Abstract
Objective
To investigate the long-term efficacy and safety of the IL-6 receptor antibody tocilizumab in patients with Takayasu arteritis (TAK).
Methods
Patients completing the randomized, double-blind, placebo-controlled period of the TAKT (Takayasu arteritis Treated with Tocilizumab) trial were followed up during open-label extended treatment with weekly s.c. tocilizumab 162 mg for up to 96 weeks or longer, with oral glucocorticoid tapering performed at the investigators’ discretion. Endpoints of the extension analysis included steroid-sparing effects of tocilizumab, imaging data, patient-reported outcomes (36-Item Short Form Health Survey) and safety.
Results
All 36 patients enrolled in the double-blind period entered the open-label extension; 28 patients received tocilizumab for 96 weeks. The median glucocorticoid dose was 0.223 mg/kg/day at the time of relapse before study entry, 0.131 mg/kg/day (interquartile range 0.099, 0.207) after 48 weeks and 0.105 mg/kg/day (interquartile range 0.039, 0.153) after 96 weeks. Overall, 46.4% of patients reduced their dose to <0.1 mg/kg/day, which was less than half the dose administered at relapse before study entry (mean difference –0.120 mg/kg/day; 95% CI −0.154, −0.087). Imaging evaluations indicated that most patients’ disease was improved (17.9%) or stable (67.9%) after 96 weeks compared with baseline. Mean 36-Item Short Form Health Survey physical and mental component summary scores and 7 of 8 domain scores were clinically improved from baseline and maintained over 96 weeks of tocilizumab treatment. No unexpected safety issues were reported.
Conclusion
These results in patients with Takayasu arteritis provide evidence of a steroid-sparing effect and improvements in well-being during long-term treatment with once-weekly tocilizumab 162 mg, with no new safety concerns.
Trial registration
JAPIC Clinical Trials Information, http://www.clinicaltrials.jp/user/cteSearch_e.jsp, JapicCTI-142616.
Keywords: biological therapies, immunosuppressants, quality of life, Takayasu arteritis, vasculitis
Rheumatology key messages
In this long-term extension, Takayasu arteritis patients received tocilizumab and glucocorticoid tapering resembling clinical practice.
A steroid-sparing effect was observed in Takayasu arteritis patients treated with tocilizumab for up to 96 weeks.
Takayasu arteritis patients experienced improved well-being and stable/improved disease on imaging evaluation, with no new safety concerns.
Introduction
Takayasu arteritis (TAK) is a rare disease characterized by inflammation of large blood vessels, resulting in vascular occlusion and aneurysm formation [1, 2]. The classic pathological feature of TAK is granulomatous inflammation in the adventitia and media of the involved arteries; typical changes are inflammatory cell infiltration with granulomatosis formation, resulting in thickening of the adventitia and intima or disruption of elastic fibres, often accompanied by thrombus formation [1].
TAK occurs most frequently in females, with a peak onset around 20 years of age [3]; globally, the estimated annual incidence is 1–3 cases/million [4], but the prevalence may be higher in Japan (∼60 cases/million) [2]. Disease manifestations include systemic symptoms (fever, malaise), head and neck symptoms (neck pain, dizziness, headache), upper limb problems (pulselessness, fatigue) and body pain [2]. In addition, patients with TAK experience impaired health-related quality of life (HRQoL) compared with healthy controls [5].
For many years, the first-line treatment for TAK has been glucocorticoids (GCs) [6]; however, long-term use is associated with adverse events (AEs), and patients with TAK frequently experience relapse during GC tapering [4]. Elevated IL-6 levels are detectable in the peripheral blood and aortic tissue of patients with TAK and correlate with disease activity [7]. Clinical responses and a steroid-sparing effect in patients with TAK treated with the recombinant, humanized, anti-IL-6 receptor mAb tocilizumab were demonstrated in case studies [8–10]. In the randomized, double-blind, placebo-controlled, phase 3 Japanese TAKT (Takayasu arteritis Treated with Tocilizumab) study, the primary endpoint of time to relapse was not met for tocilizumab vs placebo (hazard ratio 0.41; 95.41% CI 0.15, 1.10; P = 0.0596), while background GC dose was tapered [11].
Results of the double-blind period of the TAKT study were based on a short observation period and mandatory GC tapering of 10% per week. The TAKT extension evaluated long-term efficacy and safety of tocilizumab during which GC doses could be adjusted at the investigators’ discretion, as in a real-life clinical setting. This article reports final results from the TAKT study, including the open-label extension, and describes the steroid-sparing effects, HRQoL outcomes, imaging data and safety associated with long-term tocilizumab treatment.
