Abstract
Objectives
Incomplete SLE (iSLE) patients display symptoms typical for SLE but have insufficient criteria to fulfil the diagnosis. Biomarkers are needed to identify iSLE patients that will progress to SLE. IFN type I activation, B-cell-activating factor (BAFF) and B-cell subset distortions play an important role in the pathogenesis of SLE. The aim of this cross-sectional study was to investigate whether B-cell subsets are altered in iSLE patients, and whether these alterations correlate with IFN scores and BAFF levels.
Methods
iSLE patients (n = 34), SLE patients (n = 41) with quiescent disease (SLEDAI ≤4) and healthy controls (n = 22) were included. Proportions of B-cell subsets were measured with flow cytometry, IFN scores with RT-PCR and BAFF levels with ELISA.
Results
Proportions of age-associated B-cells were elevated in iSLE patients compared with healthy controls and correlated with IgG levels. In iSLE patients, IFN scores and BAFF levels were significantly increased compared with healthy controls. Also, IFN scores correlated with proportions of switched memory B-cells, plasma cells and IgG levels, and correlated negatively with complement levels in iSLE patients.
Conclusion
In this cross-sectional study, distortions in B-cell subsets were observed in iSLE patients and were correlated with IFN scores and IgG levels. Since these factors play an important role in the pathogenesis of SLE, iSLE patients with these distortions, high IFN scores, and high levels of IgG and BAFF may be at risk for progression to SLE.
Keywords: incomplete systemic lupus erythematosus, B-cell subsets, switched memory B-cells, age-associated B-cells, interferon, BAFF
Rheumatology key messages
Age-associated B-cells are elevated in incomplete SLE and correlate with IgG levels.
IFN scores and B-cell-activating factor levels are elevated in incomplete SLE patients.
In incomplete SLE, proportions of switched memory B-cells, plasma cells and autoantibodies correlate with IFN scores.
Introduction
SLE is a systemic, chronic autoimmune disease with a heterogenic clinical picture comprising a wide range of symptoms. Symptoms typically present in a relapsing–remitting pattern [1]. Given the heterogeneous nature of the disease, it is difficult to diagnose SLE. Currently, diagnosis is based on a combination of clinical and serological hallmarks, summarized in the SLICC criteria, ACR criteria and the newly formulated ACR-EULAR criteria [2–4]. Some patients with clinical symptoms and autoantibodies suggestive of lupus do not classify as having SLE. This state of disease is referred to as incomplete SLE (iSLE) [5]. This comprises a very heterogeneous group of patients, of which 20–57% will eventually develop SLE [6].
It is unclear which factors determine progression from iSLE to SLE and therefore, which iSLE patients will develop SLE. Ideally, in the future biomarkers will indicate which patients may progress from iSLE to SLE and thereby help us understand and intervene in the pathogenesis of SLE. The pathogenesis of SLE is highly complex, involving both the innate and adaptive immune system [1]. IFN—mainly type I—seems to play a key role in the pathogenesis of SLE [7, 8]. Type I IFN serves as an immune system primer that can help initiate both innate and adaptive immune processes [9, 10]. Currently, gene expression of several IFN response genes combined in an IFN score is used as a surrogate marker to quantify the amount of IFN, as IFN measurement itself is not easily applicable [11].
Type I IFN can affect B-cell activation and can prolong B-cell survival via B-cell-activating factor (BAFF), which prevents apoptosis of B-cells [8, 12]. BAFF is elevated in patients with SLE and also a target of belimumab, a novel mAb that is indicated in SLE patients who are autoantibody-positive and have active disease. Belimumab induces elimination of B-cells by binding to BAFF and thereby limits disease flares in SLE patients [13]. B-cells are central to the pathogenesis of SLE by production of autoreactive antibodies, most importantly ANAs. B-cells can also stimulate production of IFN by plasmacytoid dendritic cells, which results in a vicious cycle of autoimmune activation [14]. Several abnormalities in subsets of circulating B-cells have been described in SLE. For example, SLE patients have higher numbers of circulating plasma blasts and plasma cells compared with healthy controls (HCs) [15]. Switched memory B-cells (IgM−, IgD−, CD27+) also have effector functions, among which is the production of (auto)antibodies, and are related to disease activity in SLE [15]. Additionally, age-associated B-cells (ABCs), also known as atypical B-cells and characterized by CD19+CD21lowCD11c+ phenotype, have been found to be elevated in SLE [16–18]. Also, ABCs express the transcription factor T-bet [16]. It has been shown that T-bet/CD11+ cells can differentiate into plasma cells [19]. T-bet can be activated by triggering B-cell receptor, IFN and Toll-like receptor 7 (TLR7), leading to an increased (auto)antibody production [16, 20].
