Abstract
Objectives
To examine pregnancy outcomes among births to women with idiopathic inflammatory myopathy (IIM) in relation to time of IIM diagnosis using population-based data.
Methods
This study used Swedish nationwide registers to identify all singleton births that occurred between 1973 and 2016 among women diagnosed with IIM between 1998 and 2016 and among women unexposed to IIM. We classified births according to the IIM status of the mother at time of delivery: post-IIM (n = 68), 1–3 years pre-IIM (n = 23), >3 years pre-IIM (n = 710) and unexposed to IIM (n = 4101). Multivariate regression models were used to estimate relative risks of adverse pregnancy outcomes in post-IIM births and pre-IIM births separately, in comparison with their non-IIM comparators.
Results
We found that post-IIM births had increased risks of caesarean section [adjusted relative risk (aRR) = 1.98; 95% CI: 1.08, 3.64], preterm birth (aRR = 3.35; 95% CI: 1.28, 8.73) and low birth weight (aRR = 5.69; 95% CI: 1.84, 17.55) compared with non-IIM comparators. We also noticed higher frequencies of caesarean section and instrumental delivery in 1–3 years pre-IIM births than in the non-IIM comparators.
Conclusion
Women who gave birth after IIM diagnosis had higher risks of caesarean section, preterm birth and low birth weight. These results further underline the importance of special care and close monitoring of women with IIM. Higher frequencies of caesarean section and instrumental delivery in pre-IIM births highlight the need for future research on the influence of subclinical features of IIM on pregnancy outcomes.
Keywords: idiopathic inflammatory myopathy, adverse pregnancy outcomes, caesarean section, preterm birth, low birth weight
Rheumatology key messages
Post-IIM births were significantly associated with caesarean section, preterm birth and low birth weight.
Higher frequencies of caesarean section and instrumental delivery were observed in 1–3 years pre-IIM births.
Findings highlighted the importance of multidisciplinary maternal care for women with IIM.
Introduction
Idiopathic inflammatory myopathy (IIM) is a rare rheumatic disease characterized mainly by muscular inflammation and weakness [1]. Women with IIM, especially with active DM and PM during pregnancy, have been considered as a high-risk population, due to reported pregnancy complications [2–6]. Recent studies also report associations between IIM and pregnancy-related hypertension, antepartum haemorrhage, caesarean section and preterm birth. Data are conflicting though, and have not been reproduced using population-based design [7–9].
Risk of adverse pregnancy outcomes may not be limited to patients already diagnosed with rheumatic diseases. Existing research suggests that pregnancies antedating diagnosis of RA, SLE, primary Sjögren’s syndrome or progressive systemic sclerosis are also at greater risk of adverse pregnancy outcomes such as stillbirth, spontaneous abortion, preeclampsia, caesarean section, preterm birth, low birth weight (LBW) and small for gestational age (SGA) [10–14]. However, it is unknown if adverse pregnancy outcomes can precede a diagnosis of IIM as births that occurred in women before IIM diagnosis often have been used as a reference group in previous studies. Given that autoantibodies associated with IIM can present years preceding the diagnosis of the disease [15, 16] and anti-Jo-1 is potentially associated with pregnancy complications [4], we can speculate that women with subclinical or preclinical IIM may also be at higher risk of unfavourable pregnancy outcomes compared with women from the general population. In this study we therefore set out to determine the associations between IIM and a number of adverse pregnancy outcomes in births that occurred before and after IIM diagnosis.
Methods
Data sources
Healthcare in Sweden is mainly tax-funded and is universal to all individuals registered in Sweden [17]. The Swedish National Patient Register (NPR) has collected information on all inpatient visits at public hospitals since 1987 and also holds nationwide data on outpatient non-primary care since 2001. The overall performance of the NPR has been validated, with a coverage that ranges from 85% to 95%, depending on the studied diseases [18]. The Total Population Register (TPR) founded in 1968 has qualified data on nearly 100% of births and deaths in Sweden and is often used to randomly sample comparators in epidemiological research [19]. The Medical Birth Register (MBR) covers >98% of births in Sweden (≥ gestational week 28 before July 2008 or ≥ gestational week 22 since July 2008) from 1973 and collects comprehensive information on mothers and newborns during pregnancy, delivery and neonatal period [20]. Data on demographic characteristics, reproductive issues and major medical complications before pregnancy are reported by women in interviews performed by midwives. Information on smoking history at the first antenatal visit is available from 1982 whereas height and early pregnancy weight were introduced in 1992. Stillbirths with at least 28 or 22 gestational weeks are also recorded in the MBR.
Study population
The NPR was used to identify all women with incident IIM between 1998 and 2016. For 2001–2016, incident IIM was defined as a woman with a first and at least one subsequent visit indicating IIM within 1–12 months after the first visit. A previous validation study shows that this algorithm has a positive predictive value up to 94% and a sensitivity up to 96% [21]. Between 1998 and 2000, all women who were discharged after an IIM indication from inpatient care were also included and no follow-up visit was required as only data on inpatient care were available during that time period. We only considered the International Classification of Diseases, Tenth Revision (ICD-10) codes M33.0, M33.1, M33.2 and M33.9 from internal medicine, rheumatology, dermatology, neurology or paediatrics clinics. Each woman with IIM was matched to up to five women randomly selected from the TPR at the time of diagnosis by birth year and residential area of the patient. Women unexposed to IIM had to be alive and living in Sweden at the time of matching. All singleton births of the women with IIM and the women unexposed to IIM occurring between 1973 and 2016 were ascertained from the MBR. Women who had no birth records in the MBR were excluded, and thereafter the matching was broken.
