Abstract
With the advantages of completely controlling the phase, amplitude, and polarization in subwavelength range, metalenses have drawn intensive attentions in high resolution two-photon micro-endoscopic fluorescence imaging system. However, chromatic dispersion and severe scattering of biological tissue significantly reduce excitation-collection efficiency in the traditional two-photon imaging system based on traditional metalenses designed in the air background. Here, an excitation and emission dual-wavelength confocal and polarization-insensitive metalens designed in the biological tissue environment was proposed by adopting the composite embedding structure and spatial multiplexing approach. The metalens with numerical aperture (NA) of 0.895 can focus the excitation (915 nm) and emission (510 nm) beams to the same focal spot in the mouse cortex. According to the theoretical simulation of two-photon fluorescence imaging, the lateral resolution of the collected fluorescent spots via the proposed metalens can be up to 0.42 µm. Compared to the metalens designed in the air environment, the collection efficiency of fluorescent spot is improved from 5.92% to 14.60%. Our investigation has opened a new window of high resolution and minimally invasive imaging in deep regions of biological tissues.
1. Introduction
Two-photon fluorescence imaging has attracted much attention and become a powerful tool for deep brain imaging. Advanced research works have concentrated on the studying of deep penetration and high resolution recently, which play the key role on two-photon microscopy [1,2]. Due to the scattering in highly turbid biological tissues, particularly neurons in the cerebral cortex, the imaging depth of conventional two-photon fluorescence microscopy is limited to 1-2 millimeters [3,4]. Numerous studies on micro-endoscopy have been carried out both theoretically and experimentally, which are commonly based either on gradient refractive index microlens or fiber bundles [5–7]. However, the millimeter scale leads to significant tissue damage and displacement. Owing to tens to hundreds of micrometers size, multimode optical fiber (MMF) has become an alternative solution in minimally invasive deep tissue imaging. Ohayon et al., [8] presented a lensless micro-endoscope based on MMF, where randomized phase and mode-mixing were compensated by wavefront shaping. Nevertheless, the repeated calibration of optical fiber transmission matrix in the system induced complex design and time-consuming experimental procedure [9]. Therefore, the microlens on the end face of the optical fiber probe is an ideal candidate.
Dielectric metalenses, a kind of metasurfaces pave a new avenue to replace the traditional, bulk optical components due to their unprecedented superiority in wavefront engineering, lightweight and ultra-compact features. Composed of arrays of subwavelength scatters, metasurfaces are capable of imparting abrupt phase shifts on incident wave. So devices such as planar lenses [10–12], structured beam generator [13], and quarter-wave-plate [14] have been realized. Among them, the transmissive and achromatic metalenses have potential applications in minimally invasive high-resolution imaging deep within tissue. In this context, Arbabi et al., demonstrated a dual-wavelength confocal metalens for two-photon fluorescence imaging theoretically and experimentally [12]. However, the collection efficiency of the polarization-sensitive metalens is influenced by the polarization of light. Furthermore, the focal length would be changed because of the different refractive index and severe scattering of biological tissue as the metalens being applied to the biological tissues and the collection efficiency decreases. To the best of our knowledge, there is no report of the excitation and emission dual-wavelength confocal and polarization-independent metalenses designed directly in the biological tissue environment for two-photon micro-endoscopic fluorescence imaging system.
In this paper, a polarization-insensitive and dual-wavelength confocal metalens was designed directly in biological tissue environment to improve the collection efficiency of two-photon fluorescence micro-endoscopy in minimally-invasive deep tissue imaging. The lens with a diameter of 100 µm and NA of 0.895 in the cortical model of mouse brain shares the same focal length (f ∼ 25 µm) at the excitation wavelength λ1 = 915 nm and emission wavelength λ2 = 510 nm, which were chosen according to the two-photon excitation and emission spectra of enhanced green fluorescent protein (eGFP) [15]. According to two-photon fluorescence signal distribution at the focal plane, the corresponding lateral resolution of the two-photon fluorescence signal is up to 0.42 µm. Moreover, the collection efficiency of fluorescent spot can be improved to 14.60%, whereas it is only 5.92% using the metalens designed in the air environment.
2. Structure and designs
2.1. Necessity of designing dual-wavelength confocal metalens in brain tissue
Due to the strong chromatic dispersion, the metalenses are usually designed to operate optimally at a single wavelength. According to the reported study [16,17], the focal length is inversely proportional to the incident wavelength λ. Therefore, the focal positions at the excitation and emission wavelengths can be far apart in two-photon fluorescence microscopy, which will result in a portion of fluorescence photons not being collected. So the fluorescence collection efficiency is reduced.
Moreover, early works on metalens are typically designed in the air environment. When they are used to study subsurface features in the intact tissues and organs, the change of focal length with the refractive index and the severe scattering of biological tissue cannot be negligible. Our study showed that the simulated focal length is 44.48 µm when the metalens designed in the air environment was used to the biological tissue with refractive index of 1.35, which is rather far apart from originally designed focal length (25 µm). Moreover, it is observed in the simulation that focusing ability was greatly influenced by the biological tissue cells (see Section 3.4). Therefore, it is necessary to design an excitation and emission dual-wavelength confocal and polarization-independent metalenses under the biological tissue environment in two-photon micro-endoscopic fluorescence imaging system.