In short, distortion of B-cell subset proportions, and increased IFN scores and BAFF levels are central to the pathogenesis of SLE. We hypothesize that high IFN scores and BAFF levels promote B-cell activation and survival, leading to alterations in proportions of B-cell subsets, and thereby determine progression from iSLE to SLE. Understanding the role of B-cell subsets, IFN and BAFF might help to unravel the pathogenesis of SLE. To this end, we assessed the distribution of circulating B-cell subsets, IFN scores and serum BAFF levels in iSLE patients, SLE patients with quiescent disease (SLEDAI ≤4) and HCs.
Methods
Study population
Participants were eligible for the iSLE group if they had ANA titre of >1:80, disease duration of <5 years and at least one ACR or SLICC criterion, but not meeting ACR or SLICC criteria for SLE. Patients with iSLE who used immunosuppressive drugs, except for NSAIDs and HCQ, were excluded. As a positive control group, SLE patients with quiescent disease, i.e. SLEDAI of ≤4, were included to allow comparison with iSLE patients. SLE patients had to meet the ACR and/or SLICC criteria and had a maximum disease duration of 10 years. HCs were included if they did not have any history of autoimmune disease, no infection at time of inclusion and did not use immunosuppressive drugs.
Every participant gave informed consent before the start of the study. The study protocol was in line with the guidelines of the 1975 Declaration of Helsinki and has been approved by the medical ethics committee of the University Medical Centre Groningen (METc2015/313).
General sample processing
Blood was drawn in lithium heparin tubes and peripheral blood mononuclear cells were isolated by Ficoll-Paque density gradient centrifugation. These isolated cells were stored in liquid nitrogen until further processing. Serum samples for autoantibody measurement were separated from clotted blood by centrifugation and stored at −20°C. Additionally, blood was drawn into PaxGene tubes and stored at −80°C after 2 h storage at room temperature.
Analysis of B-cell subsets
Stored peripheral blood mononuclear cells were thawed in RPMI containing 10% fetal calf serum. mAbs were added to tubes containing 1mio cells according to the manufacturer’s instructions. After 30 min incubation at 4°C, samples were washed with cold PBS and eBioscience Fix/Perm Buffer (ThermoFisher Scientific, Waltham, MA, USA) was added. After 45 min of incubation at 4°C, samples were washed again with PBS. After addition of mouse serum, intracellular staining markers and isotype controls were added. Subsequently, cells were washed twice and resuspended in 350 μl PBS.
Flowcytometric measurements were performed on the LSR II flow cytometer (BD Biosciences, San Jose, CA, USA). Spectral overlap was corrected for with bead isotypes. Analysis and gating were performed with Kaluza Analysis Software 2.1 (see supplementary Fig. S1, available at Rheumatology online, for gating strategy). Standard gates were optically set based on cell populations of samples of 10 HCs. These standard gates were then applied to all samples. To correct for batch effects, every sample was checked individually, and the gates adapted in a standardized manner if necessary. Results are expressed as proportion of total B-cells. Absolute numbers of different B-cell subsets were calculated based on the standard lymphocyte counts.
Analysis of autoantibodies
Anti-dsDNA autoantibodies and other autoantibodies were measured by fluorescence enzyme immunoassay using the automated EliA assay (ThermoFisher Scientific, Nieuwegein, The Netherlands).
IFN score measurement
IFN scores were measured by RT-PCR. An IFN score based on the following genes was calculated: IFI44L, LY6E and MX1. These genes are most commonly used in literature and are representative for IFN type I expression [11]. First, RNA was isolated from whole blood stored in PaxGene tubes, with a kit from Qiagen (Cat No./ID: 762164). Second, RNA was converted to DNA and consecutively, expression of several IFN genes was measured by validated TaqMan assays. Results were expressed in relative expression (RE), calculated according to the formula: RE = 2−(CtTest gene−Ct GAPDH). In this formula, Ct represents the cycle threshold and glyceraldehyde 3-phosphate dehydrogenase the reference housekeeping gene. RE values were converted to logarithmic values and log(RE) values were translated to IFN scores by using the following formula: ∑(REsubject − MeanHC)/SDHC.
Based on our preliminary findings, we split iSLE patients in two subgroups for comparative analysis. This was based on high IFN scores with the median as cut-off. We compared proportions of B-cell subsets, BAFF levels and serological disease markers.
BAFF
Levels of BAFF were measured by ELISA (Duoset, R&D Systems, Minneapolis, MN, USA). High performance ELISA buffer (Sanquin, Amsterdam, The Netherlands) was used during serum incubation to prevent non-specific reactions. BAFF levels were considered elevated if they were higher than +2 s.d. above the mean of the HC group (248 pg/ml).
Statistical analysis
Non-parametric data are reported as median and interquartile range. Kruskal–Wallis tests were used for non-parametric, continuous data at group level. In case of significance, Mann–Whitney U test was used for pairwise comparisons. Correlations between B-cell subsets, IFN scores and BAFF were assessed using Spearman’s correlation.
