Abstract
Objectives
U-Act-Early was a 2-year, randomized placebo controlled, double-blind trial, in which DMARD-naïve early RA patients were treated to the target of sustained remission (SR). Two strategies initiating tocilizumab (TCZ), with and without methotrexate (MTX), were more effective than a strategy initiating MTX. The aim of the current study was to determine longer-term effectiveness in daily clinical practice.
Methods
At the end of U-Act-Early, patients were included in a 3-year post-trial follow-up (PTFU), in which treatment was according to standard care and data were collected every 3 months during the first year and every 6 months thereafter. Primary end point was disease activity score assessing 28 joints (DAS28) over time. Mixed effects models were used to compare effectiveness between initial strategy groups, correcting for relevant confounders. Between the groups as randomized, proportions of patients were tested for DMARD use, SR and radiographic progression of joint damage.
Results
Of patients starting U-Act-Early, 226/317 (71%) participated in the PTFU. Over the total 5 years, mean DAS28 was similar between groups (P > 0.20). During U-Act-Early, biologic DMARD use decreased in both TCZ initiation groups and increased in the MTX initiation group, but during follow-up this trend did not continue. SR was achieved at least once in 99% of patients. Of the 226 patients, only 30% had any radiographic progression over 5 years, without significant differences between the groups.
Conclusion
Although in the short-term the strategies initiating TCZ yielded the most clinical benefit, in the longer-term differences in important clinical outcomes between the strategies disappeared, probably due to continuation of the treat-to-target principle.
Keywords: rheumatoid arthritis, biologic therapies, DMARDs, epidemiology, clinical trials and methods
Rheumatology key messages
Post-trial effectiveness in daily practice equals efficacy of treat-to-target strategies in the U-Act-Early trial in RA patients.
Treat-to-target strategies proved to be safe also during the U-Act-Early post-trial follow-up period in RA patients.
Use of biologic DMARD remained higher in the strategy groups initiating tocilizumab early in newly diagnosed RA patients.
Introduction
The current guidelines for the treatment of RA recommend treating to target, aiming for remission, to reduce the risk of disability and improve long-term outcomes. With this aim, generally, a conventional synthetic (cs)DMARD is started immediately after diagnosis. If the treatment target is not achieved within 3–6 months, another csDMARD or a biologic (b)DMARD is added [1].
Several placebo-controlled randomized controlled trials in early RA patients have compared the effects of initial treatment strategies using a bDMARD with those of initial treatment strategies with only csDMARD(s), mostly MTX [2–8]. In the short term (<1 year), disease control and outcomes, including progression of joint damage and physical function, were better in strategies initiating a bDMARD with or without a csDMARD. The first randomized controlled trial of initiation of the bDMARD tocilizumab (TCZ) in DMARD-naïve early RA patients showed that more patients receiving TCZ (+/-MTX) achieved remission at 24 and 52 weeks compared with patients receiving MTX [6]. However, this was not a treat-to-target design like the U-Act-Early trial. In this 2-year trial involving early DMARD- and glucocorticoid (GC)-naïve RA patients with the treatment target of sustained remission (SR), efficacy and safety of step-up treatment strategies initiating treatment with TCZ, MTX or their combination were compared. When the treatment target was achieved, medication was tapered and eventually stopped, if patients remained in remission. Outcomes of U-Act-Early were in line with those of other randomized controlled trials showing improved effectiveness for strategies initiating/adding a bDMARD from the start of therapy, compared with strategy groups not including a bDMARD from the start [9]. For example, SR was achieved earlier in the TCZ strategy groups in the U-Act-Early trial [10].
However, starting TCZ immediately upon diagnosis in early RA patients does not accord with the current disease management recommendations. To justify initiation of (expensive) bDMARDs directly after diagnosis, not only clear advantages in the first months, but also a longer-term effectiveness would be warranted. Therefore, patients in the U-Act-Early trial were followed for another 3 years, to determine longer-term effectiveness. We hypothesized that during this follow-up period, the disease activity score assessing 28 joints (DAS28, based on ESR) would remain similar (continuing the treat-to-target approach) between the initial strategy groups, and that radiographic progression would be less in patients who had initiated TCZ (+/-MTX) compared with those who had initiated MTX. Moreover, based on the finding that during the 2-year trial TCZ use steadily increased in the MTX initiation strategy group and decreased in the two TCZ initiation strategy groups, we hypothesized that during follow-up, bDMARD use would further increase in the MTX initiation strategy group and further decrease in the TCZ initiation strategy groups.
Methods
This was an observational open label multicentre 3-year post-trial follow-up (PTFU) of the U-Act-Early trial. Post-trial treatment was left to the discretion of the treating rheumatologist and patients were followed during routine clinical practice, which had a focus on treat-to-target.
