but not quantifiable (fig. S4). Earlier, our ex vivo studies suggest that GA-coupled particles cross the blood-ocular barriers (25); however, such studies in dogs can be difficult in survival studies.
Fig. 2. Target tissue distribution of oral PLGA-GA2-CUR in healthy dogs and anti-inflammatory effects of oral PLGA-GA2-CUR in a canine model of acute intraocular inflammation.
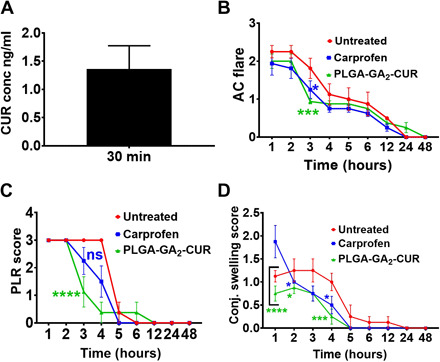
(A) Aqueous humor levels of CUR following a single oral dose of PLGA-GA2-CUR. (B to D) Following intracameral injection of lens protein at t = 0 hours, eyes were serially evaluated using the SPOTS system, including measurements of (B) aqueous flare, (C) pupillary light reflex, (D) conjunctival swelling. Statistical significance of oral PLGA-GA2-CUR administration was compared with treatment with oral carprofen and untreated controls, as determined by a two-way ANOVA. Statistical significance is denoted by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Oral efficacy of PLGA-GA2-CUR
Ocular inflammation was effectively induced in 12 dogs by 50 μl (539.32 μg) of canine lens protein solution (10.78 μg/ml) injected intracamerally. Clinical signs of ocular inflammation were semiquantitatively scored on nine different occasions (1, 2, 3, 4, 5, 6, 12, 24, and 48 hours after injection). The inflammatory response incited was immediately observed within 1 hour of the injection, with a markedly miotic pupil, and moderate amounts of protein (aqueous flare) and frank fibrin were detectable in the anterior chamber. Marked bulbar conjunctival hyperemia and swelling, along with mild serous to mucoid ocular discharge, were observed within 2 to 3 hours of lens protein administration. Clinical signs of inflammation reached a plateau by 4 to 5 hours after injection, and the response gradually subsided until complete resolution was observed in most dogs by 24 hours after injection. Corneal edema, corneal vascularization, hypopon, hyphema, iris hyperemia, lens opacification, and posterior segment lesions were not observed. The semiquantitative preclinical ocular toxicology scoring (SPOTS) system was easy and effective to use, enhancing the applicability of semiquantitative scoring criteria to the canine species. Photographic exemplars of aqueous flare and cell (fig. S5), conjunctival hyperemia (fig. S6), and conjunctival swelling (fig. S7) scoring criteria are demonstrated along with examples of frank fibrin deposition in the anterior chamber (fig. S8).
Mean ocular inflammatory scores for eyes in each group were averaged, and results are shown in Fig. 2 and fig. S9. Oral PLGA-GA2-CUR 10 mg/kg (CUR equivalent) every 12 hours showed significantly marked reduction in several ocular inflammatory parameters, such as aqueous flare (AC flare score) (Fig. 2B), miosis (PLR score) (Fig. 2C), and chemosis (conjunctival swelling score) (Fig. 2D) compared with untreated controls at various time points within the early phase (<4 hours) of lens protein–induced ocular inflammation. There was no significant difference in ocular inflammatory scores when comparing oral PLGA-GA2-CUR and untreated controls >4 hours after injection nor when comparing the PLGA-GA2-CUR and carprofen treatment groups at any time point (Fig. 2 and fig. S9). There was no significant difference in the severity of conjunctival hyperemia or conjunctival discharge scores over time in all three groups (fig. S9, A and B). There was a more rapid clearance of fibrin from the anterior chamber of dogs in the carprofen group compared to the oral PLGA-GA2-CUR and untreated control groups (fig. S9C).
Three pairwise tests are conducted for untreated versus carprofen, untreated versus PLGA-GA2-CUR, and carprofen versus PLGA-GA2-CUR. After regrouping the AC flare scores, the data from hours 1 to 4 are used in the following statistical analysis because all regrouped responses are zero after hour 4. Table 3 shows the corresponding P values of testing the interaction between treatment and time using model (2) and testing the treatment effect alone using model (3) under oral administration. All three tests of interaction term are not significant according to their corresponding large P values, which means that the drug effects over time do not change between treatments. Furthermore, we use model (3) to check whether carprofen and PLGA-GA2-CUR have constant drug effects independent of time. On the basis of those P values reported in Table 3, we know that compared with no treatment, both drugs have constant effects that are independent of time on reducing the inflammation. However, between carprofen and PLGA-GA2-CUR, their drug effects are similar because of its large P value of 0.727, although PLGA-GA2-CUR performs better than carprofen through the third hour (Fig. 2B). Overall, with n = 8, there is not enough statistical evidence to support the differences between the two treatment groups.
Table 3. P values of the test using model (2) and using model (3) for all three comparisons under oral administration.
