Abstract
Objectives
SSc is an autoimmune disease characterized by inflammation, vascular injury and excessive fibrosis in multiple organs. Secreted protein acidic and rich in cysteine (SPARC) is a matricellular glycoprotein that regulates processes involved in SSc pathology, such as inflammation and fibrosis. In vivo and in vitro studies have implicated SPARC in SSc, but it is unclear if the pro-fibrotic effects of SPARC on fibroblasts are a result of intracellular signalling or fibroblast interactions with extracellular SPARC hampering further development of SPARC as a potential therapeutic target. This study aimed to analyse the potential role of exogenous SPARC as a regulator of fibrosis in SSc.
Methods
Dermal fibroblasts from both healthy controls and SSc patients were stimulated with SPARC alone or in combination with TGF-β1, in the absence or presence of a TGF receptor 1 inhibitor. mRNA and protein expression of extracellular matrix components and other fibrosis-related mediators were measured by quantitative PCR and western blot.
Results
Exogenous SPARC induced mRNA and protein expression of collagen I, collagen IV, fibronectin 1, TGF-β and SPARC by dermal fibroblasts from SSc patients, but not from healthy controls. Importantly, exogenous SPARC induced the activation of the tyrosine kinase SMAD2 and pro-fibrotic gene expression induced by SPARC in SSc fibroblasts was abrogated by inhibition of TGF-β signalling.
Conclusion
These results indicate that exogenous SPARC is an important pro-fibrotic mediator contributing to the pathology driving SSc but in a TGF-β dependent manner. Therefore, SPARC could be a promising therapeutic target for reducing fibrosis in SSc patients, even in late states of the disease.
Keywords: SSc, SPARC, fibrosis, TGF-β, signalling, dermal fibroblasts
Rheumatology key messages
Exogenous SPARC induces a pro-fibrotic phenotype in dermal fibroblast from systemic sclerosis patients.
The pro-fibrotic of SPARC is mediated by the activation of the TGF-β signalling in systemic sclerosis patients.
Introduction
SSc is an autoimmune disease characterized by inflammation, vascular injury and fibrosis in different organs that carries a high burden of morbidity and mortality. SSc is recognized as one of the most severe connective tissue disorders, associated with the highest case-specific mortality among all the rheumatic diseases, and for which hardly any therapeutic options are available [1]. Fibrosis is the main feature of SSc pathology and is characterized by the excessive production of extracellular matrix (ECM) proteins, including collagen (Col) family members, vimentin and fibronectin, as well as by the differentiation of different cell types (mainly fibroblasts but also endothelial cells and pericytes) into myofibroblasts, which are defined by the expression of the contractile protein α smooth muscle actin [2].
SPARC (secreted protein acidic and rich in cysteine, also known as osteonectin and BM-40) is a member of a larger family of SPARC-related matricellular glycoproteins, which are ECM associated proteins, but in contrast to the classical ECM proteins (Col, laminin, fibronectin), do not have a structural role. SPARC binds to ECM components, such as fibrillar Col I, II and III and basal lamina Col IV, as well as cellular receptors and secreted growth factors, leading to the regulation of different processes involved in homeostasis and disease, including cell migration and invasion, tissue remodelling, cell proliferation, wound healing, angiogenesis, immune responses and fibrosis. SPARC is associated with several different pathologies, including obesity, diabetes, cataracts, myocardial infarct and fibrotic diseases [3, 4]. In vivo experiments have highlighted a key role for this protein in SSc pathology, as SPARC-deficient mice, as well as adenoviral or siRNA-mediated silencing of SPARC expression, reduces disease severity in the bleomycin-induced skin and lung fibrosis model [5, 6]. Importantly, SPARC expression is elevated in serum and affected skin of SSc patients [7, 8], as well in dermal fibroblasts, and SPARC silencing reduced the production of Col I, Col III and connective tissue growth factor (CTGF) by these cells [9, 10]. While these studies implicate SPARC in the fibrotic events observed in SSc pathology, it is unclear if these effects are mediated by intracellular SPARC, or extracellular SPARC which latter could be targeted by antibodies. Increasing the knowledge on SPARC biology and roles thus has relevance for therapeutic targeting of fibrosis.
Methods
Patients
Skin from patients and sex- and age-matched healthy controls (HC) was obtained from the University Medical Centre Utrecht (The Netherlands). All patients provided informed written consent approved by the local institutional medical ethics review boards prior to inclusion in this study. Samples and clinical information were treated anonymously immediately after collection. Patients fulfilled the ACR/EULAR 2013 classification criteria for SSc [11], and the demographic and clinical characteristics of the patients are detailed in Supplementary Table S1, available at Rheumatology online.