Patients who participated in the U-Act-Early trial were eligible for participation and were asked to participate at the end of U-Act-Early between Q2 2012 and Q3 2014. The only exclusion criteria were being lost to follow-up, unwillingness to give informed consent and having had too many serious protocol violations (e.g. >4 times non-adherence to protocol over a period of 1 year). Nineteen of the initial 21 hospitals in the Netherlands participating in the U-Act-Early trial also participated in the PTFU. Detailed information on the U-Act-Early trial has been reported [10].
Data collection
After the initial monthly follow-up during the U-Act-Early trial, in the PTFU period data were collected every 3 months in the first year and every 6 months thereafter up to 3 years. At every visit, all components of DAS28, physical function with the Dutch Health Assessment Questionnaire (HAQ) and information on use of NSAIDs, GCs, csDMARDs and bDMARDs were assessed, next to the occurrence of adverse events (AE) and, serious AE (SAE). Remission was defined as DAS28 < 2.6 and ≤4 swollen joints of 28 assessed joints, and SR was defined as being in remission for ≥24 weeks. Sustained drug-free remission (sDFR) was defined as having tapered and stopped all DMARDs and being in remission for ≥3 months. Radiographs from baseline U-Act-Early, end of U-Act-Early (2 years) and last available time point (5 years or end of follow-up) were scored in chronological order by an experienced professional reader, according to the Sharp–van der Heijde (SvdH) method. To make optimal use of available X-rays, if the X-ray was not taken at the 5 year time point, but ≥3 years, the 5-year progression was estimated by extrapolation using the following formula; change SvdH score between baseline and last available X-ray/(date last available X-ray − date baseline X-ray/365) × 5. The institutional review boards of the participating centers confirmed that the Medical Research Involving Human Subjects Act (WMO) was not applicable to this study, and all patients gave written informed consent.
Outcomes
The primary end point was DAS28 over 5 years. The secondary end point was medication use (NSAID use, GCs, csDMARDs and bDMARDs) during the 3-year PTFU. Other secondary end points were number of patients achieving SR and sDFR, cumulative duration of SR and sDFR, change in SvdH score, HAQ scores, and the number of patients with occurrence of any AE or SAE, all variables evaluated over 5 years.
Statistics
Data of all patients enrolled in this PTFU were used for analyses. Continuous variables were described using means with s.d. or medians with interquartile range (IQR), where appropriate. Frequencies and proportions were calculated for categorical variables. Differences between the initial strategy groups in baseline characteristics were evaluated and tested using one-way ANOVA for continuous outcomes and the χ2 test or Fisher’s exact test for categorical outcomes. Mixed model analyses were used to assess differences over time between the initial treatment strategy groups in continuous outcomes, with a random intercept and fixed effects for treatment arm, visit week, the interaction visit week × treatment arm, correcting for DAS28 at baseline and centre (i.e. both the stratification factors used for randomization). Differences between the initial treatment strategy groups in proportions of patients using medication were tested with a Cochran–Mantel–Haenszel (CMH) test. Differences between the initial treatment strategy groups in proportions of patients achieving SR and sDFR and cumulative duration of all SR and sDFR periods in individual patients were tested with CMH test and linear regression analysis, respectively, correcting for baseline DAS28 (for CMH; DAS28 <5.1 or ≥5.1, as this cut-off was used for the randomization) and centre. Differences between the initial treatment strategy groups in median change in radiographic scores were tested with the van Elteren test, correcting for baseline DAS28 and centre. The statistical analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided; a P-value ≤0.05 was considered statistically significant.
Sensitivity analysis
To evaluate the generalizability of our findings, a sensitivity analysis was performed for the primary end point, including also patients (n = 91) who had not participated in the PTFU, only in the 2-year U-Act-Early trial. Missing data for all patients in DAS28 and swollen joint count (SJC)28 over time were imputed using a joint multivariate model of two-level data. The package ‘jomo’ in R was used for multiple imputation; this package also handles categorical data and uses cluster-specific covariance matrices [11]. The variables treatment arm, visit week, duration of participation, reason for drop out, and DAS28, HAQ, age, disease duration, gender and RF status (positive/negative) at baseline were used in the imputation model. Patient was used as a cluster variable, to account for repeated measurements over time within patients. The number of imputed datasets was based on the percentages of missing values over time. By using Rubin’s rule, analysis results based on the imputed datasets were pooled to provide an overall result [12]. A second sensitivity analysis was performed to exclude the influence of acute phase reactants (APRs) on the outcome, as it is known that TCZ reduces APRs specifically [13]. However, validated disease activity indices without APR include the visual analogue scale physician, which was not assessed during the PTFU. As an alternative, the unweighted components of the DAS28, except for ESR, were analysed separately using linear mixed effects models to study the differences in outcomes between the initial strategy groups over time using observed data. Square root transformation was applied to skewed SJC28 and tender joint count (TJC)28 data.