Three comparisons under oral administration.
| Pairwise comparison | ||
| Untreated versus carprofen | 0.13827 | 0.014 |
| Untreated versus PLGA-GA2-CUR | 0.87049 | 0.00483 |
| Carprofen versus PLGA-GA2-CUR | 0.15326 | 0.7270 |
Topical efficacy of PLGA-GA2-CUR
Ocular inflammation was effectively induced in eight dogs by a second intracameral injection of 50 μl (539.32 μg) of canine lens protein (10.78 μg/ml). Clinical signs of ocular inflammation were semiquantitatively scored on nine different occasions (1, 2, 3, 4, 5, 6, 12, 24, and 48 hours after injection). There was no difference in the severity or duration of the inflammatory response incited following the second injection of canine lens protein. Therefore, scores from both treatment groups were compared with untreated control scores from the initial oral efficacy study.
Mean ocular inflammatory scores for eyes in each group were averaged, and results are shown in Fig. 3 and fig. S10. Topical installation of 500 μg of PLGA-GA2-CUR (CUR equivalent) four times daily showed significantly marked reduction in several ocular inflammatory parameters, such as aqueous flare (AC flare score) (Fig. 3A), miosis (PLR score) (Fig. 3B), and chemosis (conjunctival swelling score) (Fig. 3C) compared with untreated controls at various time points within the early phase (<4 hours) of lens protein–induced ocular inflammation. There was no significant difference between inflammatory scores when comparing topical PLGA-GA2-CUR and untreated controls >4 hours after injection (Fig. 3 and fig. S10). A significant difference was not detected between the two treatment and control groups with regard to conjunctival discharge, aqueous cell count (AC cell score), or fibrin clearance scores (fig. S10). Most of the ocular inflammatory parameters assessed did not differ between the topical PLGA-GA2-CUR and topical prednisolone treatment groups at any time point (Fig. 3 and fig. S10), except for conjunctival hyperemia, which was reduced in the topical PLGA-GA2-CUR group at 6 and 12 hours after injection (fig. S10A). No complications arose during sedation or from the intracameral injection or aqueocentesis procedures. All dogs recovered from sedation uneventfully during all procedures. Similar to the oral treatment, the drug effects over time are constant for both treatments. However, when testing against the control group under model (3), prednisolone acetate and PLGA-GA2-CUR both have effects, which are independent of time based on the small P values in Table 4.
Fig. 3. Anti-inflammatory effects of topical PLGA-GA2-CUR in a canine model of acute intraocular inflammation.
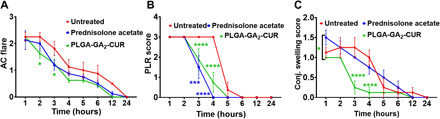
(A to C) Following intracameral injection of lens protein at t = 0 hours, eyes were serially evaluated using the SPOTS system, including measurements of (A) aqueous flare, (B) pupillary light reflex, (C) conjunctival swelling. Statistical significance of topical PLA-GA2-CUR administration was compared to treatment with topical prednisolone acetate and untreated controls, as determined by a two-way ANOVA. Statistical significance is denoted by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Table 4. P values of the test using model (2) and using model (3) for all three comparisons under topical administration.
| Pairwise comparison | ||
| Untreated versus prednisolone acetate |
0.11037 | 0.00438 |
| Untreated versus PLGA-GA2-CUR | 0.55748 | 0.00864 |
| Prednisolone acetate versus PLGA-GA2-CUR |
0.18537 | 0.506844 |
DISCUSSION
The polymer Resomer 503H PLGA used in the present study is one of the most widely used PLGA grades for drug delivery applications that typically offer sustained release capability, alongside ligand conjugation (19, 20, 22). The PLGA-GA2 synthesis was reproducible and scalable, in which the synthesis was performed at 10-g scales. Similarly, the PLGA-GA2-CUR nanoparticles were prepared at 1.5-g scales and freeze-dried, which led to multiple-dose canine study. Our previous studies with PLGA-GA (single head) or PLGA-GA2 (double headed) revealed the TfR-mediated noncompetitive active transport across the intestinal barriers, leading to improved bioavailability and drug distribution (20, 22). Our previous in silico studies also revealed the hydrophobic interaction of GA with the TfR, and such interaction is independent of transferrin binding (21).We have established the preliminary safety of PLGA-GA on repeated doses in rodents (21) that set the tone for investigating the improved versions of PLGA-GA2 in higher-order species for preliminary safety and efficacy.
No adverse events or evidence of irritation was observed with either the tested systemic doses or topical concentrations of the study drug. Our results provide valuable reference indices of the normal physiological and hematological characteristics of dogs before and after PLGA-GA2-CUR treatment, ensuring the safety of our formulated nanoparticles. The safety study (both oral and topical) is a preliminary attempt to determine whether efficacy should be pursued. A regulatory safety study is not within the scope of this work; however, the topical irritation test with one dose should be adequate to assess whether the formulation presents any irritation. The PLGA-GA2 nanoparticles used in this study had a sevenfold and twofold improvement in the maximum concentration (Cmax) compared with PLGA and PLGA-GA, respectively (22). The results presented here demonstrate the ability of PLGA-GA2-CUR to improve systemic delivery to the eye, leading to quantifiable levels of CUR upon oral administration, 30 min after dosing in aqueous humor. On the other hand, topical application led to qualitative but not quantifiable CUR levels as the particles may have been restricted to corneal epithelium, coupled with tear/blink-mediated clearance (26). We have restricted to single–time point sampling based on the Cmax of CUR (22), as it is not easy to conduct a multiple–time point aqueous humor sampling because of the availability of low volumes, its physiological role, and the invasive nature of the sampling procedure that requires sedation.