2.2. Cortical model of mouse brain
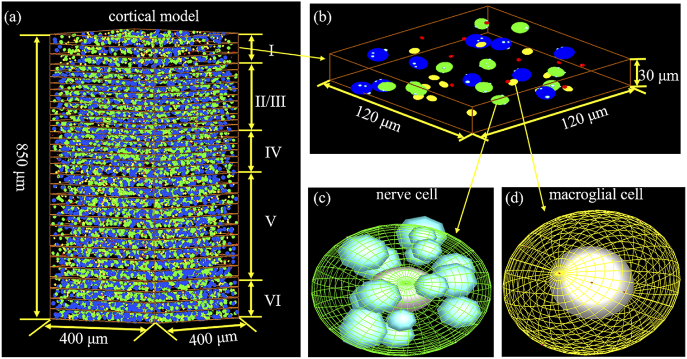
Based on the discussion above, a 3D cortical model of mouse brain (see Fig. 1(a)) was established firstly by the finite difference time domain (FDTD) method. According to the difference of cell density, the cortical model is divided into six layers in the vertical direction and composed of the nerve cells and glial cells (simply represented by microglia and macroglia) [18,19]. The number of neurons per cubic millimeter volume from the first to the sixth layers was about 0.5×105 (I), 1.4×105 (II/III), 1.7×105 (IV), 1.0×105 (V) and 1.5×105 (VI), while the number of glial cells was 0.6×105 (I), 0.5×105 (II/III), 0.6×105 (IV), 0.7×105 (V), and 0.6×105 (VI), respectively. The model of cortical layer I with the least number of cells was selected to show the structure of the mouse cortical model in detail, as shown in Fig. 1(b). Green and blue ellipsoids represent two different sizes of nerve cells, while yellow and red ellipsoids represent macroglial cells and microglial cells, respectively. For the green (blue) ellipsoid, the major radius was chosen to be 5 µm (6 µm), and the minor radius was set to 4 µm (5 µm). Meanwhile, the major and minor radii of macroglial (microglia) cell are 3 µm (1.3 µm) and 2 µm (0.9 µm), respectively. To eliminate periodicity, the location and orientation of all cells are randomly generated by random number seeds. The organelles size was randomly assigned in the range of 0.5-1.5 µm. Figure 1(c) shows the geometry of the nerve cell in detail, which is composed of homogeneous nuclei and randomly distributed organelles. Different from nerve cells, glial cells contain only homogeneous nuclei, as shown in Fig. 1(d). According to reported measurements, the refractive index values used for the organelles, cytoplasm and nuclei were n = 1.39 (1.395), n = 1.36 (1.365), and n = 1.42 (1.425), at λ1 = 915 nm (λ2 = 510 nm), respectively [20–23].
Fig. 1.
(a) Schematic of the designed cortical model with a volume of 400 µm × 400 µm × 850 µm. (b) An illustration of cortical model (layer I) used in simulation. (c) and (d) The geometry of a nerve cell and macroglial cell. The major and minor radii of the two types of nucleus are 2 µm (2.5 µm) and 1.5 µm (2 µm), respectively. The major and minor radii of nucleus of macroglia (microglia) are 1.5 µm (0.6 µm) and 1.2 µm (0.4 µm), respectively.
2.3. Design of the two-photon fluorescence system based on the metalens
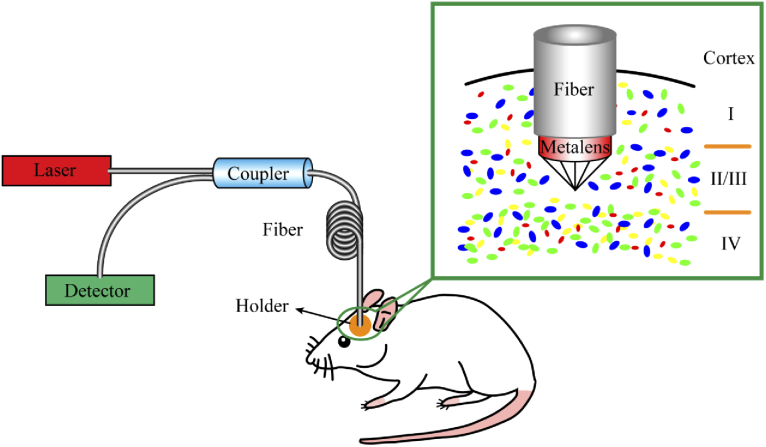
A schematic of the fiber-optical two-photon fluorescence micro-endoscopic imaging system based on an excitation and emission dual-wavelength confocal metalens is depicted in Fig. 2. It is composed of a laser source, optical fibers, an optical fiber coupler, a detector, and an ultra-compact focusing unit. An ultra-short pulsed laser beam at a central wavelength of 915 nm with pulse width τ = 100 fs and repetition rate fr = 80 MHz generated by a laser system is coupled into an optical fiber, which acts as the light delivery section. Single-mode or multi-mode fibers are widely used for delivering excitation laser pulse and fluorescence signals. The fiber coupler plays an important role in the separation of the infrared excitation beam from the fluorescence signal. An excitation and emission dual-wavelength confocal metalens fabricated on the end face of the fiber, which can be directly implanted into the mouse cortex due to the ultra-thin and lightweight features. The metalens implemented as the focusing and collecting unit. To fix the implant unit, a holder was installed on the surface of the mouse skull. Two-photon fluorescence signal excited at the focal spot was collected by the same fiber, and transported back to the detector.
Fig. 2.