Results
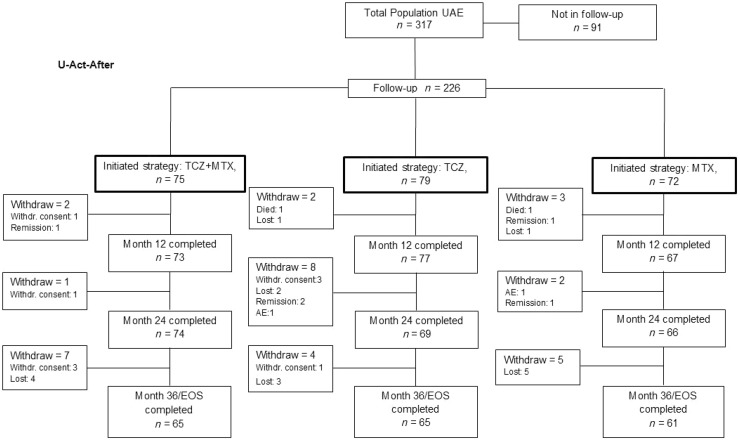
Of the 317 patients who had started in U-Act-Early, 226 patients were included in the PTFU, of whom 85% completed the study (Fig. 1). During this period one patient died due to a brainstem infarction (TCZ strategy group) and one patient due to squamous cell carcinoma of the cervix (MTX strategy group); these complications were deemed not to be related to the treatment. The patient disposition during the U-Act-Early trial is shown in Supplementary Fig. S1, available at Rheumatology online.
Fig. 1.
Patient disposition during the 3-year post-trial follow-up
AE: adverse event; EOS: end of study; Follow-up: post-trial follow-up; Lost: lost to follow-up; MTX: methotrexate + placebo–tocilizumab initiation strategy group; TCZ: tocilizumab + placebo–MTX initiation strategy group; TCZ+MTX: tocilizumab + MTX initiation strategy group; UAE: U-Act-Early; Withdr. Consent: withdrawal of consent.
Baseline characteristics of the patients included in the PTFU were not statistically significantly different between the initial strategy groups at start of the U-Act-Early trial (Table 1). Additional information about patients included and not included in the PTFU is presented in Supplementary Table S1, available at Rheumatology online.
Table 1.
Baseline characteristics at start of U-Act-Early of patients included in the post-trial follow-up study (n = 226)a
| TCZ+MTX (n = 75) | TCZ (n = 79) | MTX (n = 72) | P-value | |
|---|---|---|---|---|
| Female, n (%) | 47 (63) | 61 (77) | 48 (67) | 0.13 |
| RF+, n (%) | 53 (71) | 55 (71) | 58 (82) | 0.21 |
| Anti CCP+, n (%) | 51 (68) | 54 (69) | 56 (79) | 0.28 |
| RF+ and/or anti-CCP+, n (%) | 55 (73) | 62 (79) | 64 (89) | 0.06 |
| Caucasian, n (%) | 71 (95) | 77 (97) | 71 (99) | 0.47 |
| Smoking status, n (%) | 0.81 | |||
| Never smoked | 29 (39) | 34 (43) | 28 (39) | |
| Quit smoking | 21 (28) | 24 (30) | 25 (35) | |
| Current smoker | 25 (33) | 21 (27) | 19 (26) | |
| Age, mean (s.d.), years | 53.8 (11.2) | 55.5 (11.6) | 53.7 (12.9) | 0.63 |
| Symptom duration, median (IQR), days | 27 (18–43) | 25 (19–43) | 28 (16–46) | 0.96 |
| DAS28, mean (s.d.) | 5.1 (1.1) | 5.3 (1.1) | 5.0 (1.2) | 0.33 |
| HAQ, median (IQR) | 1.3 (0.6–1.6) | 1.3 (0.9–1.8) | 1.0 (0.5–1.4) | 0.17 |
| SvdH score, median (IQR) | 0 (0–0) | 0 (0–2) | 0 (0–1) | 0.24 |
According to their initial treatment strategy as randomized. Anti-CCP: anti-cyclic citrullinated peptide antibodies; DAS28: disease activity score assessing 28 joints; HAQ: health assessment questionnaire; IQR: interquartile range; MTX: methotrexate + placebo–tocilizumab initiation strategy group; RF: rheumatoid factor; SvdH: Sharp–van der Heijde score; TCZ: tocilizumab + placebo–methotrexate initiation strategy group; TCZ+MTX: tocilizumab + methotrexate initiation strategy group.