Methods
Patients and study design
The TAKT study design and enrolment criteria have been published [11]. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice, and was approved by the institutional review boards of the participating medical institutions. Patients aged ≥12 years at the time of informed consent (from 24 September 2014) with a confirmed diagnosis of TAK according to the 2008 Japanese Guideline for Management of Vasculitis Syndrome [3] were enrolled. TAKT was a double-blind, placebo-controlled trial in which patients with refractory TAK who experienced relapse within the previous 12 weeks despite having received treatment with oral GC at a prednisolone-equivalent dose of ≥0.2 mg/kg/day (for exclusion criteria, see published online supplement [11]) were induced into remission with oral GC therapy. Following achievement of remission, oral GCs were tapered by 10% per week to a minimum of 0.1 mg/kg/day; patients were randomly assigned 1:1 to receive tocilizumab 162 mg/week or placebo s.c. during tapering.
Patients who completed the double-blind period were followed up during open-label extended treatment with tocilizumab 162 mg/week; patients previously assigned to tocilizumab continued treatment, whereas patients assigned to placebo switched to tocilizumab until study end (September 2017). During the open-label extension, oral GC dose was tapered at the investigators’ discretion according to clinical data and patient symptoms. DMARDs and immunosuppressants were prohibited throughout the double-blind and open-label periods, and patients could not undergo surgery (excluding local surgery, such as cataract surgery).
Outcomes
Outcomes included the steroid-sparing effect of tocilizumab, imaging data, patient-reported outcomes [36-Item Short Form Health Survey (SF-36)] during the double-blind (tocilizumab-treated cohort only) and open-label extension periods, the number of TAK relapses, observed symptoms during the open-label extension and safety (see Supplementary Material, section Methods, available at Rheumatology online, for assessments and statistical analysis).
Results
Patients
Thirty-six patients were enrolled in the double-blind period; 18 received tocilizumab s.c. 162 mg/week and 18 received placebo [11]. All 36 patients entered the open-label extension period and received tocilizumab 162 mg/week s.c.; 28 patients received tocilizumab for 96 weeks (supplementary Fig. S1, available at Rheumatology online).
Baseline characteristics at the start of the double-blind period are summarized in supplementary Table S1, available at Rheumatology online. Most patients were female (86.1%), and the mean age was 30.9 years. Mean disease duration was 5.02 years, and 55.6% of patients were HLA-B52 positive. No patients with inflammatory bowel disease complications were included.
Efficacy
GC sparing
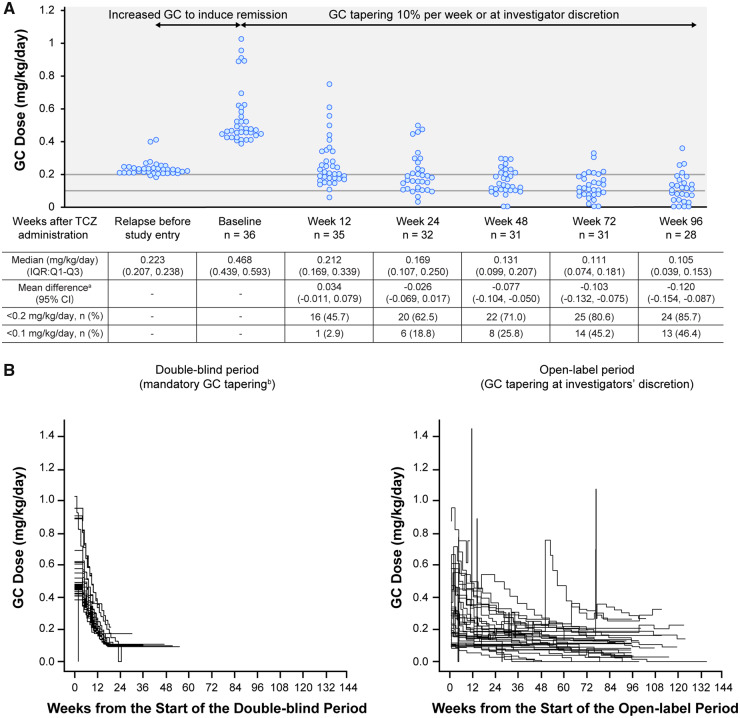
Oral GC doses during 96 weeks of treatment are shown in Fig. 1A. Median GC dose administered at the time of relapse before study entry was 0.223 mg/kg/day (interquartile range 0.207, 0.238). After 48 weeks, the median GC dose was 0.131 mg/kg/day (interquartile range 0.099, 0.207). After 96 weeks, the median GC dose was 0.105 mg/kg/day (interquartile range 0.039, 0.153), which was less than half the GC dose administered at the time of relapse before study entry (mean difference –0.120 mg/kg/day; 95% CI –0.154, –0.087).