All statistical tests were performed in IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA) and data were plotted in GraphPad Prism for Windows version 8.2.1 (GraphPad Software, Lo Jolla, CA, USA). Two-sided P < 0.05 were considered statistically significant.
Results
Demographics
A total number of 97 participants was included, of which 34 classified as having iSLE and 41 as having SLE. The age- and gender-matched HC group consisted of 22 participants (Table 1). Between groups, age was similar, and most participants were female and Caucasian.
Table 1.
Demographics, disease characteristics and medication use
| Characteristics | HC (n = 22) | iSLE (n = 34) | SLE (n = 41) |
|---|---|---|---|
| Age, years | 45 (28–58) | 45 (29–63) | 43 (30–51) |
| Female sex, n (%) | 18 (82) | 27 (79) | 34 (83) |
| Ethnicity, n (%) | |||
| Caucasian | 22 (100) | 30 (88) | 37 (90) |
| Asian | NA | 2 (6) | 1 (2) |
| Other | NA | 2 (6) | 3 (7) |
| Time since first complaints, months | NA | 14 (5–36) | 32 (18–52) |
| Number of SLICC criteria | NA | 3 (2–3) | 5 (5–6) |
| Number of ACR criteria | NA | 3 (2–3) | 5 (4–6) |
| SLEDAI score | NA | 0 (0–2) | 2 (0–4) |
| Increased total IgG (>16 g/l) | 1 (5) | 7 (21) (n = 33)a | 3 (7) |
| Positive anti-dsDNA (>15 IU/ml) | 0 | 8 (24) (n = 33)a | 17 (42) |
| Positive anti-SSA (>10 U/ml) | 0 | 16 (47) | 12 (29) |
| Positive anti-Smith (>10 U/ml) | 0 | 1 (3) | 5 (12) |
| C3 decreased (<0.9 g/l) | NA | 5 (15) | 12 (29) |
| C4 decreased (<0.1 g/l) | NA | 2 (6) | 7 (17) |
| Medication use | |||
| NSAIDs | NA | 9 (28) | 8 (20) |
| Prednisone | NA | 0 | 12 (29) |
| HCQ | NA | 10 (29) | 34 (83) |
| AZA | NA | 0 | 6 (15) |
| MMF | NA | 0 | 8 (20) |
Data are reported as median (interquartile range) or n (%). Baseline data not available for all participants. HCs: healthy controls; iSLE: incomplete SLE; NA: not applicable.
B-cell subsets
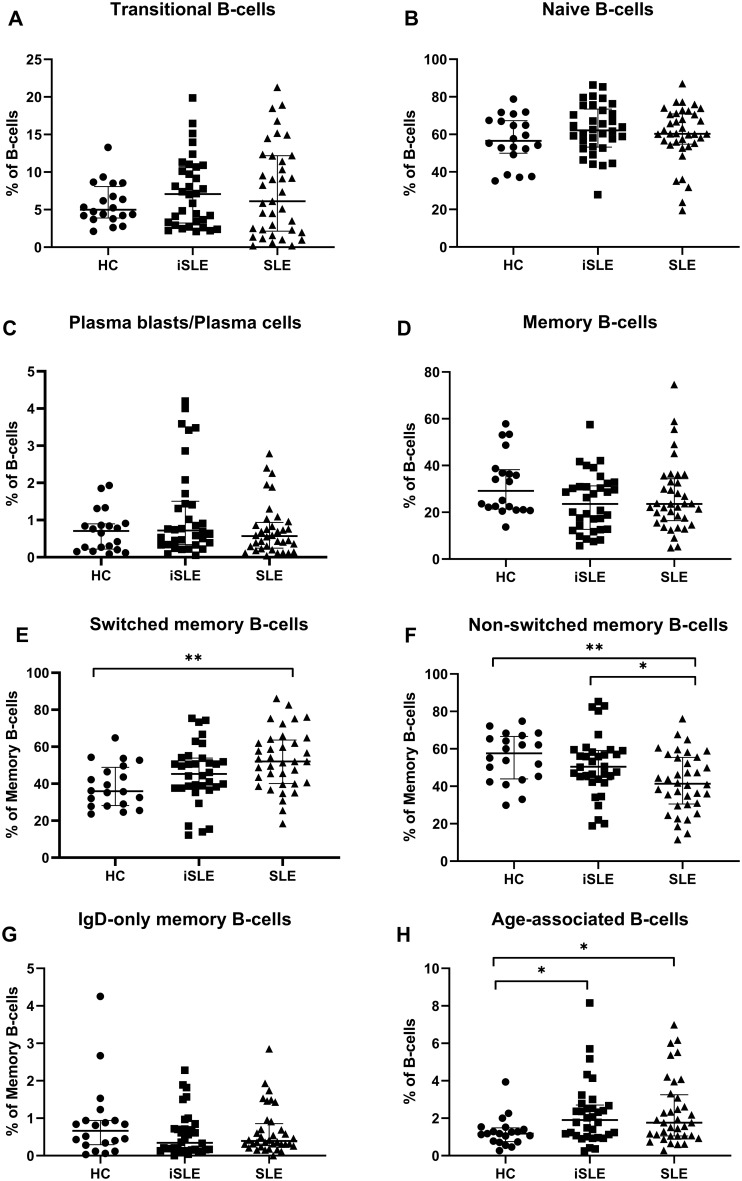
The proportions of transitional and naïve B-cells were similar between all groups (Fig. 1A and B). Also, we did not find significant differences between proportions of memory B-cells or plasma blasts/plasma cells between all groups (Fig. 1C and D). Proportions of switched memory B-cells were slightly increased in iSLE patients compared with HCs [45.3% (37.6–53.8) vs 35.9% (28.2–48.9); P = 0.07] and significantly increased in SLE patients compared with HCs [52.0% (40.1–63.6); P < 0.01] (Fig. 1E). Proportions of switched memory B-cells were not significantly different between iSLE and SLE patients. The proportion of