Births in relation to time of IIM diagnosis
The course of IIM development is generally over years and its impact on mothers and offspring may vary along with the development. We therefore classified all births in women with IIM according to when a birth took place in relation to the time of IIM diagnosis in the mothers, and also to the interval between birth and an IIM diagnosis if a birth occurred before IIM diagnosis: (i) births that occurred in women after, or up to a year before, IIM diagnosis (since the lag-time from the first symptom until diagnosis could be up to a year [22]); (ii) births that occurred in women within 1–3 years before IIM diagnosis; and (iii) births that occurred in women >3 years before IIM diagnosis (since IIM-related autoantibodies can occur ∼3–10 years prior to diagnosis of autoimmune disease [15, 16]). We hereafter use post-IIM to represent group (i) and pre-IIM to represent groups (ii) and (iii).
Outcomes and covariates
Maternal outcomes included hypertensive diseases during pregnancy (preeclampsia, eclampsia or gestational hypertension), caesarean section (elective or acute), instrumental delivery (use of forceps or vacuum extractor) and labour induction. Neonatal outcomes were preterm birth [<37 weeks of gestation, which was further classified into moderate preterm birth (32–37 weeks) and very preterm birth (<32 weeks)], LBW (<2500 g), SGA (<10th percentile), small head circumference (≤31.5 cm), low 5-min Apgar score (<7), congenital malformations at birth and diagnosed within 1 year after birth, neonatal infections at birth, severe paediatric infections that required hospitalization within 1 and 5 years of age, and stillbirth. LBW, SGA and small head circumference were studied as measures of intrauterine growth restriction. Outcomes assessed after birth were identified in the NPR. Maternal characteristics including age at IIM diagnosis (years), birth year (years), country of birth (Nordic or outside Nordic countries), residence (Southern Sweden or Northern Sweden), parity (first birth or not), age at delivery (years), self-reported smoking history (smoker or non-smoker) and BMI (≤18.5, 18.5–25, 25–30, >30 kg/m2) were described. Further, other rheumatic diagnoses before IIM, i.e. RA, SLE or other overlap syndromes in systemic connective tissues diseases, at least one visit of either disease, were identified from the NPR among women of post-IIM births and non-IIM comparators. ICD10 codes are listed in Supplementary Table S1, available at Rheumatology online.
Statistical analyses
Maternal characteristics were compared in strata comprising post-IIM births and pre-IIM births with their corresponding matched non-IIM comparators. Medians with interquartile range (IQR) are presented for continuous variables and frequencies with proportions are shown for categorical variables. Data on smoking history and BMI are not presented for pre-IIM births and their non-IIM comparators as a large proportion of them (36% or 67%) occurred before 1982 or 1992 and therefore lacked this information. Significant statistical differences were tested with the χ2 test, Mann–Whitney U test or Kruskal–Wallis test, depending on the type of variable and the number of comparative groups.
Among post-IIM births, a log-linear model with binomial distribution, an exchangeable covariance matrix and robust sandwich estimator was used to compute the unadjusted relative risks (RRs) for adverse pregnancy outcomes [23]. Correlations among births from the same mother were controlled by clustering analysis. To avoid a convergence problem when doing adjustments in analyses with small sample sizes, the adjusted RRs (aRRs) were estimated by fitting a marginal structural binomial regression model with robust variance and an exchangeable covariance matrix [24]. Inverse probability weights were calculated in a logistic regression model including IIM as the response variable and maternal age at delivery, delivery year, parity (number of children), residence, BMI and smoking as the independent variables. Multiple imputation with five iterations was used to deal with missingness of BMI and smoking, and data were imputed for each outcome. Covariates included in the imputed models were the studied outcome, maternal age at delivery, delivery year, parity (number of children), BMI, smoking, maternal height in centimetres, country of birth, family situation (living with partner or not), education level (≤9 years, 10–12 years, >12 years) and other rheumatic diagnoses before IIM [25]. Information on family situation and education level was ascertained from the MBR and the Swedish Education Register, respectively. Summarized parameter estimates of five sets of imputed data were analysed with the MIANALYZE procedure in SAS, version 9.4 (SAS Institute, Cary, NC, USA). Stillbirths were excluded in the models of outcomes assessed after birth such as congenital malformations within 1 year after birth, severe infant (within 1 year) and paediatric (within 5 years) infections after birth. Furthermore, three additional analyses were performed to examine (i) the impact of other rheumatic diseases before diagnosis of IIM by excluding these conditions from post-IIM births, (ii) the differences in risk estimates between post-IIM births in women identified from the inpatient register and in women identified from the outpatient register, and (iii) the differences in adverse pregnancy outcomes between births that occurred in women within a year before to 3 years after IIM diagnosis (≤3 years post-IIM births) and in births that occurred in women >3 years after IIM diagnosis (>3 years post-IIM births). Only outcomes with ≥5 events in both comparative groups in the main analyses were studied in the additional analyses.