The following results are based on data of the 226 patients who were included in the PTFU.
DAS28
In line with the results of the U-Act-Early trial, the mean DAS28 over time was statistically significantly lower for the TCZ initiation strategy groups compared with the MTX initiation strategy group at year 1 and 2 of U-Act-Early. There were no statistically significant differences in average DAS28 over time between the initial treatment strategy groups during the 3-year PTFU period, nor over the total duration of 5 years (see Table 2 and Fig. 2).
Table 2.
Mean difference in DAS28 between initial treatment strategy groups over 5 years and at the end of every year (n = 226)
| Period and strategy comparison | Mean difference | 95% CI of mean difference |
|---|---|---|
| Over 5 years | ||
| TCZ+MTX vs MTX | −0.11 | −0.32, 0.10 |
| TCZ vs MTX | −0.12 | −0.32, 0.09 |
| TCZ+MTX vs TCZ | 0.00 | −0.20, 0.21 |
| 1 | ||
| TCZ+MTX vs MTX | −0.75 | −0.89, −0.61 |
| TCZ vs MTX | −0.65 | −0.79, −0.51 |
| TCZ+MTX vs TCZ | −0.10 | −0.23, 0.04 |
| 2 | ||
| TCZ+MTX vs MTX | −0.63 | −0.77, −0.49 |
| TCZ vs MTX | −0.55 | −0.69, −0.40 |
| TCZ+MTX vs TCZ | −0.08 | 0.23, 0.06 |
| 3 | ||
| TCZ+MTX vs MTX | 0.07 | −0.19, 0.33 |
| TCZ vs MTX | 0.09 | −0.17, 0.34 |
| TCZ+MTX vs TCZ | −0.02 | −0.27, 0.24 |
| 4 | ||
| TCZ+MTX vs MTX | 0.09 | −0.17, 0.35 |
| TCZ vs MTX | 0.10 | −0.16, 0.36 |
| TCZ+MTX vs TCZ | −0.01 | −0.27, 0.24 |
| 5 | ||
| TCZ+MTX vs MTX | 0.11 | −0.16, 0.37 |
| TCZ vs MTX | 0.12 | −0.14, 0.38 |
| TCZ+MTX vs TCZ | −0.01 | −0.27, 0.25 |
All analyses were corrected for baseline DAS28 category (DAS28 <5.1 or ≥5.1) and centre. Outcomes are based on mixed model analyses with random intercept for repeated measurements, and fixed effects for treatment arm, visit week, interaction visit week × treatment arm. DAS28: disease activity score assessing 28 joints; MTX: methotrexate + placebo–tocilizumab initiation strategy group; TCZ: tocilizumab + placebo–methotrexate initiation strategy group; TCZ+MTX: tocilizumab + methotrexate initiation strategy group.
Fig. 2.
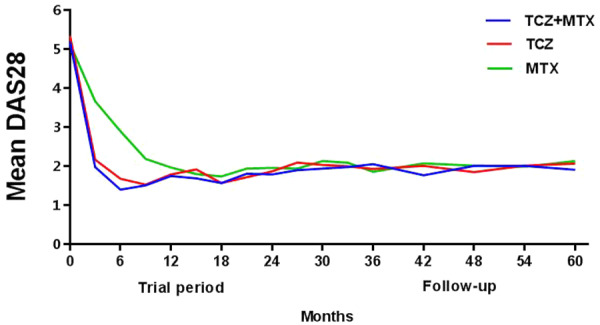
DAS28 over 5 years as predicted by model
DAS28: disease activity score assessing 28 joints; Follow-up: post-trial follow-up starting after 24 months; MTX: methotrexate + placebo–tocilizumab initiation strategy group; TCZ: tocilizumab + placebo–methotrexate initiation strategy group; TCZ+MTX: tocilizumab + methotrexate initiation strategy group; UAE: U-Act-Early.