Schematic of the fiber-optical two-photon fluorescence micro-endoscopic imaging system based on an excitation and emission dual-wavelength confocal metalens in mouse cortex. The inset illustrates the details of the focusing unit.
2.4. Design of the dual-wavelength confocal lens in mouse brain environment
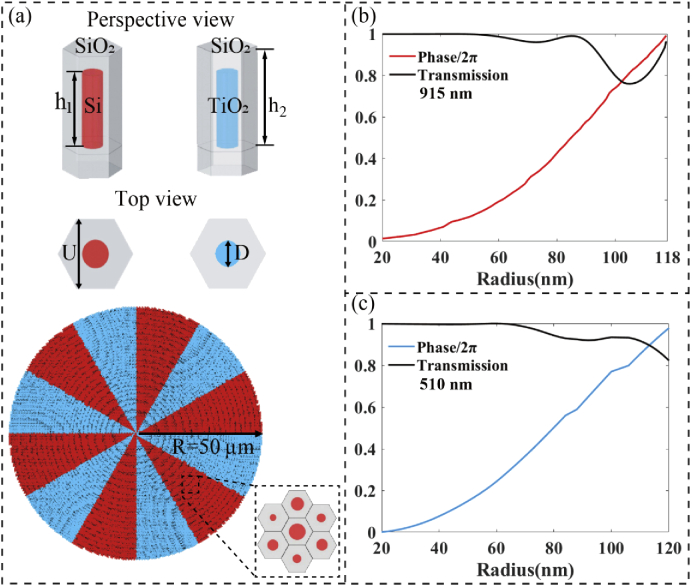
Based on the environment of mouse cortex, a dual-wavelength confocal metalens (Fig. 3(a)) with a diameter of 100 µm, and a focal length of 25 µm was designed by FDTD method. According to the requirements of high refractive index and low loss at the two operation wavelengths, silicon (Si) is chosen to manipulate the wavefront at 915 nm and titanium dioxide (TiO2) is suitable for 510 nm. Then Si and TiO2 nanoposts with circular cross section were arranged in hexagonal lattice, as shown in the top of Fig. 3(a). To function like a spherical lens in the brain tissue background, the necessary phase profile φ(x, y, λ) of the metalens should be governed by:
| (1) |
where λm is the operating wavelengths (i.e., the excitation wavelength λ1 = 915 nm and emission wavelength λ2 = 510 nm), f is the designed focal length, and nm is the refractive index of intercelluar substance related to the wavelength. The refractive indices are n1 = 1.35 at wavelength λ1 = 915 nm and n2 = 1.355 at wavelength λ2 = 510 nm, respectively. The required phase profile is imparted by varying the nanoposts diameter. To achieve full 2π phase coverage with high transmission, the nanoposts height is h1 = 0.6 µm and the lattice constant is U = 0.25 µm at both wavelengths. Simulated phase shifts and transmission as functions of post radii at wavelengths λ1 = 915 nm and λ2 = 510 nm are shown in Figs. 3(b) and 3(c), respectively. It can be observed that full 2π phase coverage with high transmission at both wavelengths were obtained.
Fig. 3.
(a) A schematic illustration of the dual-wavelength confocal metalens. Perspective and top views of the Si and TiO2 nanoposts arranged in a hexagonal lattice are depicted in the top of (a). The height of Si (TiO2) nanoposts is h1 = 0.6 µm and the lattice period of Si (TiO2) nanopost is U = 0.25 µm. (b) and (c) The simulated phase shifts and transmission for the nanoposts with different radii at wavelengths 915 nm and 510 nm, respectively.
The dual-wavelength confocal metalens comprised of 12 radial parts with equal area was designed with the area-division method, as shown in Fig. 3(a). The metalens is divided into red and blue regions, which respectively focus the wavelength of 915 nm and 510 nm to the same focal position. Unlike previous nanopost-substrate structures (the dielectric nano-posts are isolated and direct contact with the air, without anything covered around them), the Si (TiO2) nanopost is covered by a SiO2 capping layer with height h2 = 0.8 µm to isolate it from the external environment. The purpose of the encapsulating structure is to improve the mechanical robustness of the metalens and protect the nanopost. Due to the central symmetry of the structure, the designed metalens is polarization insensitive and will be more favorable for imaging application.
We proposed the fabrication processes in the future experimental project of the multi-sector metalenses samples. Firstly, the six-sector SiO2 sheet spaced 30° apart is processed to limit the deposition area and adjacent sectors, which is thicker than the Si layer to ensure sufficient thickness of silicon deposition. The Si layer is deposited on a fused silica substrate using the plasma enhanced chemical vapor deposition (PECVD) technique. Secondly, the SiO2 sheet is removed that used in first step. Another same SiO2 sheet is placed on the Si layer to protect silicon deposition. The TiO2 layer is deposited by atomic layer deposition (ALD) with same thickness as Si layer in different sectors. Then, the e-beam resist was spin coated and the design pattern is exposed in an electron beam lithography system. Finally, this multisector pattern is covered with silica grown by ALD.