non-switched memory B-cells was highest in HCs [57.6% (43.9–66.7)] and lowest in SLE patients [41.3% (30.5–55.5)] (Fig. 1F). There were no significant differences in proportions of IgD-only memory B-cells between all groups (Fig. 1G). Lastly, we found an elevated proportion of ABCs in iSLE patients compared with HCs [1.9% (1.1–2.7) vs 1.2% (0.8–1.5); P < 0.05] (Fig. 1H). Absolute B-cell counts are shown in supplementary Table S1, available at Rheumatology online.
Fig. 1.
Proportions of B-cell subsets in HCs, iSLE patients and SLE patients
Medians and IQR are depicted for every group. Proportions of transitional B-cells (A), naïve B-cells (B), plasma blasts/plasma cells (C), memory B-cells (D) and IgD-only memory B-cells (G) were not different between all groups. Switched memory B-cells were elevated in SLE patients compared with HCs (E). Non-switched memory B-cells were decreased in SLE patients compared with HCs and iSLE patients (F). ABCs were elevated in iSLE and SLE patients compared with HCs (H). *P < 0.05, **P < 0.01. HCs: healthy controls; iSLE: incomplete SLE; IQR: interquartile range; ABCs: age-associated B-cells.
IFN score
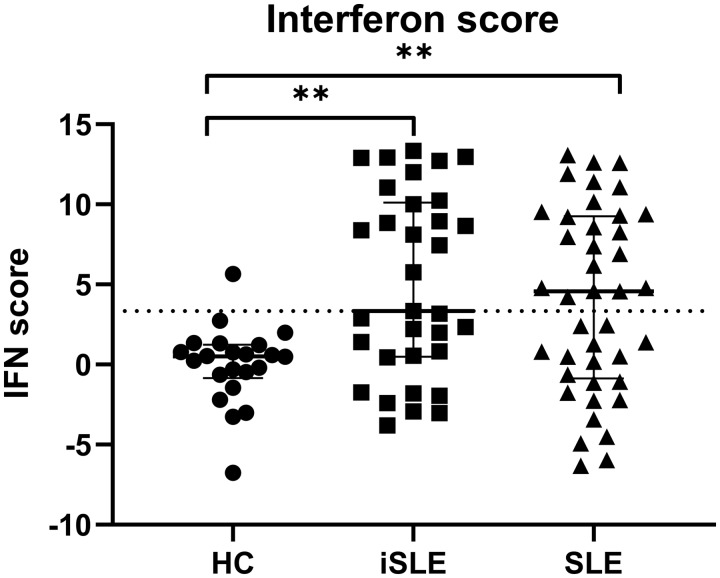
We found higher IFN scores in iSLE patients compared with HCs [3.3 (0.5–10.1) vs 0.5 (−0.8 to 1.2); P < 0.01]. IFN scores were not significantly different between iSLE and SLE patients (Fig. 2). Based on the distribution of IFN scores, iSLE patients were divided into two distinct subgroups: IFNhigh (n = 16) and IFNlow (n = 17). IFNhigh iSLE patients had IFN scores above the median.
Fig. 2.
IFN scores in HCs, iSLE patients and SLE patients
Medians and IQR are depicted for every group. The dotted line represents the median IFN score of the iSLE group. IFN scores were increased in iSLE and SLE patients compared with HCs. *P < 0.05, **P < 0.01. HCs: healthy controls; iSLE: incomplete SLE; IQR: interquartile range.
IFNhigh iSLE patients had a higher proportion of transitional B-cells [10.4% (5.1–13.6) vs 3.7% (2.4–7.4); P = 0.001], switched memory B-cells [51.3% (39.5–62.7) vs 41.0% (32.4–50.6); P = 0.04] and a lower proportion of non-switched memory B-cells [45.7% (34.3–55.9) vs 56.8% (46.7–64.2); P = 0.02] compared with IFNlow iSLE patients. There was a trend for proportions of plasma blasts/plasma cells to be elevated in IFNhigh iSLE patients compared with IFNlow iSLE patients [1.1% (0.5–2.7) vs 0.5% (0.2–1.0); P = 0.08]. Proportions of other B-cell subsets were not significantly different between IFNhigh and IFNlow iSLE patients.