A model of lens-induced uveitis using an intracameral injection of homologous lens protein was used to induce acute intraocular inflammation by stimulating the arachidonic acid cascade, as previously described (27–32). This method was chosen as a minimally invasive technique to mimic the clinical problems of uveitis following cataract surgery, as the management of postoperative ocular inflammation is a major challenge observed in both human and veterinary ophthalmology (33–36). Dogs were chosen as a large-animal model because the treatment of uveitis is a major clinical problem in spontaneous disease as well as after intraocular surgery, and canine models of uveitis have successfully evaluated the pharmacokinetics and clinical effects of drugs on ocular tissues (28, 37, 38).
The intracameral injection of canine lens protein in this study resulted in an immediate inflammatory response characterized by miosis, moderate conjunctival hyperemia, moderate chemosis, mild serous to mucoid ocular discharge, moderate aqueous flare and cell, intraocular fibrin formation, and mild transient ocular hypotension. No changes to the cornea, lens, or posterior segment were observed. The most severe clinical signs were noted within 2 to 3 hours of the injection, and complete resolution of clinical signs, with no evidence of long-term sequelae, was observed within 24 to 48 hours after injection, regardless of treatment. This represents a model of acute and transient intraocular inflammation. Although uveitis was induced twice in eight dogs enrolled in both the oral and topical efficacy studies, sensitization to lens proteins was not observed similar to those reported in the literature (28), as there was no difference in severity of clinical signs after the second injection. This may be because of the efficiency by which aqueous humor is cleared of foreign protein, as canine eyes have a calculated aqueous humor flow rate of 4.54 to 5.58 μl/min, or the relatively low antigenicity of homologous lens protein when introduced in this manner (28, 39). Therefore, the present study represents a useful model of acute anterior uveitis; however, this method is not sufficient for the evaluation of chronic, severe, or posterior forms of uveitis.
Oral administration of PLGA-GA2-CUR (10 mg/kg; CUR equivalent) every 12 hours offered improved protection compared with untreated controls, indicated by a reduction in aqueous flare (AC flare score), miosis (PLR score), and chemosis (conjunctival swelling score) in the early phase (<4 hours) of lens protein–induced ocular inflammation. The inflammatory parameters evaluated were comparable between the PLGA-GA2-CUR and carprofen groups, indicating a clinically applicable anti-inflammatory effect in the oral PLGA-GA2-CUR group.
A standard, commercial NSAID was elected in the oral efficacy study for comparison. Oral carprofen, a selective cyclooxygenase (COX-2) inhibitor, plays an important role in veterinary ocular therapeutics to suppress prostaglandin-mediated anterior uveitis, as seen following intraocular surgery (37, 38, 40). Although COX-2 inhibitors are thought to have wider safety margins compared with COX-1 inhibitors, adverse side effects may still occur including nausea, diarrhea, gastrointestinal ulceration, nephrotoxicosis, platelet dysfunction, and idiosyncratic hepatotoxicosis (37, 38, 41). Therefore, it is important to evaluate the efficacy of safer alternatives in the systemic treatment of uveitis.
Further, our results also show that four-times-daily topical instillation of 500 μg of PLGA-GA2-CUR (CUR equivalent) offered improved protection compared with untreated controls, indicated by a reduction in aqueous flare (AC flare score) and chemosis (conjunctival swelling score) in the early phase (<4 hours) of lens protein–induced ocular inflammation. In addition, conjunctival hyperemia decreased >6 hours after injection in the PLGA-GA2-CUR group compared with the prednisolone group. The remaining inflammatory parameters evaluated were comparable between the PLGA-GA2-CUR and prednisolone groups, indicating a clinically relevant anti-inflammatory effect in the topical PLGA-GA2-CUR group. Although the aqueous humor levels were not quantifiable, clinical outcomes may reflect multiple administrations.
A standard, commercial corticosteroid was elected in the topical efficacy study for comparison. Prednisolone acetate 1% ophthalmic suspension, a phospholipase A2 inhibitor, blocks the synthesis of prostaglandins and is a potent anti-inflammatory drug used to treat uveitis and control intraocular inflammation following cataract surgery in both human and veterinary patients (33, 34, 42, 43). Because corticosteroids target an early stage of the arachidonic acid cascade, they have a wide spectrum of activity as well as a greater range of adverse effects. This includes increased IOP, increased risk of ocular infection, impaired wound healing, and keratomalacia (33). Therefore, it is imperative to identify safer alternatives to the topical treatment of uveitis.
Our study used a semiquantitative ocular scoring system optimized for use in modern preclinical drug development and toxicology (SPOTS) (44). We acknowledge that more objective methods to measure the integrity of the blood-aqueous barrier exist, such as anterior chamber fluorophotometry and laser flare photometry (29, 45, 46). While photometry methods are more sensitive compared with clinical assessment scores, they only measure protein leakage, which correlates to the breakdown of the blood-aqueous barrier (29). A slit-lamp scoring system is semiobjective, based on the observer’s interpretation, and prone to bias; however, this method provides a more detailed and comprehensive picture of the inflammatory response of the eye by recording multiple parameters (29). In addition, quantitative measurements via photometry correlate well with clinical grades of aqueous flare when a standardized method of scoring is used (46). The SPOTS system was elected over other scoring systems as it provides enhanced applicability and validity of scoring criteria to canine species and incorporates ophthalmic findings commonly observed in preclinical studies involving ocular therapeutics (44).