3. Results and discussion
3.1. Focusing performance of dual-wavelength confocal metalens
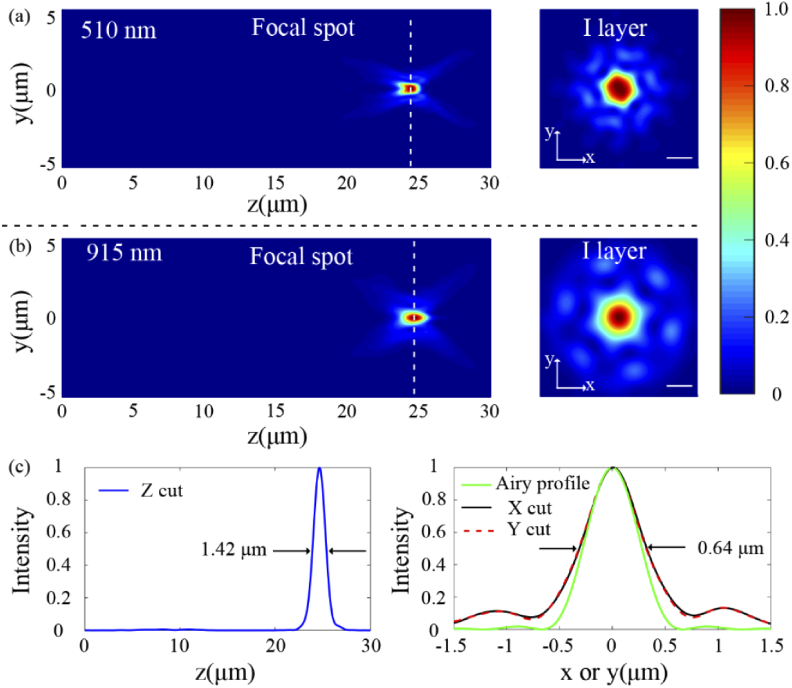
Radially polarized Bessel-Gauss beam was chosen as the incident light source due to the non-diffractive and tight focusing characteristics [24–28]. Figures 4(a) and 4(b) show the simulated electric field intensity distributions at the focal spot in the cortical layer I at 510 nm and 915 nm, respectively. It is obvious that both the excitation (915 nm) emission (510 nm) beams are focused at nearly the same position. It is worth noting that there is no second focal spot before the main focal spot in the axial plane at both wavelengths, which is mainly due to small lattice constant, optically small nanoposts, and the high contrast in the effective refractive index of Si and TiO2 nanoposts. So the cross-talk in nanoposts between 915 nm and 510 nm is rarely weak [29]. Therefore, it has little effect on two-photon imaging. In focal plane, residual unwanted light spots regularly distributed near focal spot are observed, which are induced by the area-division method. Table 1 shows the focal length in different layers of mouse cortex, verifying the effectiveness of the dual-wavelength confocal approach in mouse cortex. Figure 4(c) shows the intensity distribution along the axial and lateral directions through the center of the focal spot at 915 nm. The simulated full width at half-maximum (FWHM) along z axis is FWHMz = 1.42 µm, while the lateral resolutions along x and y axis both are 0.64 µm, which reveals the symmetry of the focal spot. In addition, to investigate the ability of diffraction-limited focusing at the excitation wavelength of 915 nm, Airy profile is also depicted in Fig. 4(c). Obviously, the simulated intensity profile along lateral direction of the focal spot is in agreement with the theoretical Airy profile. Calculated from 0.514λ/NA, the theoretical diffraction-limited FWHM of Airy spot should be 0.53 µm. The slight difference of FWHM is attributed to the scattering of biological tissue and the grid spacing used in our simulation.
Fig. 4.
(a) and (b) The normalized electric field intensity distribution in the axial (left) and focal (right) planes at emission wavelength of 510 nm with a focal length of 24.40 µm and excitation wavelength of 915 nm with a focal length of 24.67 µm, respectively. Scale bar: 0.5 µm. (c) The electric field intensity profile along the axial (left) and lateral (right) directions through the center of the focal spot at 915 nm. The green curve: intensity profile of the ideal Airy spot.
Table 1. The focal length in different layers of mouse cortex (Unit: µm).
| Layer | I | II/III | IV | V | VI |
|---|---|---|---|---|---|
| f1 (excitation wavelength) | 24.67 | 24.61 | 24.61 | 24.56 | 24.73 |
| f2 (emission wavelength) | 24.40 | 24.27 | 24.33 | 24.40 | 24.43 |
The focusing efficiency, defined as the ratio of the power passing through a 5-µm diameter circular aperture at the focal plane to the total power of incident light [12], was simulated to be 31.24% at the excitation wavelength of 915 nm. It is slightly lower than the reported result in Ref. [29], where a focusing efficiency of 40.60% was achieved at 915 nm by the dual-wavelength confocal metalens with NA=0.46 designed with area-division method. The higher NA is the main reason for the lower focusing efficiency of metalens proposed in this work because the focusing efficiency of metalens decreases with increasing NA [30]. Besides, the mouse cortex is a turbid imaging environment, more tissue cells will bring strong scattering, and the scattering in biological tissue will further reduces the focusing efficiency. Owing to the slightly low focusing efficiency at excitation wavelength λ1 = 915 nm, optimization of an incident power is required for not only ensuring excitation process but also avoiding photobleaching and photodamage of fluorescent protein and biological tissue. Furthermore, the low focusing efficiency could worsen fluorescence collection efficiency. However, according to the theory of fluorescence collection in Section 3.3 (see Eq. (3)), a lens with a higher NA has a higher fluorescence collection efficiency, which can compensate for the decrease in collection efficiency caused by the focusing efficiency to some extent.