BAFF levels tended to be higher in IFNhigh iSLE patients compared with IFNlow iSLE patients [208 pg/ml (117–335)] vs 149 pg/ml (100–188); P = 0.06]. In addition, IgG levels were significantly elevated in IFNhigh iSLE patients compared with IFNlow iSLE patients [15.6 g/l (10.4–20.4) vs 11.3 g/l (9.4–12.8); P = 0.007], as well as anti-SSA autoantibodies [141.5 U/ml (0.3–240.0) vs 0.0 U/ml (0.0–16.5); P = 0.01). Anti-Smith and anti-dsDNA autoantibodies were not elevated in IFNhigh iSLE patients.
Five iSLE patients (14.7%) had low C3 levels (cut-off <0.9 g/l) and also showed higher IFN scores compared with iSLE patients with normal C3 levels (P = 0.01). Low C4 levels were observed in two iSLE patients (6.1%), of which one patient showed high IFN scores.
BAFF levels
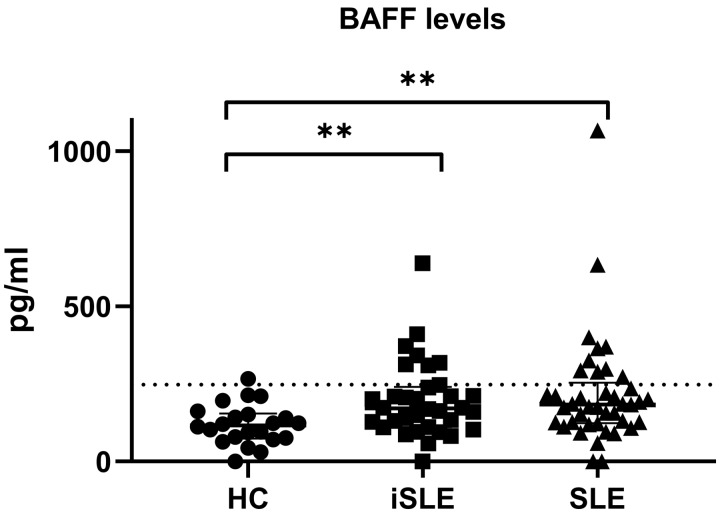
Median levels of BAFF were significantly higher in iSLE patients compared with HCs [171 pg/ml (111–240) vs 117 pg/ml (74–154); P < 0.01], but similar to SLE patients [184 pg/ml (124–254)]. BAFF levels were elevated in 7 (21%) iSLE patients and 10 (24%) SLE patients (Fig. 3).
Fig. 3.
Levels of BAFF in HCs, iSLE patients and SLE patients
Medians and IQR are depicted for every group. The dotted line represents +2 s.d. above the mean of the HC group, which was used as cut-off for BAFF positivity. BAFF levels were higher in the iSLE and SLE groups compared with HCs. *P < 0.05, **P < 0.01. BAFF: B-cell-activating factor; HCs: healthy controls; iSLE: incomplete SLE; IQR: interquartile range.
Correlations in iSLE patients
In iSLE patients, IFN scores were correlated with proportions of transitional B-cells, switched memory B-cells, non-switched memory B-cells and plasma blasts/plasma cells (Table 2). Also, IFN scores were positively correlated with total IgG, anti-SSA and anti-Smith antibodies, and negatively correlated with C3 and C4 in iSLE patients. In addition, proportions of plasma blasts/plasma cells and ABCs were positively correlated with total IgG, while proportions of naïve B-cells were negatively correlated with total IgG. Proportions of switched memory B-cells were correlated with anti-Smith antibodies and with anti-SSA antibodies, while non-switched memory B-cells were negatively correlated with anti-SSA and anti-Smith antibodies. There was a negative correlation between BAFF levels and proportions of total memory B-cells and anti-dsDNA antibodies. BAFF levels were not significantly correlated with IFN scores.
Table 2.
Correlations between B-cell subsets, interferon scores, BAFF levels, autoantibodies and complement factors in iSLE and SLE patients
| IFN |
BAFF |
IgG |
Anti- dsDNA |
Anti- SSA |
Anti- Smith |
C3 |
C4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| iSLE | SLE | iSLE | SLE | iSLE | SLE | iSLE | SLE | iSLE | SLE | iSLE | SLE | iSLE | SLE | iSLE | SLE | |
| Trans | 0.50** | 0.49** | 0.47** | |||||||||||||
| Naïve | −0.36* | |||||||||||||||
| Memory | −0.38* | −0.35* | −0.39* | |||||||||||||
| SMB | 0.47** | 0.40* | ||||||||||||||
| NSMB | −0.51** | −0.44* | −0.40* | |||||||||||||
| PB/PC | 0.38* | 0.48** | ||||||||||||||
| ABC | 0.39* | |||||||||||||||
| IFN | NA | NA | 0.35* | 0.44* | 0.51* | 0.39* | 0.41* | 0.42* | −0.46** | −0.32* | −0.48** | |||||
| BAFF | 0.35* | NA | NA | −0.42* | ||||||||||||
Correlation coefficients were calculated with Spearman correlation.