Overall, the data together should pave the way to use comparative ophthalmology as a method to accelerate the development of human medications by introducing animal patients with naturally occurring pathologies to test the nonconventional dosage forms. The preliminary safety and efficacy data should allow testing of PLGA-GA2 with other traditional drugs; examples include the control drugs used in this study.
In conclusion, the therapeutic usefulness of oral and topical PLGA-GA2-CUR is safe, effective, and clinically comparable to current routinely used anti-inflammatory medications. This study highlights the potential of PLGA-GA2 nanoparticles for systemic delivery of drugs across the ocular barriers. Further testing is necessary to see whether such delivery strategies are clinically viable by both routes of administration.
MATERIALS AND METHODS
Synthesis and characterization of PLGA-GA2
The polymers used in particle preparation were prepared according to prior optimized protocols in our laboratory (22). In brief, the linker, tris(2-aminoethyl)amine-diBoc (Di-tert-butyl dicarbonate), (TREN-diBoc) is coupled to polymer [polylactide-co-glycolide (PLGA) 50:50, Resomer 503H] using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) coupling method. The deprotection of boc anhydride enables two free amines that were subsequently conjugated with GA via EDC coupling. PLGA-GA2 was characterized using ultraviolet, gel permeation chromatography, NMR, and FTIR (22).
Preparation and characterization of PLGA-GA2-CUR nanoparticles
CUR encapsulation into PLGA-GA2 was achieved by single emulsification process as described previously (22). We have thoroughly optimized the CUR loading and entrapment efficiencies in our laboratory; briefly, 500 mg of PLGA-GA2 and 75 mg of CUR were dissolved in 50 ml of ethyl acetate under stirring. This organic phase is then emulsified into aqueous phase made of 500 mg of polyvinyl alcohol in 50 ml of water under stirring. The emulsion is added to 200 ml of water to facilitate diffusion of organic solvent into aqueous phase that was evaporated overnight. The resultant suspension is centrifuged at 15,000g for 30 min at 4°C. The pellet was suspended in 25 ml of 5% (w/v) sucrose solution and frozen overnight at −80°C. The freeze-drying was carried out using benchtop freeze drier (Labconco FreeZone Triad −85°C Benchtop Freeze Dryers) at −55°C for 54 hours, followed by heating at 20°C for 20 hours under vacuum (0.008 mbar). The freeze product is crimp sealed and stored at 4°C until further use. The freeze-dried formulations were characterized for particle size and entrapment efficiency. The freeze-dried formulations for oral administrations or bacterial cultures were suspended in sterile water, while topical formulations used Dulbecco’s phosphate-buffered saline pH 7.4 sterile. A blood agar plate (BAP), a tergitol plate, and a phenylethyl alcohol plate were used for the aerobic culture, and a single BAP was used for the anaerobic culture. Plates were inoculated for each test sample and streaked for colony isolation. The anaerobic plates were placed in a sealed container along with a gas-generating pack and anaerobic indicator strip and incubated at 36° to 38°C for 48 hours before opening. All aerobic plates were placed in a CO2 (5 to 10%) incubator at 36° to 38°C overnight. Because no growth was observed the next day, aerobic plates were returned to the CO2 incubator for an additional 18 to 24 hours before final results were reported.
Canine participants
Studies were conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (47) and in compliance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research (https://arvo.org/About/policies/statement-for-the-use-of-animals-in-ophthalmic-and-vision-research/). The Texas A&M University Institutional Animal Care and Use Committee (animal use protocol no. 2017-0279) approved the protocols. Twelve healthy purpose-bred juvenile intact male beagles (Ridglan Farms, Mount Horeb, WI) were included in the study and maintained at Texas A&M University for 3 months before adoption. All dogs were 4 to 6 months old throughout the study. Dogs were both single housed and pair housed in runs (2.1 m L by 1.2 m W by 3.0 m H). The facility provided controlled light cycle (12-hour light/12-hour dark), temperature (21° to 22°C), and humidity (55 to 60%) conditions. Water was available at all times, and a maintenance diet was provided twice daily. Environmental enrichment consisted of daily exposure to toys, socialization, leash walks, and exercise in outdoor pens. Dogs received basic behavior training to increase adoptability. Dogs were well accustomed to handling and acclimated to ophthalmic examinations for 2 weeks before the study was initiated. At the completion of the study, all 12 dogs were neutered and adopted to private homes.
Complete ophthalmic examinations using slit-lamp biomicroscopy and indirect ophthalmoscopy were performed on all 12 dogs before the study by a board-certified veterinary ophthalmologist (E.M.S.) to ensure all dogs were free of ocular disease. Measurements of aqueous tear production (Schirmer tear test I, Alcon Laboratories, Ft. Worth, TX) and IOP via rebound tonometry (TonoVet, iCare, Raleigh, NC) were also performed.
Canine lens protein isolation
Homologous lens protein was used to incite uveitis. Lenses were collected from one adult dog, free of ocular disease, immediately following euthanasia for reasons unrelated to the study. Lens protein solution was aseptically prepared as previously described (27, 28) and stored in individual 1.5-ml Eppendorf tubes at −80°C for until use. The soluble protein concentration of the prepared solution was estimated at 10.78 μg/ml using the Bradford assay.