Dermal fibroblast culture and stimulation
SSc dermal fibroblasts were isolated from 3 to 4 mm skin biopsies obtained from a clinically affected area. HC dermal fibroblasts were obtained from skin biopsies taken from resected material after cosmetic surgery. Dermal fibroblast isolation was performed using a whole skin dissociation kit (Miltenyi Biotec, Leiden, The Netherlands) following the manufacturer’s instructions and fibroblasts were routinely maintained in Fibroblast Basal Medium supplemented with Fibroblasts Growth Kit-Low Serum (both from ATCC, Manassas, Virginia, USA). Cells were used for experiments between passages 3 and 5 and stimulations were performed after overnight culture. Fibroblasts were left unstimulated or were stimulated with different concentrations of SPARC (0.1–10 μg/ml, R&D Systems, Abingdon, United Kingdom) alone or in combination with TGF-β1 (10 ng/ml, Biolegend, London, United Kingdom) for 1, 6, 24 or 48 h. Alternatively, fibroblasts were pre-incubated for 1 h at 37°C in the presence of the TGFR1 inhibitor Galunisertib (10 nM, Selleck, Munich, Germany) and further stimulated with SPARC (1 μg/ml) alone or in in combination with TGF-β1 (10 ng/ml) for 24 and 48 h.
RT-PCR and quantitative (q)PCR
RNA from dermal fibroblasts was isolated using the RNeasy micro Kit and RNase-Free DNase Set (Qiagen, Venlo, The Netherlands). Total RNA was reverse-transcribed using an iScript cDNA Synthesis kit (Biorad, Veenendaal, The Netherlands). Duplicate PCR reactions were performed using SYBR green (Applied Biosystems, Bleiswijk, The Netherlands) with a StepOne Plus Real-Time PCR system (Applied Biosystems). cDNA was amplified using specific primers (all from Integrated DNA Technologies, Inc. (IDT), see Supplementary Table S2, available at Rheumatology online, for primer sequences). Relative levels of gene expression were normalized to B2M housekeeping gene. The relative quantity of mRNA was calculated using 2−ΔΔCt.
Immunoblotting
Dermal fibroblasts were lysed in Laemmli’s buffer and protein content was quantified with a BCA Protein Assay Kit (Thermo Fisher Scientific, Bleiswijk, The Netherlands). Equivalent amounts of total protein lysate were subjected to electrophoresis on NuPAG 4–12% Bis-Tris Protein Gels (Thermo Fisher Scientific) and proteins were transferred to polyvinylidene difluoride membranes (Millipore). Membranes were incubated overnight at 4°C with primary antibodies specific for SPARC, histone 3 (H3), SMAD family member 2 (SMAD2) (all from Cell Signalling, Leiden, The Netherlands), Col I (Millipore, Amsterdam, The Netherlands), Col IV, TGF-β (both from Abcam, Oxford, UK), fibronectin (Bio-Techne, Abingdon, UK) and p(hospho)-SMAD2 (Thermo Fisher Scientific). Membranes were then washed and incubated in tris-buffered saline / Tween 20 containing horseradish peroxidase–conjugated secondary antibody. Protein was detected with Lumi-lightplus Western Blotting Substrate (Roche Diagnostics, Almere, The Netherlands) using a ChemiDoc™ MP System (Biorad). Densitometry analysis was performed with Image J software. Relative protein expression and relative SMAD2 activation was normalized to H3 and SMAD2 expression, respectively.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7 software (GraphPad Software Inc., San Diego, CA, US). Potential differences between experimental groups were analysed by a non-parametric, Kruskal–Wallis test or Friedman test, as appropriate. P < 0.05 was considered statistically significant.