Medication use during the 3-year PTFU
Any medication for RA was used by 75 patients (100%) in the TCZ+MTX initiation strategy group, 77 (96%) in the TCZ initiation strategy group and 71 (99%) in the MTX initiation strategy group (P=0.39). NSAIDs were used by 68 (91%) patients in the TCZ+MTX initiation strategy group, 72 (91%) in the TCZ initiation strategy group and 65 (90%) in the MTX initiation strategy group (P=0.98). The number of patients using GCs was higher (although not statistically significantly) in the MTX initiation strategy group (n = 47, 67%) compared with the TCZ+MTX (n = 41, 55%) and TCZ (n = 39, 49%) initiation strategy groups (P=0.09). csDMARDs were used by more patients in the MTX initiation strategy group (n = 65, 90%) compared with the TCZ+MTX (n = 56, 75%) and TCZ (n = 58, 73%) initiation strategy groups (P = 0.02). In contrast, the number of patients using bDMARDs was numerically higher in the TCZ+MTX (n = 40, 53%) and TCZ (n = 36, 46%) initiation strategy groups than in the MTX initiation strategy group (n = 26, 36%), although not statistically significantly (P = 0.11). Any other bDMARD than TCZ was used by 8% of all patients (8% TCZ+MTX, 8% TCZ and 10% MTX, respectively). Additional information about medication use is provided in Supplementary Table S4 and Supplementary Figs S2–6, available at Rheumatology online.
Sustained (drug free) remission
Over 5 years, SR was achieved at least once by 224 of the 226 patients (99%) and the proportions of patients achieving SR were not statistically significantly different (P = 0.15; Table 3) between the initial strategy groups. The cumulative duration of SR was statistically significantly longer in the TCZ initiation strategy groups compared with the MTX initiation strategy group (P < 0.01; Table 3). However, including only the PTFU period, no differences were found between the strategy groups [median (IQR) duration 121 (74–154), 116 (53–145) and 109 (65–140) weeks in TCZ+MTX, TCZ and MTX, respectively (P = 0.62)]. Fifty-nine of the 226 patients (26%) achieved sDFR at least once during the 5 years, without statistically significant differences between the initial strategy groups, nor for the cumulative duration of sDFR (Table 3).
Table 3.
Sustained (drug free) remission over 5 years (n = 226)
| TCZ+ MTX ( n = 75) | TCZ ( n = 79) | MTX ( n = 72) | P-value | |
|---|---|---|---|---|
| Patients achieving SR at least once, n (%) | 75 (100) | 77 (98) | 72 (100) | 0.15a |
| Cumulative duration of SR, median (IQR), weeks | 216 (152–251) | 190 (135–240) | 172 (129–202) | <0.01b |
| Patients achieving sDFR at least once, n (%) | 26 (35) | 19 (26) | 14 (19) | 0.10a |
| Cumulative duration of sDFR, median (IQR), weeks | 119 (76–157) | 107 (53–157) | 83 (37–146) | 0.27b |
The treatment strategy groups are according to the initial randomization at start of U-Act-Early. All analyses were corrected for baseline DAS28 category (DAS28 <5.1 or ≥5.1) and centre. aCochran–Mantel–Haenszel test. bLinear regression. IQR: interquartile range; MTX: methotrexate + placebo–tocilizumab initiation strategy group; sDFR: sustained drug free remission; SR: sustained remission; TCZ: tocilizumab + placebo–methotrexate initiation strategy group; TCZ+MTX: tocilizumab + methotrexate initiation strategy group.
Radiographic progression
Of the 226 patients, only 30% had any radiographic progression over 5 years, without significant differences between the groups (P=0.09; Fig. 3). The median changes in both total SvdH score and erosion score over 5 years were 0 in all strategy groups (Table 4). The median change in JSN score was statistically significantly lower for the TCZ+MTX initiation strategy group compared with the MTX initiation strategy group (IQR:TCZ+MTX: 0–0; MTX: 0–1; P = 0.03).
Fig. 3.
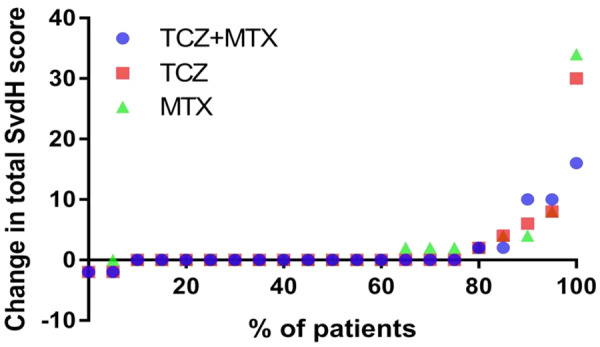
Cumulative probability plot for absolute change in total Sharp–van der Heijde score over 5 years
Change in SvdH score is based on radiographs from baseline U-Act-Early and last available time point (5 years or end of follow-up if earlier than 5 years). Median duration of radiographs from start of U-Act-Early was 5 years in all initial treatment strategies. MTX: methotrexate + placebo–tocilizumab initiation strategy group; SvdH: Sharp–van der Heijde score; TCZ: tocilizumab + placebo–methotrexate initiation strategy group; TCZ+MTX: tocilizumab + methotrexate initiation strategy group.
Table 4.