Single-dose preliminary safety assessment of PLGA-GA2-CUR
Four dogs were administered single oral dose of PLGA-GA2-CUR (10 mg/kg), and blood was collected at 24 hours and 7 days after dosing for clinical pathology tests. Detailed safety assessments of oral PLGA-GA2-CUR consisted of adverse event monitoring, vital signs, hematology, and serum biochemistry tests.
Single oral dose target levels: Aqueous humor
Three dogs were administered a single oral dose of PLGA-GA2-CUR (10 mg/kg) within 1 hour of sample collection. Within 1 hour of oral dosing, each dog was administered intravenous maropitant (1 mg/kg) to prevent emesis and then sedated with intravenous butorphanol (0.2 to 0.3 mg/kg) and dexmedetomidine (2 to 4 μg/kg) for aqueous humor sample collection. While sedated, several parameters were monitored and recorded by a board-certified veterinary anesthesiologist (M.A.L.), including electrocardiography, pulse oximetry (SpO2), indirect blood pressure (noninvasive blood pressure) (Datascope Passport 2, Mindray, Mahwah, NJ), pule rate, respiration rate, and temperature. Both eyes were aseptically prepared in a routine manner with dilute Betadine for ophthalmic use, and one drop of proparacaine 0.5% ophthalmic solution was instilled in each eye for topical anesthesia. Once peak sedation was attained, 200 to 300 μl of aqueous humor was collected from each eye via aqueocentesis using 30-gauge needles. Following the procedure, one drop of antibiotic ophthalmic solution (ofloxacin 0.3%) and one drop of corticosteroid ophthalmic suspension (prednisolone acetate 1%) were placed in each eye. Sedation was reversed when indicated with intramuscular atipamezole (10 to 20 μg/kg). Following aqueocentesis, dogs were administered one dose of carprofen (4 mg/kg) subcutaneously and one drop of topical prednisolone acetate 1% ophthalmic suspension in both eyes twice daily for 3 days. Aqueous humor samples were stored in −80°C until further use. CUR quantification in aqueous humor was performed using the liquid chromatography–tandem mass spectrometry (LC-MS/MS) method developed in our laboratory (fig. S4).
Topical ocular irritation study
Before evaluating the topical application of PLGA-GA2-CUR, two dogs were randomly selected and observed for evidence of ocular irritation following topical instillation to the eye. One drop (35 μl) of PLGA-GA2-CUR [~500 μg of CUR equivalent (to be precise 507.5 μg, calculated)] ophthalmic suspension was instilled in each eye hourly for 2 hours. Eyes were then observed for evidence of ocular irritation using the SPOTS system (44). Before the irritation test, the PLGA-GA2-CUR samples were analyzed for microbial burden.
Single topical dose target levels: Aqueous humor
Three dogs were administered one drop of PLGA-GA2-CUR (500 μg of CUR equivalent) ophthalmic suspension in each eye within 30 min of sample collection. The dogs were sedated, and eyes were prepared as mentioned above. Once peak sedation was attained, 20 to 30 μl of aqueous humor was collected from each eye via aqueocentesis using 30-gauge needles. Following aqueocentesis, dogs were administered one dose of carprofen (4 mg/kg) subcutaneously and one drop topical prednisolone acetate 1% ophthalmic suspension in both eyes twice daily for 3 days. Aqueous humor samples were stored in −80°C until further use.
Oral efficacy of PLGA-GA2-CUR
Twelve dogs were randomly assigned to three treatment groups. Four dogs served as untreated negative controls (group 1), four dogs were administered a commercially available NSAID (carprofen 2.2 mg/kg) by mouth every 12 hours for 3 days (group 2), and four dogs were administered PLGA-GA2-CUR (10 mg/kg) by mouth every 12 hours for 3 days (group 3). The investigator who performed ophthalmic examinations and measured inflammatory responses (E.M.S.) was masked to the treatment groups until the conclusion of the study.
Within 1 hour of oral dosing, each dog was administered intravenous maropitant (1 mg/kg) to prevent emesis and then sedated as mentioned above. An intravenous catheter was placed, and a systemic antibiotic (intravenous cefazolin 22 mg/kg) was administered once. Once peak sedation was attained, both eyes were aseptically prepared in a routine manner with dilute Betadine for ophthalmic use. One drop of antibiotic ophthalmic solution (ofloxacin 0.3%) was placed in each eye, followed by one drop of proparacaine 0.5% ophthalmic solution for topical anesthesia. The prepared canine lens protein solution was thawed, and 50 μl (539.32 μg) was injected intracamerally in both eyes of 12 dogs (24 eyes) using 30-gauge needles. Sedation was reversed when indicated with intramuscular atipamezole (10 to 20 μg/kg).
The intraocular inflammatory response of each eye was assessed using an ocular scoring system optimized for use in modern preclinical drug development and toxicology (SPOTS system) (44), along with tonometry. Scores were obtained and recorded at 1, 2, 3, 4, 5, 6, 12, 24, and 48 hours after injection. Additional safety measures consisted of comfort level, which was assessed throughout the study by the severity of blepharospasm and attempts to rub the eyes.