Logistic regression was used to estimate the odds ratios (ORs) of outcomes among births that occurred in women within 1–3 years and >3 years before IIM diagnosis taking non-IIM comparators as the reference group. The controlled variables were the same as the model for post-IIM births, but smoking and BMI were not adjusted for due to lack of information. Moreover, outcomes assessed after birth were not studied for pre-IIM births since most of births took place before the NPR became nationwide.
For outcomes with <5 events, only frequencies with proportions were presented. For labour induction, the analyses only included births that occurred in 1990 and onwards. This study was approved by the regional ethics board (2017/2000-31).
Results
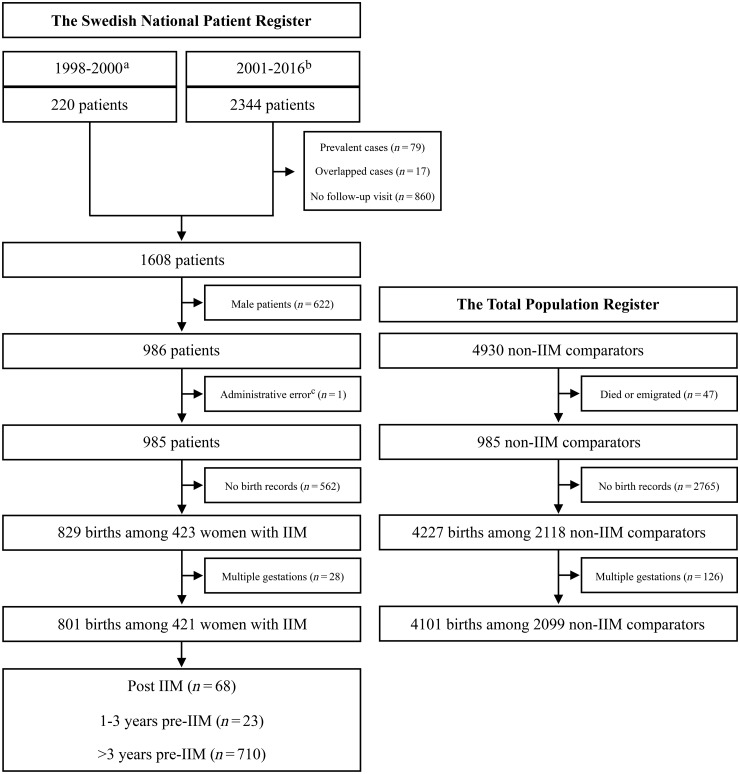
During the study period, we identified 985 women with IIM; 421 (42.74%) of them had history of singleton births, compared with 2099 (42.99%) out of 4883 women unexposed to IIM (Fig. 1). In total, these women had 4902 singleton births (IIM, n = 801, and non-IIM, n = 4101) registered in the MBR. Of the 801 births in women with IIM, 68 were post-IIM and 736 were pre-IIM, corresponding to 236 and 3865 non-IIM comparators, respectively. The median (IQR) age at IIM diagnosis and disease duration at delivery of post-IIM births was 28 (15–29) years and 4.43 (1.69–9.48) years, respectively. Table 1 presents the maternal characteristics of births after and before IIM diagnosis and for the non-IIM comparators. Women who delivered after IIM diagnosis were born in more recent years [1981 (IQR 1975–1988) vs 1975 (IQR 1972–1981), P < 0.001], and had higher prevalence of other rheumatic diagnoses (20.59% vs 0%, P < 0.001). They were also less likely to smoke (4.41% vs 9.50%, P = 0.17) and were more likely to be overweight (30 ≥ BMI > 25, 23.53% vs 19.42%, P = 0.63) compared with non-IIM comparators although this did not reach statistical significance. Among pre-IIM births, the closer the IIM diagnosis was after delivery, the later the mother was born and the older the mother was at delivery. For other characteristics, no significant differences were found between post-IIM and pre-IIM births and their matched non-IIM comparators.
Fig. 1.
Flow chart of the study population
Linkage to the Swedish Medical Birth Register for birth history in women with IIM identified from the National Patient Register and women unexposed to IIM randomly sampled from the Total Population Register. aA hospitalization indicating incident IIM. bDiagnosis of incident IIM with at least one follow-up visit within 1–12 months after the first IIM indication. cAdministrative error; comparators were kept in the study. IIM: idiopathic inflammatory myopathies.
Table 1.