3.2. Two-photon fluorescence excitation
Utilizing two-photon fluorescence micro-endoscopic imaging system based on the proposed dual-wavelength confocal metalens to observe the mouse cortex, the focus spot with a peak intensity of 5.15 MW/cm2 was obtained under the illumination of radially polarized Bessel-Gauss beam, which is in the intensity range required for two-photon fluorescence excitation process. Subsequently, the total number of photons [Nemit(t)] emitted by per fluorescent molecule per unit time can be expressed as [31]:
| (2) |
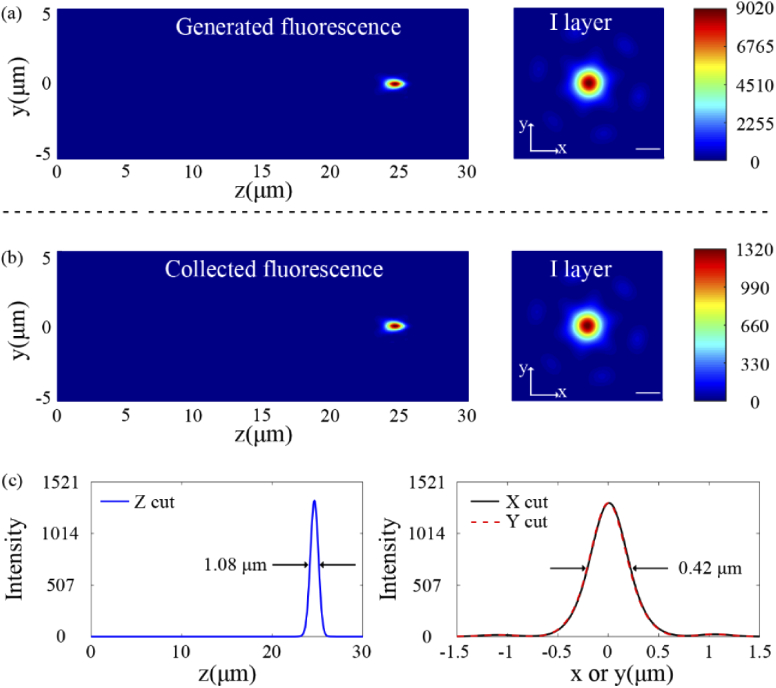
where σn is the two-photon excitation cross section, gp is a dimensionless factor depending on the pulse shape (gp = 0.66 for a Gaussian temporal profile pulse), <I(t)> is the time-averaged intensity of the excitation source, C is the fluorophore concentration, and V is the illuminated sample volume. The relevant parameters used in the simulation were as follows: C = 500 µmol, σn = 41.21 GM (1GM=10−50 cm4 s/photon). In addition, the excitation threshold value was set to 10 GW/cm2 (∼1028 photons/cm2s). Then the intensity distribution of generated fluorescent spot was calculated and depicted in Fig. 5(a).
Fig. 5.
The generated (a) and collected (b) fluorescence intensity distribution of the proposed dual-wavelength confocal metalens in the axial (left) and focal (right) planes. Scale bar: 0.5 µm. (c) The collected fluorescence intensity profile along the axial (left) and lateral (right) directions through the center of fluorescence spot.
3.3. Fluorescence collection
Based on the previous studies, the fluorescence collection efficiency Φ especially in turbid medium can be expressed as [32,33]:
| (3) |
Where θNA is the numerical-aperture angle, θf denotes the angular-acceptance angle for backward detection (θf = 0.17 in this paper) [33], σ is defined by and ns is given by . The scattering mean free path ls of 50 µm and the scattering anisotropy g of 0.9 are representative for wavelengths near 500 nm in young-rat brain [33,34]. The collected fluorescence intensity distribution is shown in Fig. 5(b). The calculated FWHMz, FWHMx, and FWHMy of the fluorescence intensity profile were reduced to 1.08, 0.42 and 0.42 µm respectively, as shown in Fig. 5(c). According to Eq. (3), the collection efficiency of the proposed metalens is 14.60%.
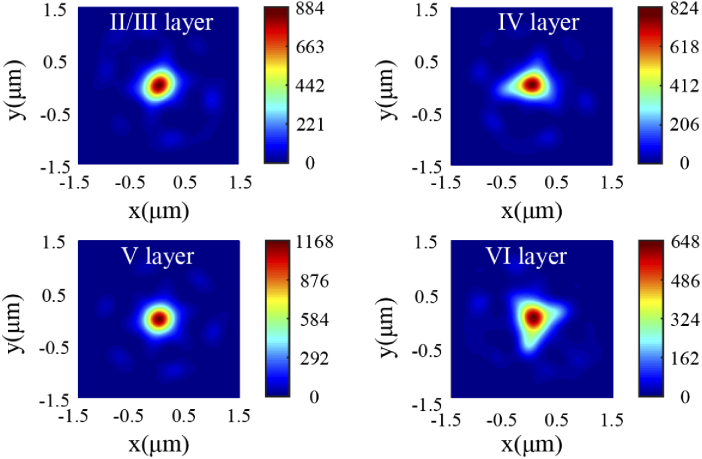
In order to investigate the influence of scattering cells in different layers of the mouse cortex on focal spot, the intensity distributions of collected fluorescence in different layers of mouse cortex under the same incident light source are presented in Fig. 6. Meanwhile, the FWHMs in every layer are listed in Table 2. It is apparent from Fig. 5(b) and Fig. 6 that Icmax (the peak value of focal intensity) is largest in the layer I and smallest in the layer VI. This is because that the smallest number of cells in the layer I leads to mild scattering effect. Since the placement of the nuclei near the focal volume, the ellipsoidal nuclei can work as a lens and result in the enhancement of focused light intensity in the focus region [23]. Though the total number of cells is similar, the Icmax of the cortical layer V is significantly higher than that of cortical layer II/III, which is also observed between layers IV and VI. Moreover, resulting from the random distribution of cells, the collected fluorescent spots are no longer center symmetrical. Therefore, randomly distributed cells in different layers have little effect on focal length, whereas they will cause the uncertainty of focal spot shape and size.
