Abstract
Objectives
To investigate changes in BMD in RA patients receiving 3-year biological/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) or conventional synthetic DMARD (csDMARD).
Methods
Patients with RA were recruited from September 2014 until March 2019. Clinical characteristics, BMD and evidence of fragility fractures at enrolment were documented. Participants were treated according to the National Institute for Health and Care Excellence (NICE) guidelines over a 3-year observation period. Repeated BMD was measured at the end of the study period. Participants were grouped into those receiving b/tsDMARD or csDMARD and by propensity score matching (1:2).
Results
A total of 388 participants completed the 3-year follow-up. After propensity score matching, 92 and 184 participants were allocated to the b/tsDMARD (Group I) and csDMARD (Group II), respectively. After 3 years, BMD remained stable at the femoral neck (FN), hip (total) (TH) and lumbar vertebra (L1-4) (P =0.09, 0.15, 0.87) in Group I. However, BMD decreased significantly in Group II (P=0.045, <0.001, 0.004) at corresponding sites. Participants receiving combined b/tsDMARD and anti-osteoporosis therapy experienced a greater BMD preserving effect than other subgroups.
Conclusion
Long-term b/tsDMARDs therapy had protective effects on bone loss for patients with RA. Patients receiving concomitant anti-osteoporosis therapy and b/tsDMARDs therapy experienced the greatest BMD preserving effect.
Keywords: rheumatoid arthritis, biological therapies, bone, osteoporosis, DMARDs
Rheumatology key messages
Long-term b/tsDMARDs benefit RA patients not only in ameliorating disease activity but also preserving bone mass.
RA patients who underwent csDMARDs, compared to b/tsDMARDs, experienced more substantial bone loss.
Introduction
RA, an autoimmune disease primarily affecting peripheral joints, also leads to deteriorated skeletal microarchitecture and bone strength. This deterioration is due to the release of proinflammatory cytokines such as TNF-α, IL-6 and IL-17, which interferes with the balance between bone formation by osteoblasts and resorption by osteoclasts. The chronic inflammation in RA not only results in peripheral bony erosion but in generalized bone loss. It has been demonstrated that the annual bone loss rate in patients with active RA ranges between 5.5% and 10% [1]. Compared with the general population, the prevalence of osteoporosis is approximately two-fold greater in patients with RA [2], ranging from 7% to 26% in the hip and 11% to 32% in the spine [2–5]. In addition, the risk of developing clinical or hip/vertebral fractures in patients with RA has been reported to be 2.25 or 2–6 fold, respectively, and was higher than that of controls [6].
In recent decades, several cytokines inhibitors, in particular biological/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) have been developed. The b/tsDMARD therapy directly targets pathological cytokines and halts the inflammatory cascade and has demonstrated anti-inflammatory effects for patients with RA. Since the advent of b/tsDMARDs, the regimens have demonstrated marked clinical symptom alleviation, improved quality of life, and decelerated joint damage in patients with RA. In addition, the notion of treat-to-target strategy accelerated the optimisation of patient management and outcomes [7].
In previous studies [8–13], b/tsDMARDs had demonstrated a potentially beneficial effect on bone loss. However, these studies either focused on changes in bone turnover markers, [10–13] had a short-term observation period, [8, 14–17] or lacked an adequate control group [8, 9]. Therefore, the long-term effect of b/tsDMARDs on generalized bone loss in patients with RA remained unknown. Medications for the treatment of postmenopausal osteoporosis including denosumab, bisphosphonates and parathyroid hormone (hPTH 1–34) have been assessed in terms of preventing bone loss in patients with RA. They have demonstrated efficacy in the prevention of systemic bone loss or in reducing localized bone erosions [18–20].
In the current study, we aimed to investigate the impact of long-term b/tsDMARDs therapy on BMD changes in patients with RA via a 3-year real-world, prospective, cohort and observational study. In addition, we explored the synergistic effect of a 3-year treatment with b/tsDMARDs and with or without anti-osteoporosis therapy (AOT) on BMD changes in patients with RA.
Methods
Study population
The inclusion criteria for participants and the methods for the current study have been previously published [21]. In brief, this was an interim analysis of an RA-related osteoporosis/fracture registry study conducted at Chang Gung Memorial hospital, Kaohsiung (CGMHK), Taiwan. In the registry, consecutive patients with RA who fulfilled the 1987 ACR revised criteria [22] or the 2010 ACR/European League Against Rheumatism (EULAR) classification criteria [23] and who had visited the rheumatology clinic at CGMHK since 1 September 2014, were enrolled. We excluded subjects who were <20 years of age, had any malignancy during the previous 5 years, or were unwilling to join in the study.