Topical efficacy of PLGA-GA2-CUR
After a 1-week washout following the oral efficacy study, a second intracameral injection of canine lens protein was performed in eight dogs (16 eyes) using the same sedation and procedural protocols and ocular preparation described above. Eight dogs were randomly assigned to two treatment groups. Four dogs were administered one drop (35 μl containing 500 μg CUR) of PLGA-GA2-CUR ophthalmic suspension in both eyes four times daily for 3 days (group 4), and four dogs were administered one drop (50 μl containing 500 μg) of prednisolone acetate 1% ophthalmic suspension in both eyes four times daily for 3 days (group 5). The intraocular inflammatory response was assessed for each eye using the SPOTS system and tonometry measurements at 1, 2, 3, 4, 5, 6, 12, and 24 hours after injection. Additional safety measures consisted of comfort level, which was assessed throughout the study by the severity of blepharospasm and attempts to rub the eyes. The investigator who performed ophthalmic examinations and measured inflammatory responses (E.M.S.) was masked to the treatment groups until the conclusion of the study.
Statistics
Statistical analyses presented in Figs. 2 and 3 and figs. S9 and S10 were performed using GraphPad Prism 7.02 software. Data were presented as the means with SE. To compare the difference in the PLGA-GA2-CUR and untreated groups and/or carprofen/prednisolone acetate, we used two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. A P value of less than 0.05 (typically ≤0.05) is considered statistically significant. Data are representative of at least eight eyes from four beagles from each group.
A detailed statistical analysis was performed to identify the reduction speed of inflammation in the treatment groups, compared with untreated or within the groups. For this analysis, we focused on one of the three measurement scores for inflammation, AC flare score (presented in Figs. 2A and 3A). All response scores are originally recorded discretely into five categories based on the number of anterior vitreous cells labeled as 0, 0.5, 1, 2, and 3. Considering that any AC flare score that is 1 or less can be seen as mild inflammation, for simplicity, we aggregate the responses into two categories. For AC flare score less than or equal to 1, the response is relabeled as 0, and for AC flare score greater than 1, the response is relabeled as 1. This enables us to simplify the statistical model to logistic regressions for binary responses. In doing so, the explanatory value is time, which is treated as a continuous predictor. The regrouped response values are binary. We use logistic regression to model the relationship between time and AC flare scores.
Let y be the regrouped response variable and t be the predictor. We denote the probability that the regrouped response is 1 at time t as π(t), i.e., P(y = 1) = π(t). Define the logarithm of the odds (log odds, hereafter) as
In logistic regressions, the log odds is directly modeled by a linear regression of time t
| (1) |
The slope β1 can be interpreted as proportional to the reduction speed of inflammation for the treatment. For the comparison between the control/untreated group and the treatment group (or between two treatment groups), additional treatment term and its interaction with time are added to the right-hand side of (1)
| (2) |
where S = 0 for the control group and S = 1 for the treatment group. To test the significance of the treatment effect over time is equivalent to test whether the coefficient of the interaction term t · S is zero, i.e., test . If there is no statistical evidence that the drug effect over time changes with the treatment, we further test whether there are constant treatment effects independent of time using the following model
| (3) |
which is the same as testing null hypothesis . For computation, we use the glm function in the R software (version 3.6.1) for logistic regressions.
Supplementary Material
Acknowledgments
We acknowledge G. Kaur for assistance with initial polymer preparation; J. Presby, B. Ridenhour, and K. Chapman for assistance with animal handling, care, training, and adoption; W. Serem, TAMU Materials Characterization Facility, for assistance with AFM imaging, and TAMU Integrated Metabolomics Analysis Core for assistance with LC-MS. Funding: The NIH (grant no. R01EY028169) supported this research. This research is also supported in part by R01CA194391 and NSF grant CCF-1934904. Author contributions: R.G., M.A., M.A.L., and E.M.S. planned the projects, performed the experiments, conducted data analysis, and edited the manuscript. E.M.S. and M.N.V.R.K. prepared and edited the manuscript. Y.N. and B.K.M. performed statistical analysis and edited the manuscript. S.C.P. edited the manuscript. M.N.V.R.K. conceived and planned the projects. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/35/eabb7878/DC1
REFERENCES AND NOTES