Fig. 6.
The collected fluorescence intensity distribution at the focal plane in different layers of mouse cortex.
Table 2. The resolution of collected fluorescence in different layers of mouse cortex (Unit: µm).
| Layer | FWHMx | FWHMy | FWHMz |
|---|---|---|---|
| I | 0.42 | 0.42 | 1.08 |
| II/III | 0.44 | 0.44 | 1.02 |
| IV | 0.50 | 0.42 | 0.97 |
| V | 0.42 | 0.42 | 1.02 |
| VI | 0.48 | 0.56 | 0.97 |
3.4. Comparison with the dual-wavelength confocal metalens designed in the air environment
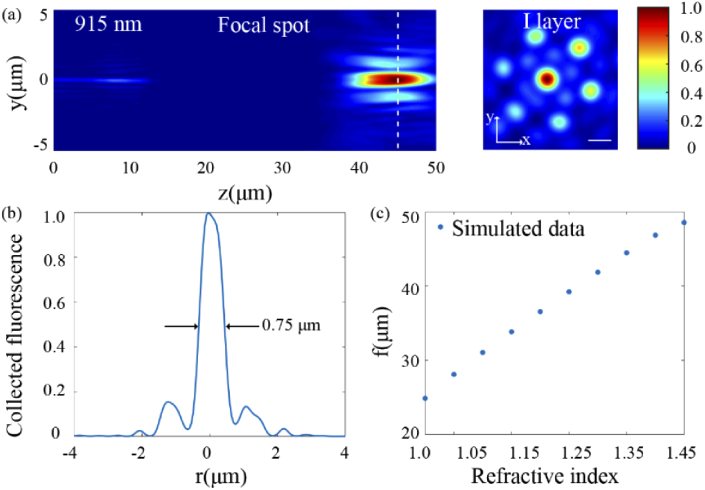
To further illustrate the strength of the proposed metalens designed in the background of mouse cortex in improving fluorescence collecting efficiency and imaging resolution, a dual-wavelength confocal metalens designed in the air environment with the same parameters (f = 25 µm, NA = 0.895, R = 50 µm) and the same area-division method was designed. For comparison, Figs. 7(a) and 7(b) show the electric field intensity distribution of excitation beam and collected fluorescence in the case of the dual-wavelength confocal metalens designed in the air environment is used for imaging in the mouse cortex. It is clear that the electric field intensity distribution suffers from severe scattering of biological tissue and the focal length at wavelength 915 nm deviates from the pre-defined focal length (25 µm) to 44.48 µm. Figure 7(b) shows that the lateral resolution of the collected fluorescent spot is 0.75 µm. In contrast, the dual-wavelength confocal metalens designed in the environment of mouse cortex can effectively achieve a smaller fluorescent spot with lateral resolution ranging from 0.42 µm to 0.56 µm (Table 2). In addition, Fig. 7(c) shows the simulated focal length at different refractive indices. Since the severe deviation of the focal length will lead to a decrease in spatial collection angle (i.e. a decrease of NA), the collection efficiency decreases according to the theory of fluorescence collection. Calculated by Eq. (3), the fluorescence collecting efficiency of the metalens designed in the air background is 5.92%, while the metalens designed directly in the mouse cortex is 14.60%. Obviously, the dual-wavelength confocal metalens designed in biological tissue can effectively improve the fluorescence collection efficiency and imaging resolution.
Fig. 7.
(a) The normalized electric field intensity distribution of the dual-wavelength confocal metalens designed in the air environment at excitation wavelength 915 nm. Scale bar: 0.5 µm. (b) The collected fluorescence intensity distribution of the dual-wavelength confocal metalens designed in the air environment along the lateral direction through the center of the fluorescent spot. (c) The simulated focal length versus the refractive index of background.
4. Conclusions
In summary, excitation and emission dual-wavelength confocal metalens designed directly in the biological tissue environment was realized by combining the spatial multiplexing method and composite embedding nano-post structure. The excitation (915 nm) and emission (510 nm) beams were focused at the same spot (focal length ∼25 µm) in all layers of the mouse cortex through the proposed polarization-insensitive metalens with high NA of 0.895. Based on the theory of two-photon excitation and fluorescence collection, the electric field intensity distribution of fluorescence signal with the high lateral resolution of 0.42-0.56 µm at focal spot was obtained. The fluorescence collection efficiency is 14.60%, which is significantly increased compared with the collection efficiency of metalens designed in the air environment. Therefore, with the lightweight and ultracompact features and high focusing performance in the biological tissue imaging, we believe that the proposed metalens has promising applications in fiber-optical two-photon fluorescence and stimulated emission depletion (STED) microendoscopy to achieve minimally-invasive deep-tissue imaging. Moreover, the present design concept can be readily applied to other organs of living animals, such as kidney, heart, skin etc.