Fig. 1.
GC dosing in the TAKT study
(A) Individual GC dose at each time point. (B) Individual GC dose reductions over time. aChange from dose at relapse before study entry. bBy 10% per week to a minimum of 0.1 mg/kg/day. Data were collected at 12 weeks (day 85), 24 weeks (day 169), 48 weeks (day 337), 72 weeks (day 506) and 96 weeks (day 673) after TCZ administration. If data were unavailable for the specified time point (definition date) but the patient had other visits within the time window, the most recent data (definition date minus 7 days) were used for dose calculations. The GC doses at each time point were calculated according to each patient’s weight at baseline. GC: glucocorticoid; IQR: interquartile range; Q: quartile; TAKT: Takayasu arteritis Treated with Tocilizumab trial; TCZ: tocilizumab.
A GC dose reduction to <0.1 mg/kg/day was achieved by 18.8, 25.8 and 46.4% of patients at weeks 24, 48 and 96, respectively. By week 96, four patients had entirely discontinued GC treatment, and another three discontinued GC treatment after week 96; none of these seven patients experienced relapse while they were GC-free.
Baseline factors associated with disease activity (HLA-B52 and CRP) [3] were evaluated in patients who had their GC dose reduced to <0.1 mg/kg and those whose dose remained ≥0.1 mg/kg at week 96; baseline HLA-B52-positive status was 46.2 and 60.0%, respectively, and mean ± s.d. CRP levels were 0.342 ± 0.425 mg/dl and 0.459 ± 0.594 mg/dl, respectively.
During the open-label extension period of TAKT, the GC dose reduction occurred at the investigators’ discretion and proceeded slowly compared with the mandatory 10% per week GC tapering (to a minimum of 0.1 mg/kg/day) during the double-blind period (Fig. 1B).
Imaging
Imaging evaluations (per physician assessment) after 96 weeks of tocilizumab administration indicated that the conditions of 24 of 28 patients (85.7%) were improved (5/28; 17.9%) or stable (19/28; 67.9%) compared with baseline. Worsening TAK was observed in 4 of 28 patients (14.3%), 3 of whom continued to show worsening from baseline until study end; 1 patient had a single episode of worsening at week 96 that was no longer observed at week 114. All four patients whose imaging evaluations worsened had worsening progression of aorta wall thickness compared with baseline; three had new stenosis/occlusions and one showed worsening of vascular expansion. Only one patient with worsened imaging data met the protocol-defined clinical criteria for TAK relapse.
Patient-reported outcomes (SF-36)
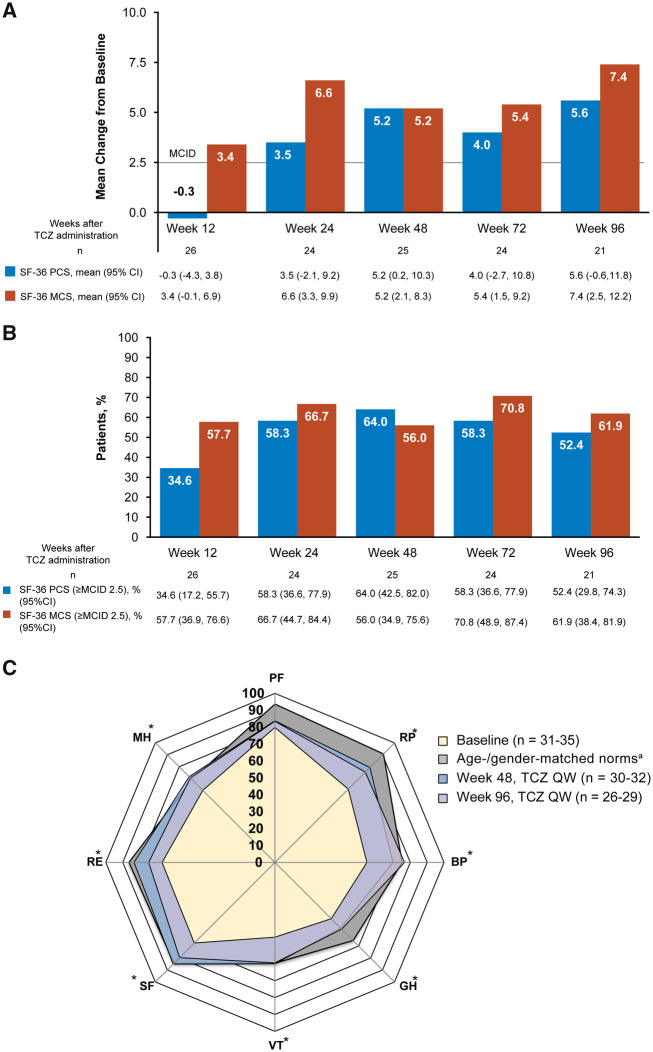
Mean SF-36 mental component summary scores improved rapidly by week 12 of tocilizumab treatment (Fig. 2A). By 24 weeks, the physical and mental component summary mean scores were clinically improved from baseline (minimum clinically important difference of >2.5) and this was maintained over 96 weeks. More than half the patients achieved clinically meaningful improvements (minimum clinically important difference >2.5) in mental and physical component summary scores at weeks 48 and 96 (Fig. 2B). Individual SF-36 domain scores were impaired at baseline in patients with TAK compared with age- and gender-matched norms (Fig. 2C, Supplementary Table S2, available at Rheumatology online). Data of patients treated with tocilizumab were closer to age- and gender-matched data for all SF-36 domain scores at weeks 48 and 96 compared with baseline. In patients with TAK, seven of eight domain mean scores improved from baseline by week 96 of treatment; only the physical functioning score was not meaningfully improved (minimum clinically important difference <5.0) at weeks 48 and 96.