- 1.Tsirouki T., Dastiridou A., Symeonidis C., Tounakaki O., Brazitikou I., Kalogeropoulos C., Androudi S., A focus on the epidemiology of uveitis. Ocul. Immunol. Inflamm. 26, 2–16 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Agrawal R. V., Murthy S., Sangwan V., Biswas J., Current approach in diagnosis and management of anterior uveitis. Indian J. Ophthalmol. 58, 11–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan F., Luo Y., Lu Y., Liu X., Reasons for early ocular hypertension after uneventful cataract surgery. Eur. J. Ophthalmol. 24, 712–717 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Sharma T., Fong A., Lai T. Y., Lee V., Das S., Lam D., Surgical treatment for diabetic vitreoretinal diseases: A review. Clin. Experiment. Ophthalmol. 44, 340–354 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Ilhan O., Ilhan N., Coşkun M., Dağlioğlu M. C., Tuzcu E. A., Ayintap E., Keskın U., Oksüz H., The effect of enoxaparin-containing irrigation fluid used during cataract surgery on postoperative inflammation in patients with diabetes. Am. J. Ophthalmol. 156, 1120–1124.e3 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Pleyer U., Ursell P. G., Rama P., Intraocular pressure effects of common topical steroids for post-cataract inflammation: Are they all the same? Ophthalmol Ther 2, 55–72 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaynes B. I., Fiscella R., Topical nonsteroidal anti-inflammatory drugs for ophthalmic use: A safety review. Drug Saf. 25, 233–250 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Overby D. R., Bertrand J., Tektas O.-Y., Boussommier-Calleja A., Schicht M., Ethier C. R., Woodward D. F., Stamer W. D., Lütjen-Drecoll E., Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Invest. Ophthalmol. Vis. Sci. 55, 4922–4933 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennessy A. L., Katz J., Covert D., Kelly C. A., Suan E. P., Speicher M. A., Sund N. J., Robin A. L., A video study of drop instillation in both glaucoma and retina patients with visual impairment. Am. J. Ophthalmol. 152, 982–988 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Stone J. L., Robin A. L., Novack G. D., Covert D. W., Cagle G. D., An objective evaluation of eyedrop instillation in patients with glaucoma. Arch. Ophthalmol. 127, 732–736 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Fassbender Adeniran J. M., Jusufbegovic D., Schaal S., Common and rare ocular side-effects of the dexamethasone implant. Ocul. Immunol. Inflamm. 25, 834–840 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Bernal-Mizrachi C., Weng S., Feng C., Finck B. N., Knutsen R. H., Leone T. C., Coleman T., Mecham R. P., Kelly D. P., Semenkovich C. F., Dexamethasone induction of hypertension and diabetes is PPAR-α dependent in LDL receptor-null mice. Nat. Med. 9, 1069–1075 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Bahar I., Rosenblat I., Erenberg M., Eldar I., Gaton D., Avisar R., Weinberger D., Effect of dexamethasone eyedrops on blood glucose profile. Curr. Eye Res. 32, 739–742 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Zhou P.-Z., Zhu Y.-M., Zou G.-H., Sun Y.-X., Xiu X.-L., Huang X., Zhang Q.-H., Relationship between glucocorticoids and insulin resistance in healthy individuals. Med. Sci. Monit. 22, 1887–1894 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain S. K., Rains J., Jones K., Effect of curcumin on protein glycosylation, lipid peroxidation, and oxygen radical generation in human red blood cells exposed to high glucose levels. Free Radic. Biol. Med. 41, 92–96 (2006). [DOI] [PubMed] [Google Scholar]
- 16.du Preez R., Pahl J., Arora M., Kumar M. N. V. R., Brown L., Panchal S. K., Low-dose curcumin nanoparticles normalise blood pressure in male wistar rats with diet-induced metabolic syndrome. Nutrients 11, 1542 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar P. A., Suryanarayana P., Reddy P. Y., Reddy G. B., Modulation of α-crystallin chaperone activity in diabetic rat lens by curcumin. Mol. Vis. 11, 561–568 (2005). [PubMed] [Google Scholar]
- 18.Tang C., Koulajian K., Schuiki I., Zhang L., Desai T., Ivovic A., Wang P., Robson-Doucette C., Wheeler M. B., Minassian B., Volchuk A., Giacca A., Glucose-induced beta cell dysfunction in vivo in rats: Link between oxidative stress and endoplasmic reticulum stress. Diabetologia 55, 1366–1379 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Ganugula R., Arora M., Jaisamut P., Wiwattanapatapee R., Jørgensen H. G., Venkatpurwar V. P., Zhou B., Hoffmann A. R., Basu R., Guo S., Majeti N. V. R. K., Nano-curcumin safely prevents streptozotocin-induced inflammation and apoptosis in pancreatic beta cells for effective management of type 1 diabetes mellitus. Br. J. Pharmacol. 174, 2074–2084 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saini P., Ganugula R., Arora M., Kumar M. N. V. R., The next generation non-competitive active polyester nanosystems for transferrin receptor-mediated peroral transport utilizing gambogic acid as a ligand. Sci. Rep. 6, 29501 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora M., Ganugula R., Kumar N., Kaur G., Pellois J.-P., Garg P., Kumar M. N. V. R., Next-generation noncompetitive nanosystems based on gambogic acid: In silico identification of transferrin receptor binding sites, regulatory shelf stability, and their preliminary safety in healthy rodents. ACS Appl. Bio. Mater. 2, 3540–3550 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur G., Arora M., Ganugula R., Kumar M. N. V. R., Double-headed nanosystems for oral drug delivery. Chem. Commun. (Camb.) 55, 4761–4764 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabs D. A., Rosenbaum J. T., Foster C. S., Holland G. N., Jaffe G. J., Louie J. S., Nussenblatt R. B., Stiehm E. R., Tessler H., Van Gelder R. N., Whitcup S. M., Yocum D., Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. Am. J. Ophthalmol. 130, 492–513 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Yefimova M. G., Jeanny J. C., Guillonneau X., Keller N., Nguyen-Legros J., Sergeant C., Guillou F., Courtois Y., Iron, ferritin, transferrin, and transferrin receptor in the adult rat retina. Invest. Ophthalmol. Vis. Sci. 41, 2343–2351 (2000). [PubMed] [Google Scholar]