P < 0.05,
P < 0.01.
BAFF: B-cell-activating factor; iSLE: incomplete SLE; Trans: proportions of transitional B-cells; Naïve: proportions of naïve B-cells; Memory: proportions of memory B-cells; SMB: proportions of switched memory B-cells; NSMB: proportions of non-switched memory B-cells; PB/PC: proportions of plasma blasts/plasma cells; ABCs: proportions of age-associated B-cells; C3 and C4: complements 3 and 4; NA: not applicable.
Correlations in SLE patients
Apart from transitional B-cells, none of the B-cell subsets was correlated with IgG levels in SLE patients (Table 2). There was a negative correlation between proportions of plasma blasts/plasma cells and C4 levels. IFN scores were positively correlated with proportions of transitional B-cells, but negatively correlated with proportions of total memory B-cells and C3. In addition, IFN was correlated with anti-SSA and anti-Smith antibodies. In SLE patients, BAFF levels were correlated with IFN score. There was no correlation between BAFF levels and proportions of B-cell subsets.
Discussion
The aim of this cross-sectional study was to investigate whether there are distortions in B-cell subsets, IFN scores and BAFF levels in iSLE patients, in order to better understand the pathogenesis and maybe—in the future—predict progression. Our findings highlight that elevated levels of ABCs, switched memory B-cells and increased IFN scores might play a crucial role in the pathogenesis of SLE. We found that iSLE patients have increased proportions of ABCs compared with HCs. In addition, IFN scores and BAFF levels were similarly elevated in iSLE and SLE patients compared with HCs. In iSLE patients, IFN score was correlated with proportions of transitional B-cells, switched memory B-cells, plasma blasts/plasma cells and established serological markers for SLE including total IgG, anti-SSA and anti-Smith antibodies, and complement levels. This finding suggests that a more activated B-cell compartment, in terms of increased proportions of plasma cells, ABCs and switched memory B-cells, is reflected by higher (auto)antibody production. Also, we found that iSLE patients with high IFN scores had higher proportions of switched memory B-cells and transitional B-cells and serological disease markers than iSLE patients with low IFN scores.
It has been demonstrated that IFN type I-induced gene transcripts are increased in blood and tissue of the majority of SLE patients, as a consequence of a persistent self-directed immune reaction, and are found to be of importance in the early phase of SLE [21, 22]. Chiche et al. [11] showed that IFN-induced genes can be clustered in three different modules, including a stable module (M1.2), driven by IFN-α, and variable modules (M3.4 and M5.12), also induced by IFN-β and -γ. In order to improve comparability with previous studies, we calculated an IFN score based on three IFN genes (IFI44L, LY6E and MX1) from M1.2, that are among the most frequently tested in SLE patients [11, 23]. We found that this IFN score was elevated in iSLE and in SLE patients compared with HCs. Interestingly, a clear dichotomy was observed in the distribution of IFN scores within the iSLE group, since approximately half had elevated IFN scores. Although there is considerable heterogeneity among iSLE patients, others have found similar distributions of IFN scores in iSLE patients [24]. It is unclear why this division occurs. Possibly, iSLE patients with high IFN scores have higher risk of progressing to SLE. However, this needs to be confirmed in future longitudinal studies.
It has been reported that IFN-α activity increases gradually before diagnosis of SLE and that higher IFN-α activity is linked with an increase in autoantibodies [25]. Additionally, Li et al. [24] showed that IFN-induced gene expression was correlated with SLE autoantibody profiles, measured with an autoantibody proteomic array, in iSLE patients. In line with the study by Li et al. [24] we demonstrated a positive correlation between IFN score and anti-SSA and anti-Smith antibodies. In the present study, IFN scores were similar in iSLE patients and SLE patients. This might be explained by the fact that all the included SLE patients had quiescent disease and received immunosuppressive therapy [26].
IFN can also stimulate BAFF production, which stimulates B-cell maturation and, when dysregulated, can prevent apoptosis of autoreactive B-cells leading to autoimmunity [25]. In our study, BAFF levels were also elevated in iSLE (and SLE) patients compared with HCs, which supports its role in the pathogenesis of SLE. Others have also found increased BAFF levels in iSLE patients [27]. In a study by Munroe et al. [28] BAFF levels were measured in relatives of SLE patients. Interestingly, relatives who later developed SLE showed elevated BAFF levels at baseline compared with unaffected relatives. However, BAFF did not independently predict transitioning to classified SLE in the study by Munroe et al. Nonetheless, it has been described that belimumab, which targets BAFF, has therapeutic benefit in active SLE patients [13]. As class-switched memory B-cells and plasma blasts were substantially depleted under belimumab, belimumab could be of interest for iSLE patients [13].