Fig. 2.
Patient well-being in the TAKT study
(A) Patient-reported outcomes measured by the SF-36 component summary scores. (B) Patients with clinically meaningful improvements (MCID >2.5) from baseline. (C) Individual domain scores. Patients with missing baseline data were excluded for these analyses. *Change from baseline MCID of >5.0 for TCZ QW at weeks 48 and 96. aFor calculation of age- and gender-matched norms, patients younger than 20 years of age were calculated as 20 years of age. MCS consists of social functioning, mental health, role emotional and vitality domains. PCS consists of physical functioning, role physical, bodily pain and general health domains. SF-36 MCID has not been validated in patients with TAK. BL: baseline; BP: bodily pain; GH: general health; MCID: minimum clinically important difference; MCS: mental component summary; MH: mental health; PCS: physical component summary; PF: physical function; QW: once weekly; RE: role emotional; RP: role physical; SF: social function; SF-36: 36-Item Short Form Health Survey; TAKT: Takayasu arteritis Treated with Tocilizumab trial; TCZ: tocilizumab; VT: vitality.
TAK relapses
During the open-label extension (median 96 weeks), 18 relapses were recorded in 14 patients [29.4 events per 100 patient-years (PY)]. The most common symptoms of relapse were malaise (12/18; 66.7%), spontaneous pain in chest or back region (8/18; 44.4%) and tenderness or spontaneous pain in carotid artery (7/18; 38.9%) (Supplementary material, section Results, and supplementary Tables S3–S5, available at Rheumatology online).
Safety
During the total duration of the TAKT study (double-blind and open-label periods), patients received tocilizumab 162 mg/week s.c. for a median of 108.07 weeks.
The most frequently reported AEs were infections and infestations (88.9%; 218.8/100 PY) (see Supplementary material, section Results, and supplementary Table S6, available at Rheumatology online, for additional safety results). Serious AEs were reported by 25% of patients (17.4/100 PY); the most common were gastroenteritis (5.6%; 2.9/100 PY) and pneumonia (5.6%; 2.9/100 PY). All serious infections resolved without sequelae. No patients withdrew from the study because of an AE.
Discussion
In this report from a prospective, multicentre trial to assess long-term (>2 years) efficacy and safety of tocilizumab therapy for the treatment of patients with TAK, there was a clear steroid-sparing effect observed with tocilizumab treatment from week 24 through week 96 compared with the GC dose at relapse before study entry. In a previous study, >70% of patients with TAK experienced relapse while receiving <10 mg/day GC for 6 months [12]. In contrast, the results of the TAKT study indicate that most patients receiving tocilizumab 162 mg/week can taper GC to doses <0.2 mg/kg/day for longer than 48 weeks (equivalent to <10 mg/day for patients weighing 50 kg). No clear relationship was identified between baseline HLA-B52 status or elevated CRP levels and efficacy in patients who had their GC dose reduced to <0.1 mg/kg.