Clinical assessments included demographic data, presence of anti-cyclic citrullinated peptide antibodies (anti-CCP) and RF. Disease duration was defined as the time elapsed between the onset of the first disease-related symptoms and enrolment in the study. RA activity was assessed using the DAS in 28 joints based on ESR (DAS28-ESR). Information on current medications at the time of registration was collected. In addition, lifestyle factors, evidence of previous fragility fractures (history or radiographic), and risk factors for fragility fracture based on the FRAX® tool were recorded. The 10-year probability of major and hip fractures was calculated and recorded. Prescription of systemic glucocorticoid (oral, intravenous, subcutaneous or intramuscular administration) was recorded at baseline and during the study period, converting to a prednisolone-equivalent dose. We defined baseline exposure as current steroid usage at the start date of study for >3 months or having been exposed for >3 months before the start date, and calculated the average dose within the latest 3 months. Cumulative exposure was defined as any systemic glucocorticoid exposure during the study cohort, and the cumulative dose was determined at the end of study, recording all the available, systemic glucocorticoid prescription and changing to daily, prednisolone-equivalent dose.
The BMD of each patient was measured at enrolment using a dual-energy X-ray absorptiometry scanner (Delphi A; Hologic Corp., Waltham, MA, USA) for femoral neck (FN), hip (total) (TH) and lumbar vertebra (L1–L4). Body height, body weight and BMI were recorded at baseline. And DAS28-ESR, laboratory assessments for each participant were documented every 3–6 months during the 3-year observation period. Repeated BMD measurements and radiographs at the same site as enrolment were performed for each participant at the end of the 3-year observation period. Each image was read by an independent radiologist, according to Genant’s semiquantitative assessment of vertebral fractures [24] to assess the evidence of vertebral compression fracture at enrolment and 3 years later. Data ceased to be collected on 19 March 2019. During the study period, participants were treated according to National Institute for Health and Care Excellence (NICE) guidelines or 2013/2016 ACR/EULAR recommendations targeted at sustaining low disease activity or remission [25, 26]. Those who completed the 3-year study period were recruited for the analysis.
The participants were grouped based on medications used in the study period. Those who received continuous b/tsDMARDs therapy for at least one year were in the b/tsDMARD group, while those who did not receive any b/tsDMARDs and took only csDMARDs was categorized into the csDMARDs group. In this study, b/tsDMARDs included anti-TNFα (etanercept, adalimumab, golimumab, certolizumab), anti-IL6 receptor (tocilizumab), CTLA4 analogue (abatacept), anti-CD 20 (rituximab) and JAK inhibitor (tofacitinib). The csDMARDs included methotrexate, sulfasalazine, hydroxychloroquine, azathioprine and leflunomide. The propensity score for each participant was calculated based on imbalanced covariates between the b/tsDMARD and csDMARDs groups by logistic regression. Participants were then matched by using an optimal method with a 0.2 calliper width to create 1:2 (b/tsDMARD: csDMARDs) matched groups using NCSS software (NCSS 9; NCSS statistical software, 2013, Kaysville, Utah, USA). Each participant provided written informed consent and the study was conducted with the approval of the Regional Ethical Review Board of CGMHK (106–0047 C).
Statistics and propensity score matching
Continuous variables were analysed using Student's t test and the Mann–Whitney U test, whereas categorical variables were evaluated by the Chi-squared test or Fisher's exact test. P-values <0.05 were considered statistically significant. The change in BMD of each participant from baseline was calculated by paired t test. We performed the statistical analyses using the Statistical Package for the Social Sciences (SPSS, version 20.0, Chicago, IL, USA). Trend analyses of BMD changes were achieved by one-way analysis of variance (ANOVA) under each category of treatment regimen.