Maternal characteristics of births in women that occurred after and before IIM diagnosis
| Variable | After IIM diagnosis |
Before IIM diagnosis |
|||||
|---|---|---|---|---|---|---|---|
| Post-IIM (n = 68) | Non-IIM (n = 242) | P a | 1–3 years pre-IIM (n = 23) | >3 years pre-IIM (n = 710) | Non-IIM (n = 3859) | P b | |
| Age at diagnosis, median (IQR), years | 28 (15–29) | — | — | 33 (29–36) | 52 (46–60) | — | — |
| Maternal birth year, median (IQR) | 1981 (1975–1988) | 1975 (1972–1981) | <0.001 | 1974 (1970–1977) | 1955 (1950–1963) | 1956 (1951–1965) | <0.001 |
| Maternal country of birth, Nordic, n (%) | 59 (86.76) | 209 (86.36) | 0.93 | 18 (78.26) | 639 (90.00) | 3524 (91.32) | 0.15 |
| Maternal country of birth missing | 0 | 0 | — | 0 | 2 (0.28) | 14 (0.36) | 0.91 |
| Living in Southern Sweden, n (%) | 61 (89.71) | 230 (95.04) | 0.11 | 21 (91.30) | 614 (86.48) | 3260 (84.48) | 0.27 |
| Other rheumatic diagnosesc, n (%) | 14 (20.59) | 0 | <0.001 | — | — | — | — |
| First births, n (%) | 28 (41.18) | 116 (47.93) | 0.32 | 10 (43.48) | 279 (39.30) | 1489 (38.59) | 0.84 |
| Age at delivery, median (IQR), years | 30 (25–34) | 28 (25–32) | 0.27 | 31 (26–33) | 28 (25–32) | 29 (25–33) | <0.001 |
| Smoking in early pregnancyd, n (%) | |||||||
| Smoker | 3 (4.41) | 23 (9.50) | 0.17 | — | — | — | — |
| Non-smoker | 62 (91.18) | 206 (85.12) | — | — | — | — | |
| Smoking missing | 3 (4.41) | 13 (5.37) | 0.75 | — | — | — | — |
| Maternal BMI in early pregnancyd, kg/cm2, n (%) | |||||||
| ≤18.5 | 1 (1.47) | 2 (0.83) | 0.63 | — | — | — | — |
| 18.5–25 | 40 (58.82) | 138 (57.02) | — | — | — | — | |
| 25–30 | 16 (23.53) | 47 (19.42) | — | — | — | — | |
| >30 | 6 (8.82) | 21 (8.68) | — | — | — | — | |
| Maternal BMI missing | 5 (7.35) | 34 (14.05) | 0.14 | — | — | — | — |
Comparing post-IIM deliveries to their comparators; P from χ2 test for categorical variable and Mann–Whitney U test for continuous variable.
Comparing pre-IIM deliveries in relation to time of IIM diagnosis to their comparators; P from χ2 test for categorical variable and Kruskal–Wallis test for continuous variable.
Including (at least one visit in the National Patient Register) RA (18.85% in post-IIM births), SLE (3.08% in post-IIM births) and other overlap syndromes in systemic connective tissues diseases (12.31% in post-IIM births).
Information on smoking and BMI was available in 1982 and onwards. IIM: idiopathic inflammatory myopathies; IQR: interquartile range.
Table 2 shows the RRs of adverse pregnancy outcomes in births that occurred in women after IIM diagnosis compared with non-IIM comparators. Post-IIM births were at significantly higher risk of caesarean section (aRR = 1.98; 95% CI: 1.08, 3.64), preterm birth (aRR = 3.35; 95% CI: 1.28, 8.73) and LBW (aRR = 5.69; 95% CI: 1.84, 17.55). Moreover, very preterm birth (5.88% vs 0.83%, P = 0.01) and moderate preterm birth (8.82% vs 4.55%, P = 0.17) were more common in post-IIM births compared with non-IIM comparators. There were no differences between post-IIM births and the non-IIM comparators in risks of instrumental delivery, labour induction, or infant and paediatric infections. Due to few outcomes we were not able to assess RRs of preeclampsia, small head circumference, low 5-min Apgar score, congenital malformations, neonatal infections at birth and stillbirth in post-IIM births. In the analyses including post-IIM births that were unaffected by other rheumatic diagnoses vs their non-IIM comparators, stronger associations were detected for caesarean section (aRR = 2.13; 95% CI: 1.17, 3.90), preterm birth (aRR = 4.27; 95% CI: 1.75, 10.41) and LBW (aRR = 7.31; 95% CI: 2.55, 20.93) than those found in the main analyses (Supplementary Table S2, available at Rheumatology online). We observed no significant differences in risk estimates for births of women with IIM retrieved from the inpatient register vs the outpatient register (Supplementary Tables S3 and S4, available at Rheumatology online). For the occurrences of adverse pregnancy outcomes regarding time of delivery after IIM diagnosis, a higher proportion of caesarean section (34.48% vs 15.38%) was observed in ≤3 years post-IIM births than in >3 years post-IIM births, where severe infant (3.45% vs 12.82%) and paediatric (6.90% vs 17.95%) infections were more likely to occur (Supplementary Table S5, available at Rheumatology online).
Table 2.