Results
Exogenous SPARC induces the expression of ECM components and fibrotic mediators by SSc patient dermal fibroblasts
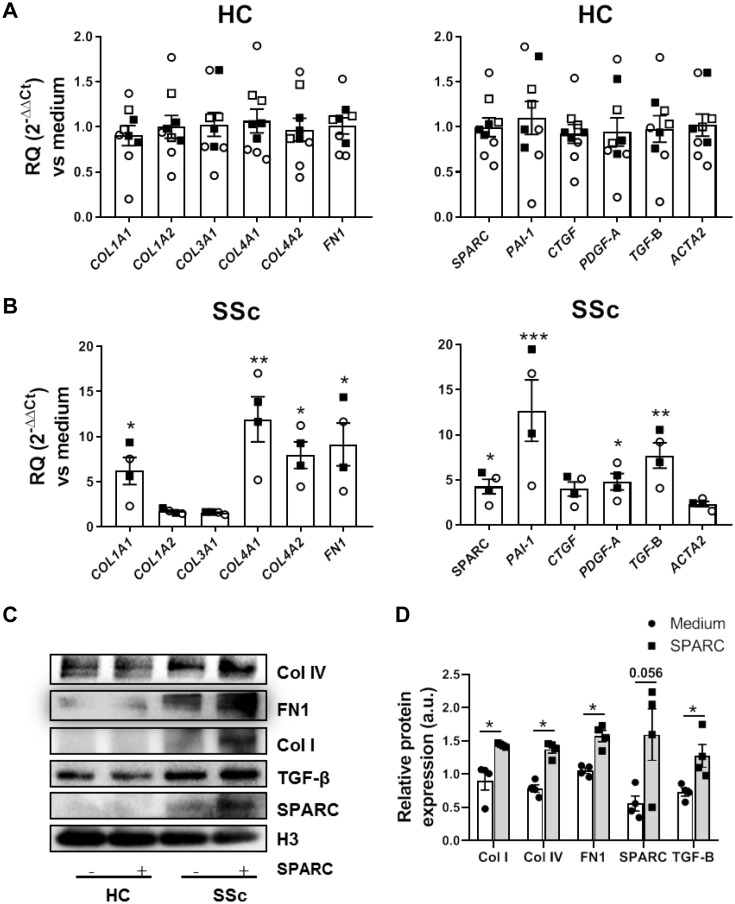
SPARC silencing reduces the expression of fibrotic mediators by dermal fibroblasts of SSc patients [10], but the effect of exogenous administration of SPARC is unknown. We first determined the effect of exogenous SPARC administration on the mRNA expression of ECM or fibrotic genes in dermal fibroblasts from HC and SSc patients. As the phenotypic characteristics of fibroblasts are dependent of the location [12], we analysed the effect of SPARC in fibroblast obtained from forearm, breast and abdominal tissue of HC. We observed that SPARC does not regulate the mRNA expression of ECM or fibrotic genes in HC fibroblast, independently of the tissue location (Fig. 1A). In contrast, exogenous SPARC significantly up-regulated the mRNA expression of the ECM components COL1A1, COL4A1, COL4A2 and fibronectin-1 (FN1), and the fibrosis-related genes TGF-β1, PAI-1, PDGF-A and SPARC itself by SSc patient dermal fibroblasts. We also observed a trend towards up-regulation of CTGF and ACTA2 (Fig. 1B). We further validated these findings at the protein level as we found that exogenous SPARC induced the expression of Col I, Col IV, FN1 and TGF-β1 in fibroblasts from SSc patients, but not from HC (Fig. 1C and D). To determine whether the effect of exogenous SPARC was dose-dependent, we stimulated fibroblasts from HC and SSc patients with different concentrations of SPARC. In fibroblasts from HC, SPARC did not induce expression of fibrosis-related genes in any of the concentrations used. In dermal fibroblasts of SSc patients, exogenous SPARC induced the expression of COL1A1, COL4A1, COL4A2, FN1 and SPARC in a dose-dependent manner in the range of 0.01–1 µg/ml (Supplementary Fig. S1, available at Rheumatology online). These results provide the first direct evidence that exogenous SPARC can regulate pro-fibrotic gene expression in SSc fibroblasts.
Fig. 1.
SPARC induces the expression of ECM components and fibrotic mediators in dermal fibroblast of SSc patients
(A and B) mRNA expression of ECM components and fibrotic genes by dermal fibroblast from breast (open squares), abdomen (filled squares) or forearm (open circles) of HC (A) and from forearm of lcSSc (open circles) and dcSSc patients (filled squares) (B) stimulated with SPARC (1 µg/ml) for 24 h. (C and D) Representative immunoblots (C) and densitometric analysis (D) of Col I, Col IV, fibronectin, TGF-β and SPARC protein expression in dermal fibroblasts of HC and SSc patients stimulated with SPARC (1 µg/ml) for 48 h. Data is shown as relative expression with respect to H3 expression. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with medium. HC: healthy controls; SPARC: secreted protein acidic and rich in cysteine; ECM: extracellular matrix.