Change in SvdH scores over 5 years (n = 226)
|
Change in SvdH score, median (IQR) [min, max]
|
P-value
a
|
|||||
|---|---|---|---|---|---|---|
| TCZ+MTX ( n = 75) | TCZ ( n = 79) | MTX ( n = 72) | TCZ+MTX vs MTX | TCZ vs MTX | TCZ+MTX vs TCZ | |
| Total SvdH score | 0 (0–1) [−2, 15] | 0 (0–1) [−2, 30] | 0 (0–2) [0, 35] | 0.41 | 0.05 | 0.67 |
| Erosion score | 0 (0–0) [0, 9] | 0 (0–1) [−2, 17] | 0 (0–1) [0, 35] | 0.62 | 0.80 | 0.62 |
| JSN score | 0 (0–0) [−2, 8] | 0 (0–0) [−1, 13] | 0 (0–1) [0, 6] | 0.03 | 0.11 | 0.31 |
The treatment strategy groups are according to the initial randomization at start of U-Act-Early. All analyses were corrected for baseline DAS28 category (DAS28 <5.1 or ≥5.1) and centre. aVan Elteren test. IQR: interquartile range; JSN: joint space narrowing; max: maximum; min: minimum; MTX: methotrexate + placebo–tocilizumab initiation strategy group; SvdH: Sharp–van der Heijde score; TCZ: tocilizumab + placebo–methotrexate initiation strategy group; TCZ+MTX: tocilizumab + methotrexate initiation strategy group.
Physical function
No statistically significant differences in HAQ scores over 5 years were observed between the strategy groups (Table 5).
Table 5.
Mean difference in HAQ scores between initial treatment strategy groups over 5 years and at the end of every year (n = 226)
| Period and strategy comparisons | Mean difference | 95% CI of mean difference |
|---|---|---|
| Over 5 years | ||
| TCZ+MTX vs MTX | −0.05 | −0.22, 0.11 |
| TCZ vs MTX | 0.03 | −0.14, 0.20 |
| TCZ+MTX vs TCZ | −0.08 | −0.25, 0.08 |
| 1 | ||
| TCZ+MTX vs MTX | −0.10 | −0.26, 0.05 |
| TCZ vs MTX | −0.04 | −0.20, 0.11 |
| TCZ+MTX vs TCZ | −0.06 | −0.21, 0.09 |
| 2 | ||
| TCZ+MTX vs MTX | −0.09 | −0.25, 0.06 |
| TCZ vs MTX | −0.03 | −0.19, 0.12 |
| TCZ+MTX vs TCZ | −0.06 | −0.21, 0.09 |
| 3 | ||
| TCZ+MTX vs MTX | −0.05 | −0.23, 0.12 |
| TCZ vs MTX | 0.02 | −0.15, 0.20 |
| TCZ+MTX vs TCZ | −0.08 | −0.25, 0.09 |
| 4 | ||
| TCZ+MTX vs MTX | −0.05 | −0.23, 0.12 |
| TCZ vs MTX | 0.02 | −0.15, 0.20 |
| TCZ+MTX vs TCZ | −0.08 | −0.25, 0.09 |
| 5 | ||
| TCZ+MTX vs MTX | −0.05 | −0.23, 0.12 |
| TCZ vs MTX | 0.03 | −0.15, 0.20 |
| TCZ+MTX vs TCZ | −0.08 | −0.25, 0.09 |
All analyses were corrected for baseline DAS28 category (DAS28 <5.1 or ≥5.1) and centre. Outcomes are based on mixed model analyses with random intercept for repeated measurements, and fixed effects for treatment arm, visit week, interaction visit week × treatment arm. HAQ: Health Assessment Questionnaire MTX: methotrexate + placebo–tocilizumab initiation strategy group; TCZ: tocilizumab + placebo–methotrexate initiation strategy group; TCZ+MTX: tocilizumab + methotrexate initiation strategy group.
Subgroup analyses for (non-) severe RA are shown in Supplementary Tables S2 and S3, available at Rheumatology online. In general, results are in line with the overall results, probably except for total SvdH score.
Safety
No statistically significant differences over 5 years were found in safety outcomes between strategy groups as initially randomized (Table 6). Of the patients in the TCZ+MTX initiation strategy group, 21 (28%) experienced at least one SAE compared with 23 (29%) in the TCZ initiation strategy group and 15 (21%) in the MTX initiation strategy group (P = 0.47). Infections, benign/malignant neoplasms and cardiac disorders were the most common SAEs and occurred at least once 15, 8 and 8 times, respectively. Occurrence of at least one serious infection was numerically more frequent in the TCZ initiation strategy groups compared with the MTX initiation strategy group (7 in TCZ+MTX, 5 in TCZ vs 1 in MTX; P=0.11).