The RRs of adverse pregnancy outcomes in post-IIM births
| Outcome | Post-IIM, n (%) | Non-IIM, n (%) | Unadjusted RR (95% CI) | Adjusted RR (95% CI)a |
|---|---|---|---|---|
| Hypertensive diseases during pregnancy | 2 (2.94) | 11 (4.55) | — | — |
| Caesarean section | 16 (23.53) | 43 (17.77) | 1.42 (0.79, 2.58) | 1.98 (1.08, 3.64) |
| Instrumental delivery | 5 (7.35) | 14 (5.79) | 1.24 (0.47, 3.25) | 1.12 (0.39, 3.18) |
| Labour inductionb | 7 (10.29) | 34 (14.47) | 0.80 (0.36, 1.74) | 0.63 (0.27, 1.46) |
| Preterm birth | 10 (14.71) | 13 (5.37) | 2.67 (1.12, 6.33) | 3.35 (1.28, 8.73) |
| Low birth weightc | 8 (11.76) | 6 (2.49) | 4.73 (1.68, 13.31) | 5.69 (1.84, 17.55) |
| Small for gestational agec | 7 (10.29) | 20 (8.30) | 1.07 (0.43, 2.68) | 0.90 (0.35, 2.33) |
| Small head circumferenced | 3 (4.69) | 9 (3.88) | — | — |
| Low 5-min Apgar scoree | — | 3 (1.25) | — | — |
| Stillbirth | — | 1 (0.41) | — | — |
| Congenital malformations at birth | — | 5 (2.07) | — | — |
| Congenital malformations within 1 year after birthf | 1 (1.47) | 9 (3.77) | — | — |
| Neonatal infections at birth | — | — | — | — |
| Severe infant infections within 1 year after birthf | 6 (8.82) | 16 (6.69) | 1.35 (0.54, 3.37) | 1.32 (0.40, 4.32) |
| Severe paediatric infections within 5 years after birthf | 9 (13.24) | 21 (8.79) | 1.36 (0.56, 3.30) | 1.28 (0.46, 3.58) |
Adjusted for residential area (Southern Sweden or Northern Sweden), maternal age at delivery, parity, delivery year, smoking (smoker or non-smoker) and BMI (≤18.5, 18.5–25, 25–30, >30).
Analysis including 68 post-IIM births and 235 births in non-IIM comparators that occurred in 1990 and onwards.
The percentages of missing low birth weight and small for gestational age for the non-IIM comparators were 0.41%.
The percentages of missing small head circumference for post-IIM births and the non-IIM comparators were 5.88% and 4.13%, respectively.
The percentages of missing low 5-min Apgar score for post-IIM births and the non-IIM comparators were 1.47% and 0.83%, respectively.
The percentages of missing congenital malformation within 1 year after birth, severe infant infections within 1 year after birth and severe paediatric infections within 5 years after birth for the non-IIM comparators were 1.24%. IIM: idiopathic inflammatory myopathy; RR: relative risk.
Compared with non-IIM comparators, births that occurred in women within 1–3 years before IIM diagnosis had higher frequencies of caesarean section (17.39% vs 11.17%) and instrumental delivery (17.39% vs 5.96%) although without significant differences (Table 3). There was one stillbirth in pre-IIM births that occurred within 1–3 years before IIM diagnosis, resulting in a proportion of 4.35% vs 0.41% of the non-IIM comparators. In pre-IIM births that occurred >3 years before IIM diagnosis, significant increased odds of caesarean section [adjusted OR (aOR) = 1.32; 95% CI 1.03–1.68], labour induction (aOR = 1.59; 95% CI: 1.04, 2.43), LBW (aOR = 1.66; 95% CI: 1.14, 2.41) and SGA (aOR = 1.55; 95% CI: 1.25–1.93) were found.
Table 3.
The aORs of adverse pregnancy outcomes in pre-IIM births
| Outcome | 1–3 years pre-IIM (n = 23) |
>3 years pre-IIM (n = 710) |
Non-IIM comparators (n = 3859) | ||
|---|---|---|---|---|---|
| n (%) | aOR (95% CI)a | n (%) | aOR (95% CI)a | n (%) | |
| Hypertensive diseases during pregnancy | — | — | 20 (2.82) | 1.60 (0.97, 2.66) | 81 (2.10) |
| Caesarean section | 4 (17.39) | — | 92 (12.96) | 1.32 (1.03, 1.68) | 431 (11.17) |
| Instrumental delivery | 4 (17.39) | — | 45 (6.34) | 1.10 (0.78, 1.54) | 230 (5.96) |
| Labour inductionb | 2 (8.70) | — | 31 (12.55) | 1.59 (1.04, 2.43) | 149 (9.78) |
| Preterm birthc | 1 (4.35) | — | 38 (5.37) | 1.13 (0.79, 1.62) | 188 (4.88) |
| Low birth weightd | 1 (4.35) | — | 38 (5.37) | 1.66 (1.14, 2.41) | 127 (3.30) |
| Small for gestational agee | 3 (13.04) | — | 129 (18.27) | 1.55 (1.25, 1.93) | 467 (12.15) |
| Small head circumferencef | 1 (4.35) | — | 26 (3.77) | 1.31 (0.85, 2.04) | 105 (2.81) |
| Low 5-min Apgar scoreg | — | — | 5 (0.79) | 0.74 (0.29-1.89) | 36 (1.03) |
| Stillbirth | 1 (4.35) | — | 5 (0.70) | 1.64 (0.60, 4.50) | 16 (0.41) |
| Congenital malformation at birth | — | — | 14 (1.97) | 0.85 (0.48, 1.51) | 83 (2.15) |
| Neonatal infections at birth | — | — | 7 (0.99) | 0.69 (0.31, 1.54) | 51 (1.32) |
Adjusted for residential area (Southern Sweden or Northern Sweden), maternal age at delivery, parity and delivery year.