Results
A total of 651 participants were registered for the study cohort. A total of 388 participants completed the 3-year follow-up and were enrolled in this study. The characteristics of the participants are shown in Fig. 1. Before propensity score matching (PSM), there were 102 participants allocated to the b/tsDMARD group, and 286 were allocated to the csDMARD group. The baseline demographics and clinical characteristics of the participants are shown in the left column of Table 1. The participants in the b/tsDMARD group, before matching, were younger in age (P = 0.02), had a higher body weight (P = 0.03) and height (P = 0.01), less comorbidity (P = 0.04), higher DAS-28 and ESR (DAS28-ESR) (P = 0.001), a higher rate of RF positivity (P = 0.005), a higher mean steroid dose (P = 0.05), and a higher BMD at L1–4 (P = 0.005) than that of the csDMARD group (left column, Table 1). After appropriate propensity score matching (b/tsDMARD: csDMARD, 1:2) by adjusting the imbalanced covariables, including age, body weight and height, RF positivity, mean steroid dose and BMD at L1-4, between groups we obtained 276 matched participants, of whom 92 participants were allocated to the b/tsDMARD group (Group I) and 184 participants to the csDMARD group (Group II). After PSM, all the imbalanced covariables between the groups except baseline DAS28-ESR, mean DAS28-ESR and baseline HAQ disability index (HAQ-DI) were matched. The mean age of participants in Group I and II was 56.6 (8.7) and 57.2 (9.8) (P = 0.65), respectively. Participants in Group I had a significantly higher baseline DAS28-ESR (3.7 (1.4) vs 3.3 (1.0), P < 0.001), 3-year mean DAS28-ESR (3.3 (1.0) vs 3.1 (0.9), P = 0.046), and a higher HAQ-DI (6.1 ( 6.0) vs 4.0 (5.6), P = 0.006) than that of participants in Group II. The total duration, including before and after enrolment, of b/tsDMARD exposure in Group I was 6.1 (3.9) years. The proportion of each biologic or target-synthetic DMARD use in Group I was anti-TNF (n = 59, 64.1%), tocilizumab (n = 12, 13.0%), abatacept (n = 11, 12.0%), rituximab (n = 3, 3.3%), class-switch (n = 7, 7.6%) respectively. In those who received b/tsDMARDs class-switching therapy, three switched from anti-TNF to abatacept, two switched from anti-TNF to anti-IL 6 receptor, one switched from anti-TNF to tofacitinib, and one shifted from anti-IL 6 receptor to tofacitinib. The additional variables of both groups are presented in the Supplementary Material, available at Rheumatology online.
Fig. 1.
Disposition of participants and grouping
Table 1.
Clinical characteristics of participants before and after propensity score match (PSM)
| Groups (before PSM) |
Groups (after PSM) |
|||||
|---|---|---|---|---|---|---|
| b/tsDMARD + csDMARD n = 102 | csDMARD n = 286 | P | Group Icn = 92 | Group IIcn = 184 | P | |
| Age (years) | 56.4 (9.4) | 59.2 (10.2) | 0.02* | 56.6 (8.7) | 57.2 (9.8) | 0.65 |
| Female, n (%) | 87 (85.3) | 247 (86.4) | 0.87 | 78 (84.8) | 151 (82.1) | 0.57 |
| Menopause, n (%) | 69 (79.3) | 206 (83.4) | 0.40 | 62 (79.5) | 120 (79.5) | 0.72 |
| Body weight (kg) | 60.4 (11.6) | 57.4 (11.7) | 0.03* | 61.0 (11.0) | 59.1 (11.1) | 0.17 |
| Body height (cm) | 158.0 (7.0) | 155.9 (7.4) | 0.01* | 158.2 (6.4) | 157.6 (7.1) | 0.46 |
| BMI (kg/cm2) | 24.1 (4.0) | 23.5 ( 3.9) | 0.23 | 24.3(4.0) | 23.7 (3.9) | 0.24 |
| Comorbiditya | 56 (54.9) | 189 (66.1) | 0.04* | 49 (53.3) | 113 (61.4) | 0.20 |
| RA related factors | ||||||