In a previous study of patients with TAK, the prednisolone dose reduction rate was the only significant predictor of relapse during GC treatment [13]. In the TAKT study, the difference in the exposure-adjusted relapse rate between the double-blind period, during which GC was mandatorily tapered (101.1 events/100 PY in the tocilizumab group) [14], and the open-label extension period, during which tapering was at the investigator’s discretion (29.4/100 PY), highlights the importance of the GC dose reduction rate for management of this disease. GCs should be tapered more slowly in patients receiving tocilizumab who experience relapse during steroid reduction in order to avoid further relapses.
Although four patients showed worsened imaging data at 96 weeks, only one met protocol-defined clinical criteria for TAK relapse. Therefore, patients with TAK should be regularly monitored using appropriate imaging techniques while receiving tocilizumab, even when symptoms of TAK relapse are not apparent. Furthermore, in two patients with worsened imaging at 96 weeks who did not improve by the end of the study, the GC dose was nonetheless tapered at the investigators’ discretion because these patients were not displaying clinical TAK-related symptoms. In the real-world setting, if radiographic worsening is detected in patients receiving tocilizumab, an increase in GC dose or addition of immunosuppressant therapy should be considered to prevent further vascular damage; the investigators could have chosen to increase the GC dose or to discontinue the study and switch from tocilizumab to another immunosuppressant. Given that safety and cost issues limit the frequency of imaging examinations, there is a need to identify biomarkers of disease activity to facilitate early detection in patients who need therapy modifications.
Tocilizumab treatment has demonstrated clinically meaningful HRQoL improvement in randomized controlled trials in GCA and RA [15, 16]. HRQoL is decreased in patients with TAK compared with healthy control subjects, particularly for physical domains of SF-36 [5]. Given that TAK is commonly a disease of younger women [3] and GC administration is associated with psychological symptoms [17], improvement of HRQoL in patients receiving tocilizumab and tapered GC will likely have a positive impact on school life and work productivity.
After 96 weeks of treatment, the long-term safety of tocilizumab 162 mg/week in patients with TAK was consistent with the known safety profile in RA, with no new signals observed [18, 19].
Limitations of this study are that data collected at randomization were used as the baseline to calculate subsequent changes in outcome measures; therefore, the change from the first dose of tocilizumab in the open-label period was not evaluated for patients who received placebo during the double-blind period.
In conclusion, this prospective clinical trial is the largest reported in patients with TAK and demonstrates that tocilizumab 162 mg/week provided a steroid-sparing effect, improvements in patient well-being and stable or improved disease on imaging evaluation, with no new safety concerns.
Supplementary Material
Acknowledgements
The authors would like to acknowledge all investigators, sub-investigators, study sites, patients and the TAKT study team for their contributions to this study. Professional writing and editorial assistance were provided by Sally-Anne Mitchell, PhD, Maxwell Chang, MSc, and Sara Duggan, PhD, of ApotheCom on behalf of F. Hoffmann–La Roche Ltd and Chugai Pharmaceutical Co. Funding for manuscript preparation was provided by F. Hoffmann–La Roche Ltd and Chugai Pharmaceutical Co. Ltd. Y.N. was involved in conception and design, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content. N.N. was involved in conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content. M.I., Y.Tanaka and S.T. were involved in conception and design, acquisition of data, interpretation of data and revising the article critically for important intellectual content. S.Yokota was involved in conception and design, interpretation of data and revising the article critically for important intellectual content. T.I., S.O., H.N., N.T., S.B., H.Y., Y.S., A.K., T.A., S.F., H.K., K.S., R.H., Y.M., H.T. and Y.Takasaki were involved in acquisition of data, interpretation of data and revising the article critically for important intellectual content. N.O. and S.Yamakido were involved in conception and design, analysis and interpretation of data and drafting the article and revising it critically for important intellectual content. K.Y. was involved in interpretation of data and drafting the article and revising it critically for important intellectual content. Y.N., K.Y., N.O. and S.Yamakido had full access to all the data in the study and take responsibility for its integrity and the data analysis. Qualified researchers may request access to individual patient level data through the clinical study data request platform (www.clinicalstudydatarequest.com). For further details on Chugai’s Data Sharing Policy and how to request access to related clinical study documents, see www.chugai-pharm.co.jp/english/profile/rd/ctds_request.html. Deidentified patient data, protocols and statistical analysis plans are available upon reasonable request via www.clinicalstudydatarequest.com.