Analysis including 270 pre-IIM births and 1523 births in non-IIM comparators that occurred in 1990 and onwards.
The percentages of missing preterm birth for >3 years pre-IIM births and the non-IIM comparators were 0.28% and 0.23%, respectively.
The percentages of missing low birth weight for >3 years pre-IIM births and the non-IIM comparators were 0.28% and 0.18%, respectively.
The percentages of missing small for gestational age for >3 years pre-IIM births and the non-IIM comparators were 0.56% and 0.41%, respectively.
The percentages of missing small head circumference for >3 years pre-IIM births and the non-IIM comparators were 2.96% and 3.06%, respectively.
The percentages of missing low 5-min Apgar score for 8 months to 3 years pre-IIM births, >3 years pre-IIM births and the non-IIM comparators were 4.35%, 11.27% and 9.59%, respectively. aOR: adjusted odds ratio; IIM: idiopathic inflammatory myopathies.
Discussion
In this population-based study, births that occurred in women after IIM diagnosis had an about 3-fold risk of preterm birth (very preterm birth in particular) and a 6-fold risk of LBW, as well as a doubled risk of caesarean section, compared with non-IIM comparators. There were also increased frequencies of certain adverse pregnancy outcomes including caesarean section and instrumental delivery in births that occurred in women within 1–3 years before IIM diagnosis.
Preterm birth, caesarean section and intrauterine growth restriction are the major adverse pregnancy outcomes previously reported in births to women with an IIM diagnosis [26]. An Australian study including 17 births in 13 women with DM/PM found a 5-fold risk (aRR = 5.48; 95% CI: 2.46, 12.2) of preterm birth and a 3-fold risk of caesarean section (aRR = 3.12; 95% CI: 2.46, 3.96) in women with DM/PM compared with women from the general population [7]. Yet, the risks might be overestimated as the study required hospitalization for IIM, which might lead to selection of women with high disease activity/severity in that study. In several small scale clinic-based studies and in a review, higher risk of preterm birth was associated with active disease status [2, 4, 5, 27]. We identified women with IIM from both inpatient and outpatient clinics, and therefore disease activity may have varied from mild to severe, which might explain the difference between our findings and the Australian study although our study did not find any different risk estimates among births to women with IIM identified from the inpatient vs the outpatient register. Besides, Kolstad et al. recently found that the risk of early preterm birth (7.9% vs 1.0%, aRR = 10.0; 95% CI: 3.2, 31.1) was particularly high in 38 births of women with DM/PM compared with controls [9], which is in line with our findings. Two previous studies including women with IIM prior to pregnancy also observed lower mean birth weight among cases with active IIM compared with cases with inactive IIM [4, 28].
To our knowledge, risk of adverse pregnancy outcomes in births that occurred in women before IIM diagnosis remains unexplored and this group often acts as a negative control in non-population-based studies. In our study, women who gave birth within 1–3 years before IIM diagnosis were more likely to have caesarean section and instrumental delivery than women of non-IIM comparators, although cases were too few to make any inferences. An American study analysing births that occurred before or after IIM diagnosis together found a modestly higher proportion of caesarean section in these births vs controls (26% vs 22.5%) [8]. However, since births that occurred before or after IIM diagnosis were analysed together, the risk of caesarean section before IIM diagnosis could not be differentiated from that of after IIM diagnosis. Pinal-Fernandez et al. reported that the frequencies of caesarean section was 6% in women who delivered before IIM onset vs 18.2% in women who delivered after IIM onset, and no clear implication in risk of caesarean section before IIM diagnosis could be made since there was a lack of negative controls [29]. Although there has been little discussion about pre-IIM births and adverse pregnancy outcomes, it is interesting to compare our findings with that presented in a Swedish study in which deliveries that occurred in women within 2–5 years before SLE diagnosis were more likely to have maternal and birth complications including caesarean section and SGA than controls [12]. Moreover, our findings generally corroborated the comment of a review article concluding that pregnancies with symptoms of rheumatic disease that did not fulfil the criteria for a definite diagnosis had lower risk of pregnancy complications than women who were already diagnosed with rheumatic diseases but higher than in healthy controls [14].
Significant higher odds of SGA, as well as caesarean section, labour induction and LBW, were only detected in births that occurred >3 years before IIM diagnosis after adjusting for potential confounders such as maternal age and delivery year. However, given that the majority of births occurred >10 years before IIM diagnosis, these results need to be interpreted with caution. Other factors irrelevant to IIM such as unmeasured confounders or calendar period effects might also affect the associations.