We found that IFNhigh iSLE patients had higher proportions of switched memory B-cells and transitional B-cells and serological disease markers than IFNlow iSLE patients. Concordantly, IFN scores were correlated with proportions of switched memory B-cells and transitional B-cells in iSLE patients. It has been demonstrated that elevated proportions of transitional B-cells are present in SLE patients and reflect an early checkpoint in the generation of autoreactive B-cells [29, 30]. In a recent study, RNA sequencing of sorted transitional B-cells from untreated SLE patients revealed a predominant overexpression of IFN-stimulated genes [30].
In addition, switched memory cells tended to be elevated in iSLE patients compared with HCs. Upon activation, these cells can rapidly differentiate into plasma cells and therefore play a crucial role in the pathogenesis of SLE [31]. Conversely, proportions of non-switched memory B-cells were decreased in iSLE patients. Non-switched memory B-cells can re-enter germinal centre reaction after antigenic stimulation [31]. In line with this observation, elevated proportions of switched memory B-cells and decreased levels of non-switched memory B-cells in SLE patients have been observed previously [15]. In the current study, switched memory B-cells correlated with anti-SSA and anti-Smith antibodies, which underlines that this B-cell subset is associated with autoantibody production. Arbuckle et al. [32] investigated development of autoantibodies before the clinical onset of SLE, and showed that at least one SLE-specific autoantibody was present years before diagnosis (mean 3.3 years). They also showed that more individuals had anti-SSA autoantibodies before they became positive for anti-dsDNA. Indeed, in our iSLE patients, almost half of patients were anti-SSA positive and proportions of B-cell subsets were correlated with IgG levels or specific autoantibodies. Consequently, for studying progression from iSLE to SLE it is important to measure both specific B-cell subsets and specific autoantibodies. Also, proportions of switched memory B-cells and plasma cells were correlated with IFN scores, which supports the notion that IFN may be involved in isotype switching [12].
Lastly, we analysed proportions of ABCs (CD19+CD21lowCD11c+). As described by Rubtsova et al. [17] ABCs are a functionally unique B-cell subset that accumulate with age, and probably arise from activation driven differentiation, leading to upregulation of the transcription factor T-bet. Although measurement of T-bet was not included in our study, CD21lowCD11c+ cells can be regarded as ABCs. In a recent review, Naradikian et al. [18] proposed that ABCs are a memory subset generated by nucleic acid-containing antigens in the context of a proinflammatory cytokine milieu. Normally, B-cells that internalize nucleic acid containing self-antigens fail to survive because they lack the cognate T-cell interactions and appropriate cytokine signals to further differentiate and survive. However, inadvertent or aberrant receipt of these signals can afford survival of these cells and enable their recruitment into a long-lived memory and effector ABC pool, which can produce autoantibodies [18]. We observed elevated proportions of ABCs in iSLE patients and SLE patients compared with HCs [18]. Even more, a significant correlation between ABCs and IgG levels was found in iSLE patients, which supports their antibody-producing function in autoimmunity.
Limitations
There are some limitations to this study that need mentioning. Our data only reflect values of SLE patients with quiescent disease who were being treated at the time of inclusion. Thus, our results cannot be translated to SLE patients with active disease (SLEDAI >4). Also, SLE patients used more immunosuppressive medication than iSLE patients. This could have influenced B-cell subsets, IFN scores, BAFF levels, complement levels and antibody titres. In addition, our study design is cross-sectional and comprises a relatively small sample size. Thus, it should be noted that correlations with P < 0.05 should be regarded as borderline significance and require confirmation in future studies.
Conclusion
In summary, iSLE patients showed increased proportions of ABCs compared with HCs. IFN scores were increased and correlated with proportions of transitional B-cells, switched memory B-cells, plasma cells and anti-SSA and anti-Smith antibodies. Moreover, we found that iSLE patients with high IFN scores had higher proportions of transitional B-cells, plasma cells and switched memory B-cells and higher serological disease markers than iSLE patients with low IFN scores. Our findings suggest that IFN and distortions of B-cell subsets are involved in the early steps of the pathogenesis of SLE. Possibly, these iSLE patients are more at risk to progress to SLE. However, this should be confirmed with longitudinal data.
Funding: This work was supported by ReumaNederland (15-1-401 to K.d.L.).