- 25.Ganugula R., Arora M., Kumar M. N. V. R., Ex vivo rat eye model for investigating transport of next generation precision-polyester nanosystems. ACS Appl. Mater. Interfaces 9, 25668–25671 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Kompella U. B., Sundaram S., Raghava S., Escobar E. R., Luteinizing hormone-releasing hormone agonist and transferrin functionalizations enhance nanoparticle delivery in a novel bovine ex vivo eye model. Mol. Vis. 12, 1185–1198 (2006). [PubMed] [Google Scholar]
- 27.Miyano K., Chiou G. C., Pharmacological prevention of ocular inflammation induced by lens proteins. Ophthalmic Res. 16, 256–263 (1984). [DOI] [PubMed] [Google Scholar]
- 28.Dziezyc J., Millichamp N. J., Rohde B. H., Baker J. S., Chiou G. C., Effects of lipoxygenase inhibitors in a model of lens-induced uveitis in dogs. Am. J. Vet. Res. 50, 1877–1882 (1989). [PubMed] [Google Scholar]
- 29.Chang M. S., Chiou G. C., Prevention of lens protein-induced ocular inflammation with cyclooxygenase and lipoxygenase inhibitors. J. Ocul. Pharmacol. 5, 353–360 (1989). [DOI] [PubMed] [Google Scholar]
- 30.Xiao J., Wang Y., Wu S., Li J., Zhang S., Inhibitory effect of tetrandrine on lens proteins-induced ocular inflammation in rabbits. J. Ocul. Pharmacol. 8, 309–315 (1992). [DOI] [PubMed] [Google Scholar]
- 31.Chiou G. C., Yao Q. S., Chang M. S., Okawara T., Prevention and treatment of ocular inflammation with a new class of nonsteroidal anti-inflammatory agents. J. Ocul. Pharmacol. 10, 335–347 (1994). [DOI] [PubMed] [Google Scholar]
- 32.Chiou G. C., Yao Q. S., Okawara T., Prevention of ocular inflammation induced by lens protein, endotoxin, and interleukin-1 with synthetic interleukin-1 blockers. J. Ocul. Pharmacol. 10, 577–586 (1994). [DOI] [PubMed] [Google Scholar]
- 33.Simone J. N., Whitacre M. M., Effects of anti-inflammatory drugs following cataract extraction. Curr. Opin. Ophthalmol. 12, 63–67 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Kessel L., Tendal B., Jørgensen K. J., Erngaard D., Flesner P., Andresen J. L., Hjortdal J., Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: A systematic review. Ophthalmology 121, 1915–1924 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Sigle K. J., Nasisse M. P., Long-term complications after phacoemulsification for cataract removal in dogs: 172 cases (1995-2002). J. Am. Vet. Med. Assoc. 228, 74–79 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Klein H. E., Krohne S. G., Moore G. E., Stiles J., Postoperative complications and visual outcomes of phacoemulsification in 103 dogs (179 eyes): 2006–2008. Vet. Ophthalmol. 14, 114–120 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Gilmour M. A., Lehenbauer T. W., Comparison of tepoxalin, carprofen, and meloxicam for reducing intraocular inflammation in dogs. Am. J. Vet. Res. 70, 902–907 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Gilmour M. A., Payton M. E., Comparison of the effects of IV administration of meloxicam, carprofen, and flunixin meglumine on prostaglandin E2 concentration in aqueous humor of dogs with aqueocentesis-induced anterior uveitis. Am. J. Vet. Res. 73, 698–703 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Ward D. A., Cawrse M. A., Hendrix D. V., Fluorophotometric determination of aqueous humor flow rate in clinically normal dogs. Am. J. Vet. Res. 62, 853–858 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Brookshire H. L., English R. V., Nadelstein B., Weigt A. K., Gift B. W., Gilger B. C., Efficacy of COX-2 inhibitors in controlling inflammation and capsular opacification after phacoemulsification cataract removal. Vet. Ophthalmol. 18, 175–185 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Luna S. P. L., Basílio A. C., Steagall P. V. M., Machado L. P., Moutinho F. Q., Takahira R. K., Brandão C. V. S., Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am. J. Vet. Res. 68, 258–264 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Holmberg B. J., Maggs D. J., The use of corticosteroids to treat ocular inflammation. Vet. Clin. North Am. Small Anim. Pract. 34, 693–705 (2004). [DOI] [PubMed] [Google Scholar]
- 43.McLean N. J., Ward D. A., Hendrix D. V. H., Vaughn R. K., Effects of one-week versus one-day preoperative treatment with topical 1% prednisolone acetate in dogs undergoing phacoemulsification. J. Am. Vet. Med. Assoc. 240, 563–569 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Eaton J. S., Miller P. E., Bentley E., Thomasy S. M., Murphy C. J., The SPOTS System: An ocular scoring system optimized for use in modern preclinical drug development and toxicology. J. Ocul. Pharmacol. Ther. 33, 718–734 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Tugal-Tutkun I., Herbort C. P., Laser flare photometry: A noninvasive, objective, and quantitative method to measure intraocular inflammation. Int. Ophthalmol. 30, 453–464 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Bantseev V., Miller P. E., Bentley E., Schuetz C., Streit T. M., Christian B. J., Farman C., Booler H., Thackaberry E. A., Determination of a no-observable effect level for endotoxin following a single intravitreal administration to dutch belted rabbits. Invest. Ophthalmol. Vis. Sci. 58, 1545–1552 (2017). [DOI] [PubMed] [Google Scholar]
- 47.National Research Council, in Guide for the Care and Use of Laboratory Animals (Washington DC, ed. 8, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/35/eabb7878/DC1