Little is currently known about the biological basis of the association between IIM and adverse pregnancy outcomes. A systemic review of 78 pregnancies among 59 women with DM/PM/juvenile IIM found that active disease status and disease onset in early pregnancy were particularly associated with pregnancy complications [26]. Other characteristics such as presence of anti-synthetase antibodies, massive perivillous fibrin deposition and elevated level of creatine kinase have also been reported [3, 4, 26, 30, 31]. This information could probably fit the generally proposed mechanism in other relevant studies where endothelial dysfunction due to impaired autoimmunity may lead to abnormal placentation and eventually result in adverse pregnancy outcomes [8, 13, 14, 32]. This suggested pathogenesis may also explain the higher frequencies of adverse pregnancy outcomes among pre-IIM births that occurred close to IIM diagnosis given that autoimmune impact on pregnancy may precede a definite diagnosis [15, 16]. According to the frequency analysis of adverse pregnancy outcomes between two groups of post-IIM births, disease duration of IIM appeared to have an impact on caesarean section and severe offspring infections. However, this finding should be interpreted with caution given that it was based on few cases and without consideration of other factors like maternal age, autoimmunity in pregnancy and treatment effect.
This study has several strengths. First it is to our knowledge the largest population-based study investigating the association between IIM and adverse pregnancy outcomes. Moreover, comprehensive data on maternal characteristics were prospectively collected in the MBR, thereby minimizing recall bias and enabling adjustment of numerous important confounders. By using nationwide data and a validated algorithm to identify women with IIM, the results of our study also have high generalizability. Further the classification of pre-IIM births made it possible to examine if the risks of adverse pregnancy outcomes varied in relation to time of IIM diagnosis.
As for study limitations, the present study included only a small number of births that occurred in women after IIM diagnosis, precluding us from analysing some relevant maternal and neonatal outcomes, such as preeclampsia and low 5-min Apgar score. Secondly, some potential confounders such as chronic hypertension and pre-existing diabetes were not adjusted for in the analyses since information on these factors in the MBR was poorly reported [20]. Moreover, neonatal diagnoses might have lower ascertainment than other outcomes due to less complete reporting of neonatal diagnoses for infants referred to a neonatal ward [20]. This might affect the proportions of outcomes presented in our study, but it had little impact on the risk estimation as the proportions of missing outcomes presumably were similar in the IIM and in the non-IIM births. Thirdly, this is a register-based study and therefore we lacked information on clinical presentation of IIM-like disease activity and autoantibody profile, which is particularly important when we want to examine the pathology of pregnancy risk in women with IIM. Moreover, we also lacked information on treatments targeting IIM, which precluded us from studying the treatment effect on pregnancy outcomes. Yet, these factors are less likely to affect the overall interpretation in our study as they would only moderate the associations between post-IIM births and adverse pregnancy outcomes. Lastly, in order to increase statistical power, we did not exclude women with other rheumatic diagnoses before IIM from the regression analyses of post-IIM births. However, sensitivity analyses excluding births in women with other rheumatic diagnoses before IIM showed even stronger associations of IIM with caesarean section, preterm birth and LBW.
In summary, we found that births in women diagnosed with IIM were associated with increased risks of caesarean section, preterm birth and LBW compared with births in women of the general population. These findings further emphasize the importance of multidisciplinary maternal care for women with IIM. Although higher frequencies of caesarean section and instrumental delivery were observed in pre-IIM births that occurred in women within 1–3 years before IIM diagnosis, this evidence was inadequate to make any suggestion on clinical practice but highlights the need for future research on the potential influence that early disease phase with pro-inflammation and symptoms of preclinical IIM may have on pregnancy.
Supplementary Material
Acknowledgement
We thank Dr Jonas Söderling for giving valuable advice on statistical analyses during the study.
Funding: This work was supported by the Nanna Svartz Foundation; the Swedish Rheumatism Association; by grants provided by the Stockholm County Council (ALF project) and King Gustaf V 80 years Foundation.
Disclosure statement: IL has received research grants from Bristol Myers Squibb and Astra Zeneca; and is a scientific advisor for aTyr and Corbus Pharmaceuticals. The other authors declare no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Miller FW, Lamb JA, Schmidt J, Nagaraju K.. Risk factors and disease mechanisms in myositis. Nat Rev Rheumatol 2018;14:255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gutierrez G, Dagnino R, Mintz G.. Polymyositis/dermatomyositis and pregnancy. Arthritis Rheum 1984;27:291–4. [DOI] [PubMed] [Google Scholar]
- 3. Silva CA, Sultan SM, Isenberg DA.. Pregnancy outcome in adult-onset idiopathic inflammatory myopathy. Rheumatology (Oxford) 2003;42:1168–72. [DOI] [PubMed] [Google Scholar]