Pro-fibrotic effect of SPARC is mediated by TGF-β1 signalling
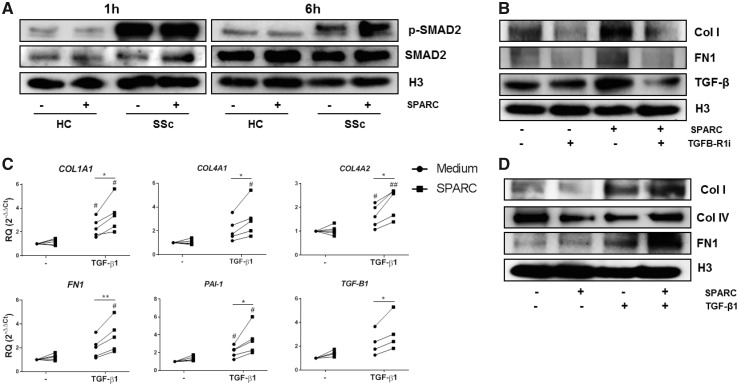
We next investigated the mechanisms that could explain the differences in the effect of exogenous SPARC between HC and SSc patient dermal fibroblasts. As previous studies have shown that SPARC may interact with TGF-β1 signalling pathways [13, 14] we analysed the phosphorylation status of SMAD2, a tyrosine kinase substrate in the TGF-β1 signalling pathway [15], in dermal fibroblasts from HC and SSc patients. Non-stimulated fibroblasts from SSc patients displayed a higher phosphorylation of SMAD2 compared with HC fibroblasts (Fig. 2A). Importantly, exogenous SPARC induced SMAD2 activation in SSc patient fibroblasts, but had no effect in fibroblasts from HC. As these results suggested SPARC may interact with autocrine TGF-β1 signalling in SSc fibroblasts, we examined the effects of SPARC on SSc fibroblasts in the absence or presence of a TGFB-R1 antagonist (Galunisertib -LY2157299-). Strikingly, the effect of SPARC was almost fully abrogated by blocking TGF-β signalling (Fig. 2B).
Fig. 2.
SPARC induces the expression of ECM components and fibrotic mediators in a TGF-β signalling dependent manner
(A) Representative immunoblots of SMAD2 activation in dermal fibroblasts of HC and SSc patients stimulated with SPARC (1 µg/ml) for 1 and 6 h. (B) Representative immunoblots of Col I, fibronectin and TGF-β protein expression in skin fibroblasts of SSc patients pre-incubated 1 h with a TGB-R1 inhibitor (10 nM) and further stimulated with SPARC (1 µg/ml) for 48 h. (C) mRNA expression of ECM components by skin fibroblast of HC stimulated with SPARC (1 µg/ml) alone or in combination with TGF-β1 (10 ng/ml) for 24 h. Data is shown as connected dots. (D) Representative immunoblots of Col I and fibronectin protein expression in skin fibroblasts of HC stimulated with SPARC (1 µg/ml) alone or in combination with TGF-β1 (10 ng/ml) for 48 h. #P < 0.05 and ## P < 0.01 compared with medium. *P < 0.05 and **P < 0.01. HC: healthy controls; SPARC: secreted protein acidic and rich in cysteine; ECM: extracellular matrix.
Reciprocally, while exogenous SPARC alone did not modulate the expression of ECM components or fibrosis-related genes in HC dermal fibroblasts, exogenous SPARC significantly enhanced the TGF-β1 induced expression of COL1A1, COL1A2, COL4A1, COL4A2, FN1 and PAI-1 (Fig. 2C). Changes observed in mRNA expression were relevant to fibroblast protein production, as we found that SPARC enhanced the TGF-β1 induced secretion of Col I and fibronectin by HC fibroblasts (Fig. 2D). Together, these results demonstrate that extracellular SPARC induces a pro-fibrotic phenotype of fibroblasts from SSc patients by sensitizing them for TGF-β1 mediated activation.