Funding: This work was supported by Chugai Pharmaceutical Co. Ltd. The sponsor was involved in the study design and conduct, collection, analysis and interpretation of data, drafting and revising the manuscript, and in the decision to submit the manuscript for publication.
Disclosure statement: Y.N. reports personal fees from Chugai as a consultant of the sponsor-initiated clinical trial (Chugai) using tocilizumab for Takayasu arteritis from Chugai during the conduct of the study; grants and personal fees from Chugai, Bayer, Astellas, Pfizer, MSD, Actelion Pharmaceuticals Japan, Daiichi Sankyo and AbbVie; grants from Takeda, Otsuka and Mitsubishi Tanabe; personal fees from Nippon Shinyaku, Novartis and Kowa outside the submitted work; M.I. reports grants from Chugai during the conduct of the study; grants and personal fees from Chugai, Mitsubishi Tanabe, Daiichi Sankyo, Teijin Pharma and ONO; personal fees from Kyorin, Sumitomo Dainippon Pharma, Nihon Medi-Physics, Biotronik, Pfizer, Bayer, MSD, Otsuka, Kowa, Asahi Kasei ZOLL Medical, Takeda, Medtronic, Sanwa Kagaku, GlaxoSmithKline, Sanofi, Teijin Home Healthcare, Astellas, Kaketsuken, Actelion Pharmaceuticals Japan, AstraZeneca, Eisai, Nippon Shinyaku, Nipro, Toa Eiyo, Nippon Boehringer Ingelheim, Amgen Astellas BioPharma, Bristol-Myers Squibb and Kyowa Hakko Kirin outside the submitted work; Y.Tanaka reports grants and personal fees from Chugai during the conduct of the study; grants and personal fees from Daiichi Sankyo, Astellas, AbbVie, Mitsubishi Tanabe, Bristol-Myers Squibb, Eisai and Takeda; grants from ONO, MSD and Taisho-Toyama; personal fees from Eli Lilly Japan, Sanofi, Pfizer, YL Biologics, GlaxoSmithKline, UCB Japan, Novartis, Janssen and Asahi Kasei Pharma outside the submitted work; T.I. reports personal fees from Chugai during the conduct of the study; personal fees from Janssen, Teijin Pharma, Eizai, AbbVie, AstraZeneca, GlaxoSmithKline, Asahi Kasei Pharma, Eli Lilly Japan, ONO, UCB Japan, Astellas and Mitsubishi Tanabe outside the submitted work; S.O. reports grants from Chugai during the conduct of the study; H.N. reports grants from Chugai during the conduct of the study; grants and personal fees from Mitsubishi Tanabe, Takeda, Astellas, Daiichi Sankyo, AbbVie, Eisai, Asahi Kasei Pharma and Teijin Pharma; personal fees from Chugai, Pfizer, Bristol-Myers Squibb, Gilead Sciences, Sanofi, Zenyaku Kogyo, Bayer, Actelion Pharmaceuticals Japan, GlaxoSmithKline, Novartis, Nippon Shinyaku, Nippon Kayaku, Eli Lilly Japan, Janssen and Ayumi outside the submitted work; N.T. reports grants from Chugai during the conduct of the study; grants and personal fees from Chugai, Mitsubishi Tanabe, Takeda, Astellas, Eisai, Ayumi, Teijin Pharma, Bristol-Myers Squibb, Novartis, Eli Lilly Japan, Janssen, Asahi Kasei Pharma and AbbVie; grants from MSD, Daiichi Sankyo, GlaxoSmithKline, Kyowa Hakko Kirin and Gilead Sciences; personal fees from UCB Japan, Otsuka and Kissei outside the submitted work; S.B. reports grants from Chugai during the conduct of the study; grants and personal fees from Chugai; personal fees from Bristol-Myers Squibb, Eisai, Janssen, Mitsubishi Tanabe and Takeda outside the submitted work; H.Y. reports personal fees from Chugai during the conduct of the study; personal fees from Nihon Medi-Physics, FUJIFILM Toyama Chemical, Nippon Shinyaku, Actelion Pharmaceuticals Japan, Mochida and Bayer; grants from Astellas Pharma and Teijin Pharma outside the submitted work; Y.S. reports grants and other from Chugai during the conduct of the study; grants and personal fees from Astellas, Bayer, Biotronik Japan, Boston Scientific Japan, Daiichi Sankyo, Edwards Lifesciences, Eisai, FUJIFILM RI Pharma, Kowa, Medtronic, Mitsubishi Tanabe, Nippon Boehringer Ingelheim, Nippon Shinyaku, Novartis, ONO, Otsuka, Pfizer, Roche Diagnostics, Sanofi, Sanwa Kagaku Kenkyusho, Sumitomo Dainippon Pharma, Takeda, Teijin