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Tsokos GC, Lo MS, Reis PC, Sullivan KE.. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol 2016;12:716–30. [DOI] [PubMed] [Google Scholar]
- 2. Cohen AS, Tan EM, Mcshane DJ. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 3. Petri M, Orbai AM, Alarcón GS. et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aringer M, Costenbader K, Daikh D. et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol 2019;71:1400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swaak AJ, van de Brink H, Smeenk RJ. et al. Incomplete lupus erythematosus: results of a multicentre study under the supervision of the EULAR Standing Committee on International Clinical Studies Including Therapeutic Trials (ESCISIT). Rheumatology (Oxford) 2001;40:89–94. [DOI] [PubMed] [Google Scholar]
- 6. Lambers WM, Westra J, Jonkman MF, Bootsma H, de Leeuw K.. Incomplete systemic lupus erythematosus – what remains after application of ACR and SLICC criteria? Arthritis Care Res (Hoboken) 2019; doi: 10.1002/acr.23894 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pascual V, Chaussabel D, Banchereau J.. A genomic approach to human autoimmune diseases. Annu Rev Immunol 2010;28:535–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Banchereau J, Pascual V.. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 2006;25:383–92. [DOI] [PubMed] [Google Scholar]
- 9. Bengtsson AA, Rönnblom L.. Role of interferons in SLE. Best Pract Res Clin Rheumatol 2017;31:415–28. [DOI] [PubMed] [Google Scholar]
- 10. Kalliolias GD, Ivashkiv LB.. Overview of the biology of type I interferons. Arthritis Res Ther 2010;12:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiche L, Jourde-Chiche N, Whalen E. et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol 2014;66:1583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiefer K, Oropallo MA, Cancro MP, Marshak-Rothstein A.. Role of type I interferons in the activation of autoreactive B cells. Immunol Cell Biol 2012;90:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang W, Quach TD, Dascalu C. et al. Belimumab promotes negative selection of activated autoreactive B cells in systemic lupus erythematosus patients. JCI Insight 2018;3:e122525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berggren O, Hagberg N, Weber G. et al. B lymphocytes enhance interferon-α production by plasmacytoid dendritic cells. Arthritis Rheum 2012;64:3409–19. [DOI] [PubMed] [Google Scholar]
- 15. Kaminski DA, Wei C, Qian Y, Rosenberg AF, Sanz I.. Advances in human B cell phenotypic profiling. Front Immunol 2012;3:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manni M, Ricker E, Pernis AB.. Regulation of systemic autoimmunity and CD11c+Tbet+B cells by SWEF proteins. Cell Immunol 2017;321:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubtsova K, Marrack P, Rubtsov AV.. Age-associated B cells: are they the key to understanding why autoimmune diseases are more prevalent in women? Expert Rev Clin Immunol 2012;8:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naradikian MS, Hao Y, Cancro MP.. Age-associated B cells: key mediators of both protective and autoreactive humoral responses. Immunol Rev 2016;269:118–29. [DOI] [PubMed] [Google Scholar]
- 19. Jenks SA, Cashman KS, Zumaquero E. et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 2018;49:725–39.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang J, Siegel R, Naiman B. et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat Commun 2018;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol 2014;192:5459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rönnblom L, Leonard D.. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med 2019;6:e000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maria NI, Brkic Z, Waris M. et al. MxA as a clinically applicable biomarker for identifying systemic interferon type I in primary Sjogren’s syndrome. Ann Rheum Dis 2014;73:1052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Q-Z, Zhou J, Lian Y. et al. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol 2010;159:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munroe ME, Lu R, Zhao YD. et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis 2016;75:2014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steiman AJ, Gladman DD, Ibañez D. et al. Lack of interferon and proinflammatory cyto/chemokines in serologically active clinically quiescent systemic lupus erythematosus. J Rheumatol 2015;42:2318–26. [DOI] [PubMed] [Google Scholar]
- 27. Aberle T, Bourn RL, Munroe ME. et al. Clinical and serologic features distinguish patients with incomplete lupus classification versus systemic lupus erythematosus patients and controls. Arthritis Care Res (Hoboken) 2017;69:1780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Munroe ME, Young KA, Kamen DL. et al. Discerning risk of disease transition in relatives of systemic lupus erythematosus patients utilizing soluble mediators and clinical features. Arthritis Rheumatol 2017;69:630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vossenkämper A, Lutalo PMK, Spencer J.. Translational mini-review series on B cell subsets in disease. Transitional B cells in systemic lupus erythematosus and Sjögren’s syndrome: clinical implications and effects of B cell-targeted therapies. Clin Exp Immunol 2012;167:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dieudonné Y, Gies V, Guffroy A. et al. Transitional B cells in quiescent SLE: an early checkpoint imprinted by IFN. J Autoimmun 2019;102:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kurosaki T, Kometani K, Ise W.. Memory B cells. Nat Rev Immunol 2015;15:149–59. [DOI] [PubMed] [Google Scholar]
- 32. Arbuckle MR, McClain MT, Rubertone MV. et al. Development of Autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.