Discussion
SPARC levels are elevated in the serum, affected skin and isolated skin fibroblasts from SSc patients. Next, lowering SPARC levels resulted in reduced fibrosis in mice models of SSc and the expression of ECM components by dermal fibroblasts of SSc patients [5, 6, 10]. These studies implicate an important role of SPARC in fibrosis and justify further research as understanding the mode of action of SPARC could provide important insight for SPARC as a therapeutic target. In the present study we provide evidence that exogenous SPARC is able to induce a pro-fibrotic phenotype in dermal fibroblasts of SSc patients. This effect was not observed in HC fibroblasts, likely due to the absence of constitutive activation of TGF-β signalling pathways. TGF-β is one of the most important fibrotic mediators involved in SSc pathology and several studies have shown that TGF-β expression is elevated in the serum and skin of SSc patients, and induces the production of ECM components and other fibrotic mediators such as CTGF, thromboposdin-1 and Serpine1, the protein encoded by the gene PAI-1 [15]. Importantly, TGF-β induces the expression of SPARC by dermal fibroblasts [13, 16] and reciprocally, SPARC induces the expression of TGF-β and regulates TGF-β induced apoptosis and fibrosis [13, 14], suggesting a functional link between these two proteins. In this study, we found that SPARC induces the expression of TGF-β and the activation SMAD2, a key transcription factor downstream of TGF-β signalling, in SSc dermal fibroblasts. Moreover, the inhibition of autocrine TGF-β1 signalling abrogated SPARC-induced gene expression, demonstrating a feedback loop between SPARC and TGF-β1 that contributes to the fibrotic phenotype observed in SSc pathology. Consistent with this, exogenous SPARC enhances TGF-β induced expression of ECM components and fibrotic mediators by HC dermal fibroblasts, resembling the phenotype of SPARC-stimulated SSc fibroblasts. This finding supports the cooperative role of SPARC in fibrotic processes induced by TGF-β signalling. In homeostatic conditions, exogenous SPARC does not induce a pro-fibrotic phenotype in dermal fibroblast. However, in a TGF-β-induced pro-fibrotic environment, exogenous SPARC enhances the fibrotic phenotype of dermal fibroblasts. Therefore, SPARC neutralization could be an interesting approach for reducing fibrosis even in advanced stages of SSc.
Supplementary Material
Acknowledgements
T.C. was supported by a grant from the Portuguese national funding agency for science, research and technology: Fundação para a Ciência e a Tecnologia [SFRH/BD/93526/2013].
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Gabrielli A, Avvedimento EV, Krieg T.. Scleroderma. N Engl J Med 2009;360:1989–2003. [DOI] [PubMed] [Google Scholar]
- 2. Ebmeier S, Horsley V, Haven N, Haven N.. Origin of fibrosing cells in systemic sclerosis. Curr Opin Rheumatol 2015;27:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal 2009;3:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradshaw AD. Diverse biological functions of the SPARC family of proteins. Int J Biochem Cell Biol 2012;44:480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strandjord TP, Madtes DK, Weiss DJ, Sage EH.. Collagen accumulation is decreased in SPARC-null mice with bleomycin-induced pulmonary fibrosis. Am J Physiol 1999;277:L628–35. [DOI] [PubMed] [Google Scholar]
- 6. Wang JC, Lai S, Guo X. et al. Attenuation of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Res Ther 2010;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davies CA, Jeziorska M, Freemont AJ, Herrick AL.. Expression of osteonectin and matrix Gla protein in scleroderma patients with and without calcinosis. Rheumatology 2006;45:1349–55. [DOI] [PubMed] [Google Scholar]
- 8. Macko RF, Gelber AC, Young BA. et al. Increased circulating concentrations of the counteradhesive proteins SPARC and thrombospondin-1 in systemic sclerosis (scleroderma). Relationship to platelet and endothelial cell activation. J Rheumatol 2002;29:2565–70. [PubMed] [Google Scholar]
- 9. Vuorio T, Kahari VM, Black C, Vuorio E.. Expression of osteonectin, decorin, and transforming growth factor-beta 1 genes in fibroblasts cultured from patients with systemic sclerosis and morphea. J Rheumatol 1991;18:247–51. [PubMed] [Google Scholar]
- 10. Zhou X, Tan FK, Guo X, Arnett FC.. Attenuation of collagen production with small interfering RNA of SPARC in cultured fibroblasts from the skin of patients with scleroderma. Arthritis Rheum 2006;54:2626–31. [DOI] [PubMed] [Google Scholar]
- 11. Van Den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sacco AM, Belviso I, Romano V. et al. Diversity of dermal fibroblasts as major determinant of variability in cell reprogramming. J Cell Mol Med 2019;23:4256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shibata S, Ishiyama J.. Secreted protein acidic and rich in cysteine (SPARC) is upregulated by transforming growth factor (TGF)-β and is required for TGF-β-induced hydrogen peroxide production in fibroblasts. Fibrogenes Tissue Repair 2013;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Francki A, Bradshaw AD, Bassuk JA. et al. SPARC regulates the expression of collagen type I and transforming growth factor-β1 in mesangial cells. J Biol Chem 1999;274:32145–52. [DOI] [PubMed] [Google Scholar]
- 15. Lafyatis R. Transforming growth factor β—at the centre of systemic sclerosis. Nat Rev Rheumatol 2014;10:706.. [DOI] [PubMed] [Google Scholar]
- 16. Reed MJ, Vernon RB, Abrass IB, Sage EH.. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol 1994;158:169–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.