Table 6.
Safety outcomes over 5 years, results based on data of patients included in the post-trial follow-up (n = 226)
| TCZ+MTX (n = 75) | TCZ (n = 79) | MTX (n = 72) | P-valuea | |
|---|---|---|---|---|
| ≥1 AE, n (%) | 75 (100) | 78 (99) | 72 (100) | 1.00 |
| AE rate per 100 patient-years | 336 | 340.96 | 382 | |
| Treatment was given for AE, % | 73 | 70 | 72 | 0.13 |
| ≥1 SAE, n (%) | 21 (28) | 23 (29) | 15 (21) | 0.47 |
| SAE rate per 100 patient-years | 7.1 | 10.5 | 6.5 | |
| ≥1 serious infection, n (%) | 7 (9) | 5 (6) | 1 (1) | 0.11 |
Extended Fisher’s exact test. AE: adverse event; MTX: methotrexate + placebo–tocilizumab initiation strategy group; SAE: serious adverse event; TCZ: tocilizumab + placebo–methotrexate initiation strategy group; TCZ+MTX: tocilizumab + methotrexate initiation strategy group.
Sensitivity analysis
For the primary end point, DAS28 over 5 years, the results of the sensitivity analysis using data of all patients who were included in U-Act-Early (n = 317), after imputation of missing PTFU data, were in line with the findings of our main analysis with DAS28 as end point (see Supplementary Table S5, available at Rheumatology online).
The sensitivity analysis, analysing the individual unweighted components of the DAS28 separately (ESR excluded), to eliminate the influence of TCZ on APR in the primary outcome yielded statistically significant differences between the treatment strategies for U-Act-Early trial period, but not for the PTFU (see Supplementary Table S3, available at Rheumatology online).
Discussion
This is the first long-term PTFU evaluating the effectiveness and safety of tight-controlled treat-to-target treatment strategies using a bDMARD from start of therapy in early DMARD- and GC-naïve RA patients up to 5 years. The high effectiveness during the trial period of 2 years was maintained in a real-world setting without differences between the treatment strategy groups over the 3-year PTFU period. This suggests that an early start of any tight-controlled treat-to-target treatment strategy is also effective in the longer-term. The cumulative duration of SR over 5 years was significantly longer for the TCZ initiation strategy groups compared with the MTX initiation strategy group. For the TCZ+MTX initiation strategy group, the median SR duration was about 4 years (216 weeks), which may have an important positive impact on daily activities and quality of life. However, this effect may be biased as patients in the MTX initiation strategy group had a considerable delay in achieving remission compared with the TCZ initiation strategy groups. Including only the PTFU period, no differences for SR were found between the strategy groups, but the duration of SR was numerically longer in the TCZ initiation strategy groups. For future research, a cost effectiveness analysis is warranted to determine whether early initiation of TCZ is cost-effective over a period up to 5 years, compared with MTX initiation.
During the trial, medication was tapered if the patients remained in SR. In the open label PTFU, the numbers of patients using a bDMARD remained numerically higher in the TCZ initiation strategy groups compared with the MTX initiation strategy group, and the number of patients using csDMARDs remained higher in the MTX initiation strategy group. This outcome and the long duration of SR as mentioned above suggest that bDMARD tapering is not yet widely applied in a real-world setting. The most frequently used bDMARD was TCZ in all strategy groups. Physicians were not completely familiar with the effects of TCZ tapering, and probably therefore TCZ was tapered cautiously in the real-world setting during the follow-up phase.
Our study also provides evidence for the safety over 5 years of the treatment strategies initiating TCZ, MTX or their combination in a step-up treat-to-target treatment strategy compared according to their randomized groups. The effectiveness and safety results are in line with those of other PTFU studies, with TCZ use, performed in established RA patients [14–16].
The limitations of this study include the non-protocolized follow-up, during the PTFU. However, this non-protocolized follow-up gives a realistic view of treatments in a daily practice setting. Not all patients who participated in the U-Act-Early trial could be included in the PTFU, but the sensitivity analysis suggests that results based on the patients with observed data are generalizable to the original U-Act-Early population. Another limitation is the number of missing radiographs at the end of the follow-up period up to 5 years. Although during the follow-up radiographs available at a previous visit could be used for our analyses, still 17% of radiographs in the TCZ+MTX group, 33% in the TCZ group and 21% in the MTX group were missing. Finally, quality of life was not assessed, as well as data about comorbidity.