| Disease duration (years) | 15.3 (9.5) | 14.1 (8.9) | 0.25 | 15.6 (9.6) | 13.6 (8.6) | 0.07 |
| DAS28-ESR | 3.7 (1.3) | 3.2 (1.1) | 0.001* | 3.7 (1.4) | 3.2 (1.1) | <0.001* |
| 3-year mean DAS 28-ESR | 3.3 (1.0) | 3.1 (0.9) | 0.03* | 3.3 (1.0) | 3.1 (0.9) | 0.046* |
| RF, + (%) | 79 (77.5) | 176 (61.8) | 0.005* | 72 (78.3) | 147 (79.9) | 0.75 |
| ACPA, + (%) | 78 (76.5) | 186 (65.5) | 0.05 | 70 (76.1) | 133 (73.1) | 0.59 |
| ESR (mm/h) | 25.3 (21.2) | 22.0 (19.4) | 0.15 | 24.8 (19.9) | 22.7 (20.7) | 0.44 |
| CRP (mg/l) | 7.2 (13.8) | 7.5 (15.7) | 0.86 | 7.2 (13.8) | 8.3 (17.6) | 0.62 |
| HAQ-DI | 6.1 (6.1) | 4.6 (6.0) | 0.04* | 6.1 (6.0) | 4.0 (5.6) | 0.006* |
| FRAX risk factorsb | ||||||
| Previous fracture +, n (%) | 28 (27.5) | 101 (35.3) | 0.15 | 26 (28.3) | 59 (32.1) | 0.52 |
| 2nd Osteoporosis +, n (%) | 3 (2.9) | 14 (4.9) | 0.58 | 3 (3.3) | 8 (4.3) | 0.66 |
| Glucocorticoidd | ||||||
| Baseline exposure +, n (%) | 87 (85.3) | 251 (87.8) | 0.50 | 78 (84.8) | 159 (86.4) | 0.71 |
| Dose (mg/day) | 4.9 (1.0) | 4.5 (1.5) | 0.05* | 4.9 (1.9) | 4.6 (1.6) | 0.19 |
| Cumulative exposure +, n (%) | 97 (95.1) | 270 (94.4) | 1.00 | 87 (94.6) | 174 (94.6) | 1.00 |
| Cumulative dose (mg/day) | 4.0 (2.5) | 4.2 (2.3) | 0.47 | 3.9 (2.5) | 4.3 (2.4) | 0.18 |
| Parent fractured hip +, n (%) | 8/102 (7.8) | 27/282 (9.6) | 0.60 | 8 (8.7) | 19 (10.3) | 0.67 |
| BMD (g/cm2) | ||||||
| FN | 0.784 (0.144) | 0.785 (0.136) | 0.92 | 0.634 (0.117) | 0.642 (0.115) | 0.54 |
| TH | 0.630 (0.115) | 0.626 (0.117) | 0.81 | 0.793 (0.146) | 0.807 (0.131) | 0.35 |
| L1-4 | 0.901 (0.171) | 0.847 (0.161) | 0.005* | 0.904 (0.168) | 0.877 (0.145) | 0.20 |
| Current smoking +, n (%) | 5 (4.9) | 18 (6.3) | 0.61 | 5 (5.4) | 16 (8.7) | 0.34 |
| Alcohol +, n (%) | 1 (1.0) | 4 (1.4) | 1.00 | 1 (1.1) | 3 (1.6) | 0.72 |
| AOT +, n (%) | 30 (29.4) | 102 (35.7) | 0.25 | 27 (29.3) | 56 (30.4) | 0.85 |
| Laba | ||||||
| Calcium (mg/dl) | 9.3 (0.3) | 9.3 (0.4) | 0.90 | 9.3 (0.3) | 9.3 (0.4) | 0.77 |
| Vit D25(OH) (ng/ml) | 22.1 (7.9) | 22.7 (7.4) | 0.54 | 22.1 (8.1) | 23.0 (7.3) | 0.39 |
| iPTH (pg/ml) | 40.9 (19.9) | 44.0 (22.8) | 0.23 | 41.5 (19.8) | 40.4 (19.2) | 0.65 |
Specific items refer to Supplementary Material, available at Rheumatology online.
Defined as in FRAX tool (www.sheffield.ac.uk/FRAX/index.aspx? lang=en).
Group I: b/tsDMARDs ± csDMARD; Group II: csDMARD.
Systemic glucocorticoid (oral, intravenous, subcutaneous or intramuscular administration) was captured as prednisolone-equivalent dose. We define baseline exposure as current steroid usage at the start date of study for >3 months or had been exposed for >3 months before the start date, and calculate the average dose within the latest 3 months. Cumulative exposure was defined as any systemic glucocorticoid exposure during the study cohort, and the cumulative dose was determined at the end of study, recording all the available, systemic glucocorticoid prescription and calculating daily dose.
*P-value < 0.05. DAS28-ESR: disease activity score in 28 joints with ESR; HAQ-DI: health assessment questionnaire disability index; AST: aspartate aminotransferase; ALT: alanine transaminase; ALK-P: alkaline phosphatase; iPTH: intact parathyroid hormone. Anti-TNF: including etanercept, adalimumab, golimumab and opinercept. FN: femoral neck; AOT: anti-osteoporosis therapy, including bisphosphonate(s), denosumab, teriparatide, selective oestrogen receptor modulator (SERM); PSM: propensity score match.