Funding
Scientific Research Project of Tianjin Educational Committee (2018KJ084); National Natural Science Foundation of China10.13039/501100001809 (61701346).
Disclosures
The authors declare no conflicts of interest.
References
- 1.Kawakami R., Sawada K., Kusama Y., Fang Y. C., Kanazawa S., Kozawa Y., Sato S., Yokoyama H., Nemoto T., “In vivo two-photon imaging of mouse hippocampal neurons in dentate gyrus using a light source based on a high-peak power gain-switched laser diode,” Biomed. Opt. Express 6(3), 891–901 (2015). 10.1364/BOE.6.000891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebina T., Masamizu Y., Tanaka Y. R., Watakabe A., Hirakawa R., Hirayama Y., Hira R., Terada S.-I., Koketsu D., Hikosaka K., Mizukami H., Nambu A., Sasaki E., Yamamori T., Matsuzaki M., “Two-photon imaging of neuronal activity in motor cortex of marmosets during upper-limb movement tasks,” Nat. Commun. 9(1), 1879 (2018). 10.1038/s41467-018-04286-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham D. J. L., Tseng S. F., Hsieh J. T., Chen D. J., Alexandrakis G., “Dependence of Two-Photon eGFP Bleaching on Femtosecond Pulse Spectral Amplitude and Phase,” J. Fluoresc. 25(6), 1775–1785 (2015). 10.1007/s10895-015-1667-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobat D., Horton N. G., Xu C., “In vivo two-photon microscopy to 1.6-mm depth in mouse cortex,” J. Biomed. Opt. 16(10), 106014 (2011). 10.1117/1.3646209 [DOI] [PubMed] [Google Scholar]
- 5.Wu Y. C., Leng Y. X., Xi J. F., Li X. D., “Scanning all-fiber-optic endomicroscopy system for 3D nonlinear optical imaging of biological tissues,” Opt. Express 17(10), 7907–7915 (2009). 10.1364/OE.17.007907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bocarsly M. E., Jiang W.-C., Wang C., Dudman J. T., Ji N., Aponte Y., “Minimally invasive microendoscopy system for in vivo functional imaging of deep nuclei in the mouse brain,” Biomed. Opt. Express 6(11), 4546–4556 (2015). 10.1364/BOE.6.004546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh G., Chung E., Yun S. H., “Optical fibers for high-resolution in vivo microendoscopic fluorescence imaging,” Opt. Fiber Technol. 19(6), 760–771 (2013). 10.1016/j.yofte.2013.07.008 [DOI] [Google Scholar]
- 8.Ohayon S., Caravaca-Aguirre A., Piestun R., Dicarlo J. J., “Minimally invasive multimode optical fiber microendoscope for deep brain fluorescence imaging,” Biomed. Opt. Express 9(4), 1492–1509 (2018). 10.1364/BOE.9.001492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caravaca-Aguirre A. M., Singh S., Labouesse S., Baratta M. V., Piestun R., Bossy E., “Hybrid photoacoustic-fluorescence microendoscopy through a multimode fiber using speckle illumination,” APL Photonics 4(9), 096103 (2019). 10.1063/1.5113476 [DOI] [Google Scholar]
- 10.Yang H., Li G., Cao G., Zhao Z., Yu F., Chen X., Lu W., “Polarization-independent metalens constructed of antennas without rotational invariance,” Opt. Lett. 42(19), 3996–3999 (2017). 10.1364/OL.42.003996 [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Luo M., Liu S., Sun D., Ma Z., “Polarization-insensitive micro-metalens for high-resolution and miniaturized all-fiber two-photon microendoscopic fluorescence imaging,” Opt. Commun. 445, 76–83 (2019). 10.1016/j.optcom.2019.04.027 [DOI] [Google Scholar]
- 12.Arbabi E., Li J. Q., Hutchins R. J., Kamali S. M., Arbabi A., Horie Y., Van Dorpe P., Gradinaru V., Wagenaar D. A., Faraon A., “Two-Photon Microscopy with a Double-Wavelength Metasurface Objective Lens,” Nano Lett. 18(8), 4943–4948 (2018). 10.1021/acs.nanolett.8b01737 [DOI] [PubMed] [Google Scholar]
- 13.Ou K., Li G., Li T., Yang H., Yu F., Chen J., Zhao Z., Cao G., Chen X., Lu W., “High efficiency focusing vortex generation and detection with polarization-insensitive dielectric metasurfaces,” Nanoscale 10(40), 19154–19161 (2018). 10.1039/C8NR07480A [DOI] [PubMed] [Google Scholar]
- 14.Yang H., Li G., Su X., Cao G., Zhao Z., Yu F., Chen X., Lu W., “Annihilating optical angular momentum and realizing a meta-waveplate with anomalous functionalities,” Opt. Express 25(15), 16907–16915 (2017). 10.1364/OE.25.016907 [DOI] [PubMed] [Google Scholar]
- 15.Blab G. A., Lommerse P. H. M., Cognet L., Harms G. S., Schmidt T., “Two-photon excitation action cross-sections of the autofluorescent proteins,” Chem. Phys. Lett. 350(1-2), 71–77 (2001). 10.1016/S0009-2614(01)01282-9 [DOI] [Google Scholar]