Conclusion
The high effectiveness of the treat-to-target treatment strategies during the U-Act-Early trial period of 2 years was maintained during the 3-year PTFU in a real-world setting. Although during the 2-year trial period TCZ-based initiation strategies yielded the most clinical benefit, in the following 3 years differences in important clinical outcomes between initial strategies disappeared, probably due to continuation of the treat-to-target principle, frequently leading to a convergence of treatment regimens. bDMARD use remained higher in the strategy groups initiating TCZ early, possibly due to the lack of a protocolized tapering strategy after the U-Act-Early trial. All treatment strategies proved to be safe.
Supplementary Material
Acknowledgements
We gratefully thank all patients for their participation, and also anyone who was involved in conducting these trials and other personnel for their contribution, especially the rheumatologists and study-nurses of the participating institutions. J.W.J.B. was the principal investigator, and contributed to the study design, data collection, data interpretation and writing of the report. J.W.G.J. contributed to the study design, data collection, data analysis, data interpretation and writing of the report. P.M.J.W. contributed to the study design, data analysis, data interpretation and writing of the report. M.E.A.B. signed off the clinical study report, and contributed to the study design, data interpretation and writing of the report. M.M.A.V. and M.J.H.dH. contributed to data analysis, data interpretation and writing of the report. J.T., A.P.-S., G.A.W.B., R.B., E.N.G., R.K., J.M.vL. and F.P.J.G.F. contributed to data interpretation and writing of the report. All authors approved the final version to be published and agree to the accountable for all aspects.
Funding: This work was supported by Roche Nederland BV.
Disclosure statement: J.W.G.J. reports financial support from Roche Nederland BV for the study. J.W.J.B. reports grants from Roche, during the conduct of the study and grants from Roche, outside the submitted work. A.P.-S. is an employee of F. Hoffmann-La Roche and reports personal fees from F. Hoffmann-La Roche during the conduct of the study and personal fees from F. Hoffmann-La Roche, outside the submitted work. M.E.A.B. is an employee of Roche Nederland BV. J.M.vL. reports grants from Roche, personal fees from Roche, personal fees from Arthrogen, grants from Thermofisher, personal fees from BMS, grants from MSD, personal fees from Eli Lilly, personal fees from Gesynta, personal fees from Leadiant, personal fees from Arxx Tx, grants from Astra Zeneca, personal fees from Sanofi, outside the submitted work. M.J.H.dH. is currently an employee of Novartis Pharma, Arnhem, The Netherlands. Novartis was not involved in this project. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Smolen JS, Landewé R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 2. St Clair EW, van der Heijde D, Smolen JS. et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 2004;50:3432–43. [DOI] [PubMed] [Google Scholar]
- 3. Quinn MA, Conaghan PG, O’Connor PJ. et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2005;52:27–35. [DOI] [PubMed] [Google Scholar]
- 4. Tak PP, Rigby W, Rubbert-Roth A. et al. Sustained inhibition of progressive joint damage with rituximab plus methotrexate in early active rheumatoid arthritis: 2-year results from the randomised controlled trial IMAGE. Ann Rheum Dis 2012;71:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Detert J, Bastian H, Listing J. et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naïve patients with early rheumatoid arthritis: hIT HARD, an investigator-initiated study. Ann Rheum Dis 2013;72:844–50. [DOI] [PubMed] [Google Scholar]
- 6. Burmester GR, Rigby WF, van Vollenhoven RF. et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis 2016;75:1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF. et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90. [DOI] [PubMed] [Google Scholar]
- 8. Takeuchi T, Yamanaka H, Ishiguro N. et al. Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Ann Rheum Dis 2014;73:536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh JA, Hossain A, Mudano AS. et al. Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta-analysis. Cochrane Database Syst Rev 2017;(5): CD012657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bijlsma JWJ, Welsing PMJ, Woodworth TG. et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016;388:343–55. [DOI] [PubMed] [Google Scholar]
- 11. Quartagno AM, Carpenter J, Quartagno MM. Package “jomo”. 2018.
- 12. Marshall A, Altman DG, Holder RL, Royston P.. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol 2009;9:57.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smolen JS, Aletaha D.. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum 2011;63:43–52. [DOI] [PubMed] [Google Scholar]
- 14. Nishimoto N, Miyasaka N, Yamamoto K. et al. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis 2009;68:1580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kremer JM, Blanco R, Halland A-M. et al. Clinical efficacy and safety maintained up to 5 years in patients with rheumatoid arthritis treated with tocilizumab in a randomised trial. Clin Exp Rheumatol 2016;34:625–33. [PubMed] [Google Scholar]
- 16. Jones G, Wallace T, McIntosh MJ. et al. Five-year efficacy and safety of tocilizumab monotherapy in patients with rheumatoid arthritis who were methotrexate- and biologic-naive or free of methotrexate for 6 months: the AMBITION Study. J Rheumatol 2016;44:3–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.