- 4. Nagy-Vincze M, Vencovsky J, Lundberg IE, Danko K.. Pregnancy outcome in idiopathic inflammatory myopathy patients in a multicenter study. J Rheumatol 2014;41:2492–4. [DOI] [PubMed] [Google Scholar]
- 5. Zhong Z, Lin F, Yang J. et al. Pregnancy in polymyositis or dermatomyositis: retrospective results from a tertiary centre in China. Rheumatology (Oxford) 2017;56:1272–5. [DOI] [PubMed] [Google Scholar]
- 6. Gupta L, Zanwar A, Ahmed S, Aggarwal A.. Outcomes of pregnancy in women with inflammatory myositis. J Clin Rheumatol 2019; doi: 10.1097/RHU.0000000000000996. [DOI] [PubMed] [Google Scholar]
- 7. Chen JS, Roberts CL, Simpson JM, March LM.. Pregnancy outcomes in women with rare autoimmune diseases. Arthritis Rheumatol 2015;67:3314–23. [DOI] [PubMed] [Google Scholar]
- 8. Kolstad KD, Fiorentino D, Li S, Chakravarty EF, Chung L.. Pregnancy outcomes in adult patients with dermatomyositis and polymyositis. Semin Arthritis Rheum 2018;47:865–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kolstad KD, Mayo JA, Chung L. et al. Preterm birth phenotypes in women with autoimmune rheumatic diseases: a population-based cohort study. BJOG 2020;127:70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siamopoulou-Mavridou A, Manoussakis MN, Mavridis AK, Moutsopoulos HM.. Outcome of pregnancy in patients with autoimmune rheumatic disease before the disease onset. Ann Rheum Dis 1988;47:982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dhar JP, Essenmacher LM, Ager JW, Sokol RJ.. Pregnancy outcomes before and after a diagnosis of systemic lupus erythematosus. Am J Obstet Gynecol 2005;193:1444–55. [DOI] [PubMed] [Google Scholar]
- 12. Arkema EV, Palmsten K, Sjowall C. et al. What to expect when expecting with systemic lupus erythematosus (SLE): a population-based study of maternal and fetal outcomes in SLE and Pre-SLE. Arthritis Care Res (Hoboken) 2016;68:988–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spinillo A, Beneventi F, Locatelli E. et al. Early, incomplete, or preclinical autoimmune systemic rheumatic diseases and pregnancy outcome. Arthritis Rheumatol 2016;68:2555–62. [DOI] [PubMed] [Google Scholar]
- 14. Spinillo A, Beneventi F, Caporali R, Ramoni V, Montecucco C.. Undifferentiated connective tissue diseases and adverse pregnancy outcomes. An undervalued association? Am J Reprod Immunol 2017;78:e12762.. [DOI] [PubMed] [Google Scholar]
- 15. Eriksson C, Kokkonen H, Johansson M. et al. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther 2011;13:R30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arbuckle MR, McClain MT, Rubertone MV. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–33. [DOI] [PubMed] [Google Scholar]
- 17. Anell A, Glenngard AH, Merkur S.. Sweden health system review. Health Syst Transit 2012;14:1–159. [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Andersson E, Ekbom A. et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ludvigsson JF, Almqvist C, Bonamy AK. et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125–36. [DOI] [PubMed] [Google Scholar]
- 20. Bengt K, Karin K.. The Swedish medical birth register—a summary of content and quality. Stockholm: Socialstyrelsen, 2003. [Google Scholar]
- 21. Svensson J, Arkema EV, Lundberg IE, Holmqvist M.. Incidence and prevalence of idiopathic inflammatory myopathies in Sweden: a nationwide population-based study. Rheumatology (Oxford) 2017;56:802–10. [DOI] [PubMed] [Google Scholar]
- 22. Wilson FC, Ytterberg SR, St Sauver JL, Reed AM.. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J Rheumatol 2008;35:445–7. [PubMed] [Google Scholar]
- 23. Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol 1986;123:174–84. [DOI] [PubMed] [Google Scholar]
- 24. Richardson DB, Kinlaw AC, MacLehose RF, Cole SR.. Standardized binomial models for risk or prevalence ratios and differences. Int J Epidemiol 2015;44:1660–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. White IR, Royston P, Wood AM.. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- 26. Di Martino SJ. Myositis and pregnancy In: Bermas BL, Sammaritano LR, eds. Contraception and pregnancy in patients with rheumatic disease. New York: Springer, 2014, 185–97. [Google Scholar]
- 27. Akalin T, Akkaya H, Buke B, Kocak I.. A case of new-onset dermatomyositis in the second trimester of pregnancy: a case report and review of the literature. Case Rep Obstet Gynecol 2016;2016:6430156.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Váncsa A, Ponyi A, Constantin T, Zeher M, Dankó K.. Pregnancy outcome in idiopathic inflammatory myopathy. Rheumatol Int 2007;27:435–9. [DOI] [PubMed] [Google Scholar]
- 29. Pinal-Fernandez I, Selva-O'Callaghan A, Fernandez-Codina A. et al. “Pregnancy in adult-onset idiopathic inflammatory myopathy”: Report from a cohort of myositis patients from a single center. Semin Arthritis Rheum 2014;44:234–40. [DOI] [PubMed] [Google Scholar]
- 30. Al-Adnani M, Kiho L, Scheimberg I.. Recurrent placental massive perivillous fibrin deposition associated with polymyositis: a case report and review of the literature. Pediatr Dev Pathol 2008;11:226–9. [DOI] [PubMed] [Google Scholar]
- 31. Hung NA, Jackson C, Nicholson M, Highton J.. Pregnancy-related polymyositis and massive perivillous fibrin deposition in the placenta: are they pathogenetically related? Arthritis Rheum 2006;55:154–6. [DOI] [PubMed] [Google Scholar]
- 32. Ostensen M, Brucato A, Carp H. et al. Pregnancy and reproduction in autoimmune rheumatic diseases. Rheumatology (Oxford) 2011;50:657–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.