- 16.Avayu O., Almeida E., Prior Y., Ellenbogen T., “Composite functional metasurfaces for multispectral achromatic optics,” Nat. Commun. 8(1), 14992 (2017). 10.1038/ncomms14992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khorasaninejad M., Shi Z., Zhu A. Y., Chen W. T., Sanjeev V., Zaidi A., Capasso F., “Achromatic Metalens over 60 nm Bandwidth in the Visible and Metalens with Reverse Chromatic Dispersion,” Nano Lett. 17(3), 1819–1824 (2017). 10.1021/acs.nanolett.6b05137 [DOI] [PubMed] [Google Scholar]
- 18.Wu J. P., Guo C. D., Chen S. B., Jiang T., He Y., Ding W. X., Yang Z. Q., Luo Q. M., Gong H., “Direct 3D Analyses Reveal Barrel-Specific Vascular Distribution and Cross-Barrel Branching in the Mouse Barrel Cortex,” Cereb. Cortex 26(1), 23–31 (2016). 10.1093/cercor/bhu166 [DOI] [PubMed] [Google Scholar]
- 19.Meyer H. S., Wimmer V. C., Oberlaender M., de Kock C. P. J., Sakmann B., Helmstaedter M., “Number and Laminar Distribution of Neurons in a Thalamocortical Projection Column of Rat Vibrissal Cortex,” Cereb. Cortex 20(10), 2277–2286 (2010). 10.1093/cercor/bhq067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollmann J. L., Horstmeyer R., Yang C., Dimarzio C. A., “Analysis and modeling of an ultrasound-modulated guide star to increase the depth of focusing in a turbid medium,” J. Biomed. Opt. 18(2), 025004 (2013). 10.1117/1.JBO.18.2.025004 [DOI] [PubMed] [Google Scholar]
- 21.Chao G. S., Sung K. B., “Investigating the spectral characteristics of backscattering from heterogeneous spherical nuclei using broadband finite-difference time-domain simulations,” J. Biomed. Opt. 15(1), 015007 (2010). 10.1117/1.3324838 [DOI] [PubMed] [Google Scholar]
- 22.Dunn A., Richards-Kortum R., “Three-dimensional computation of light scattering from cells,” IEEE J. Sel. Top. Quantum Electron. 2(4), 898–905 (1996). 10.1109/2944.577313 [DOI] [Google Scholar]
- 23.Starosta M. S., Dunn A. K., “Three-Dimensional Computation of Focused Beam Propagation through Multiple Biological Cells,” Opt. Express 17(15), 12455–12469 (2009). 10.1364/OE.17.012455 [DOI] [PubMed] [Google Scholar]
- 24.Yu W. T., Ji Z. H., Dong D. S., Yang X. S., Xiao Y. F., Gong Q. H., Xi P., Shi K. B., “Super-resolution deep imaging with hollow Bessel beam STED microscopy,” Laser Photonics Rev. 10(1), 147–152 (2016). 10.1002/lpor.201500151 [DOI] [Google Scholar]
- 25.Gohn-Kreuz C., Rohrbach A., “Light-sheet generation in inhomogeneous media using self-reconstructing beams and the STED-principle,” Opt. Express 24(6), 5855–5865 (2016). 10.1364/OE.24.005855 [DOI] [PubMed] [Google Scholar]
- 26.Kozawa Y., Sato S., “Sharper focal spot formed by higher-order radially polarized laser beams,” J. Opt. Soc. Am. A 24(6), 1793–1798 (2007). 10.1364/JOSAA.24.001793 [DOI] [PubMed] [Google Scholar]
- 27.Wang T. T., Kuang C. F., Hao X., Liu X., “Focusing properties of cylindrical vector vortex beams with high numerical aperture objective,” Optik 124(21), 4762–4765 (2013). 10.1016/j.ijleo.2013.01.070 [DOI] [Google Scholar]
- 28.Fahrbach F. O., Rohrbach A., “Propagation stability of self-reconstructing Bessel beams enables contrast-enhanced imaging in thick media,” Nat. Commun. 3(1), 632 (2012). 10.1038/ncomms1646 [DOI] [PubMed] [Google Scholar]
- 29.Arbabi E., Arbabi A., Kamali S. M., Horie Y., Faraon A., “Multiwavelength metasurfaces through spatial multiplexing,” Sci. Rep. 6(1), 32803 (2016). 10.1038/srep32803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arbabi E., Arbabi A., Kamali S. M., Horie Y., Faraon A., “Multiwavelength polarization-insensitive lenses based on dielectric metasurfaces with meta-molecules,” Optica 3(6), 628–633 (2016). 10.1364/OPTICA.3.000628 [DOI] [Google Scholar]
- 31.Xu C., Webb W. W., “Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm,” J. Opt. Soc. Am. B 13(3), 481–491 (1996). 10.1364/JOSAB.13.000481 [DOI] [Google Scholar]
- 32.Beaurepaire E., Oheim M., Mertz J., “Ultra-deep two-photon fluorescence excitation in turbid media,” Opt. Commun. 188(1-4), 25–29 (2001). 10.1016/S0030-4018(00)01156-1 [DOI] [Google Scholar]
- 33.Zinter J. P., Levene M. J., “Maximizing fluorescence collection efficiency in multiphoton microscopy,” Opt. Express 19(16), 15348–15362 (2011). 10.1364/OE.19.015348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaurepaire E., Mertz J., “Epifluorescence collection in two-photon microscopy,” Appl. Opt. 41(25), 5376–5382 (2002). 10.1364/AO.41.005376 [DOI] [PubMed] [Google Scholar]