Home Healthcare and Teijin Pharma; grants from Chugai, Abbott Vascular Japan, Johnson & Johnson, Shionogi and Taisho Biomed Instruments; personal fees from Philips Japan, Abbott Medical Japan, Actelion Pharmaceuticals Japan, Amgen Astellas BioPharma, Asahi Kasei ZOLL Medical, AstraZeneca, Bristol-Myers Squibb, Fukuda Denshi, Kyowa Hakko Kirin, MSD, Nihon Medi-Physics, Sysmex, Terumo, Toa Eiyo and Tsumura outside the submitted work; A.K. reports grants and personal fees from Chugai during the conduct of the study; grants and personal fees from AbbVie, Asahi Kasei Pharma, Astellas, Chugai, Daiichi Sankyo, Eisai, Eli Lilly Japan, Kissei, Janssen, Mitsubishi Tanabe, MSD, Pfizer, ONO, Taisho Toyama, Takeda and Teijin Pharma; personal fees from Actelion Pharmaceuticals Japan, Nippon Kayaku, Novartis and UCB Japan; grants from AstraZeneca, Alexion, Boehringer Ingelheim Japan, Bristol-Myers Squibb, Cosmic Corporation, Kowa, Kyowa Hakko Kirin, Mochida, Nihon Medi-Physics, Otsuka, Sanofi, Sumitomo Dainippon Pharma and YL Biologics outside the submitted work; T.A. reports personal fees from Chugai during the conduct of the study; grants and personal fees from Astellas, Takeda, Mitsubishi Tanabe, Chugai, Pfizer, Daiichi Sankyo, Otsuka, Eisai, AbbVie, Bristol-Myers Squibb and Daiichi Sankyo; grants from Alexion; personal fees from Eli Lilly Japan, GlaxoSmithKline, Pfizer and UCB Japan outside the submitted work; S.F. reports personal fees from Chugai during the conduct of the study; personal fees from Pfizer, Bristol-Myers Squibb and Asahi Kasei Pharma outside the submitted work; H.K. reports grants from Chugai during the conduct of the study; grants and personal fees from Chugai, Mitsubishi Tanabe, Teijin Pharma, Daiichi Sankyo, AbbVie, ONO and JBPO; grants from Novartis, Takeda, Pfizer, Astellas, Eli Lilly Japan, Asahi Kasei Pharma and Sanofi; personal fees from Ayumi outside the submitted work; K.S. reports grants from Chugai during the conduct of the study; grants, personal fees and non-financial support from Chugai, Eisai, Kissei, Bristol-Myers Squibb, AbbVie, Fuji Film, Mitsubishi Tanabe, Pfizer and Takeda; grants from Daiichi Sankyo and ONO; personal fees and non-financial support from Astellas, Eli Lilly Japan, Janssen, Shionogi and UCB Japan outside the submitted work; R.H. reports grants from Chugai during the conduct of the study; personal fees from Sanofi and ONO outside the submitted work; Y.M. reports grants from Chugai during the conduct of the study; H.T. reports grants and personal fees from Chugai during the conduct of the study; personal fees from Mitsubishi Tanabe, Janssen, Eisai, AbbVie, Bristol-Myers Squibb, Astellas, Nippon Kayaku, Asahi Kasei Pharma and Eli Lilly Japan outside the submitted work; Y.Takasaki reports personal fees from Chugai during the conduct of the study; personal fees from Mitsubishi Tanabe, Daiichi Sankyo, Eisai, Bristol-Myers Squibb, MSD, Asahi Kasei Pharma and Janssen outside the submitted work; K.Y. reports personal fees from Chugai during the conduct of the study; N.O. reports personal fees from Chugai during the conduct of the study; S.Yamakido reports personal fees from Chugai during the conduct of the study; S.T. reports personal fees from Chugai during the conduct of the study; personal fees from Teijin Pharma, AbbVie, Ayumi, Mitsubishi Tanabe, Novartis, Asahi Kasei Pharma, ONO, Taisho Toyama, Sanofi, Bristol-Myers Squibb, Eisai and GlaxoSmithKline outside the submitted work; S.Yokota reports personal fees from Chugai during the conduct of the study; personal fees from Chugai, Mitsubishi Tanabe and Shionogi outside the submitted work; and a patent for tocilizumab with royalties paid by Chugai; N.N. reports personal fees from Chugai during the conduct of the study; grants and personal fees from Chugai, Eisai, Sekisui Medical and Ayumi; personal fees from Mitsubishi Tanabe, Pfizer, AbbVie, UCB Japan and Novartis outside the submitted work; and a patent for tocilizumab with royalties paid by Chugai.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Clifford A, Hoffman GS.. Recent advances in the medical management of Takayasu arteritis: an update on use of biologic therapies. Curr Opin Rheumatol 2014;26:7–15. [DOI] [PubMed] [Google Scholar]
- 2. Watanabe Y, Miyata T, Tanemoto K.. Current clinical features of new patients with Takayasu arteritis observed from cross-country research in Japan: age and sex specificity. Circulation 2015;132:1701–9. [DOI] [PubMed] [Google Scholar]
- 3. Society JC. Guideline for management of vasculitis syndrome (JCS 2008). Japanese Circulation Society. Circulation 2011;75:474–503. [DOI] [PubMed] [Google Scholar]
- 4. Koster MJ, Matteson EL, Warrington KJ.. Recent advances in the clinical management of giant cell arteritis and Takayasu arteritis. Curr Opin Rheumatol 2016;28:211–7. [DOI] [PubMed] [Google Scholar]
- 5. Omma A, Erer B, Karadag O. et al. Remarkable damage along with poor quality of life in Takayasu arteritis: cross-sectional results of a long-term followed-up multicentre cohort. Clin Exp Rheumatol 2017;35(Suppl 103): 77–82. [PubMed] [Google Scholar]
- 6. Kim ESH, Beckman J.. Takayasu arteritis: challenges in diagnosis and management. Heart 2018;104:558–65. [DOI] [PubMed] [Google Scholar]
- 7. Noris M, Daina E, Gamba S. et al. Interleukin-6 and RANTES in Takayasu arteritis: a guide for therapeutic decisions? Circulation 1999;100:55–60. [DOI] [PubMed] [Google Scholar]
- 8. Nishimoto N, Nakahara H, Yoshio-Hoshino N. et al. Successful treatment of a patient with Takayasu arteritis using a humanized anti-interleukin-6 receptor antibody. Arthritis Rheum 2008;58:1197–200. [DOI] [PubMed] [Google Scholar]
- 9. Nakaoka Y, Higuchi K, Arita Y. et al. Tocilizumab for the treatment of patients with refractory Takayasu arteritis. Int Heart J 2013;54:405–11. [DOI] [PubMed] [Google Scholar]
- 10. Seitz M, Reichenbach S, Bonel HM. et al. Rapid induction of remission in large vessel vasculitis by IL-6 blockade: a case series. Swiss Med Wkly 2011;141:w13156. [DOI] [PubMed] [Google Scholar]
- 11. Nakaoka Y, Isobe M, Takei S. et al. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis 2018;77:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maksimowicz-McKinnon K, Clark TM, Hoffman GS.. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum 2007;56:1000–9. [DOI] [PubMed] [Google Scholar]
- 13. Ohigashi H, Haraguchi G, Konishi M. et al. Improved prognosis of Takayasu arteritis over the past decade—comprehensive analysis of 106 patients. Circ J 2012;76:1004–11. [DOI] [PubMed] [Google Scholar]
- 14. Nakaoka Y. et al. Response to: ‘Aortic ulceration in a tocilizumab-treated patient with Takayasu arteritis’ by Liebling. Ann Rheum Dis 2018;78:e117. [DOI] [PubMed] [Google Scholar]
- 15. Strand V, Dimonaco S, Tuckwell K. et al. Health-related quality of life in patients with giant cell arteritis treated with tocilizumab in a phase 3 randomised controlled trial. Arthritis Res Ther 2019;21:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strand V, Michalska M, Birchwood C. et al. Impact of tocilizumab monotherapy on patient-reported outcomes in patients with rheumatoid arthritis from two randomised controlled trials. RMD Open 2017;3:e000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buttgereit F, Matteson EL, Dejaco C. et al. Prevention of glucocorticoid morbidity in giant cell arteritis. Rheumatology (Oxford) 2018;57(Suppl 2):ii11–21. [DOI] [PubMed] [Google Scholar]
- 18. Atsumi T, Fujio K, Yamaoka K. et al. Safety and effectiveness of subcutaneous tocilizumab in patients with rheumatoid arthritis in a real-world clinical setting. Mod Rheumatol 2018;28:780–8. [DOI] [PubMed] [Google Scholar]
- 19. Koike T, Harigai M, Inokuma S. et al. Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol 2014;41:15–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.