Changes in BMD between groups
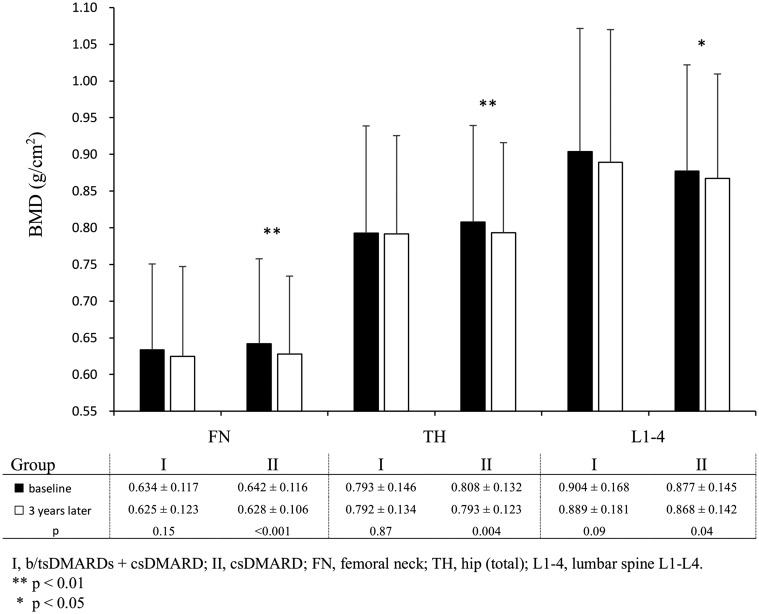
Compared with baseline, the BMD at FN, TH and L1-4 after 3 years in Group I exhibited non-significant changes (P = 0.15, P = 0.87, P = 0.09, respectively). The BMD of participants in Group II revealed significant bone loss at FN, TH and L1-4 (P < 0.001, P = 0.004, P = 0.045, respectively). (Fig. 2)
Fig. 2.
Comparison of BMD at baseline and 3 years later at FN, TH and L1-4 in Group I and II participants
The changes in BMD in participants of both groups who did or did not receive AOT is shown in Figs 3A and 3B. Compared with baseline, the BMD of the non-users of AOT in Group I participants remained stable at TH, but declined substantially at the FN and L1-4 (P = 0.046, P = 0.004, respectively). However, non-users of AOT in Group II demonstrated significant BMD reduction at FN, TH and L1-4 (all P < 0.001) (Fig. 3A). BMD at three measured sites remained stable or slightly increased in participants who received AOT in both groups, irrespective of the use of b/tsDMARD (Fig. 3B).
Fig. 3.
Difference of BMD between baseline and 3 years later in patients receiving csDMARDs or adding on b/tsDMARDs, combined AOT use or not
(A) Difference of BMD between baseline and 3 years later in patients receiving csDMARDs or adding on b/tsDMARDs, combined AOT use or not. Comparison of BMD at baseline and 3 years later in group I and II participants who did not receive anti-osteoporosis therapy during study period. (B) Difference of BMD between baseline and 3 years later in patients receiving csDMARDs or adding on b/tsDMARDs, combined AOT use or not. Comparison of BMD at baseline and 3 years later in participants who received anti-osteoporosis therapy during study period and b/tsDMARDs or not.
The subgroup analysis revealed that participants in Group II who did not receive AOT (Group II/AOT–) demonstrated clear bone loss at the FN (–2.8%), TH (–2.3%) and L1-4 (–2.4%) than the other subgroups (Group I/AOT+, Group I/AOT– and Group II/AOT+) (Fig. 4A, B and C). As demonstrated in Fig. 4, there was a trend that participants who received b/tsDMARD alone, AOT alone, or combined therapy had progressively better bone loss protection at all skeletal sites than those without either therapy. This phenomenon was most outstanding at FN and L-1-4 (P for trend = 0.018 and 0.001, respectively).
Fig. 4.
BMD change from baseline at different measured sites in group I and II, with or without use of AOT
(A) BMD change from baseline at different measured sites in group I and II, with or without use of AOT. BMD changes from baseline of FN in participants received b/tsDMARDs/csDMARD and/or AOT. (B) BMD change from baseline at different measured sites in group I and II, with or without use of AOT. BMD changes from baseline of TH in participants received b/tsDMARDs/csDMARD and/or AOT. (C) BMD change from baseline at different measured sites in group I and II, with or without use of AOT. BMD changes from baseline of L1-4 in participants received b/tsDMARDs/csDMARD and/or AOT.
Discussion
This 3-year observational cohort study revealed that b/tsDMARDs preserved BMD at the FN, TH and L1-4, whereas participants taking csDMARD experienced substantial BMD loss compared with baseline. Several studies have reported the effect of various biologics, mainly anti-TNF, on serial BMD changes in patients with RA [8, 10–17, 27–30]. However, previous studies had a short observation period (1–2 years) [8, 14–17], a small sample size [8, 11, 29], were retrospective in character [30], had no parallel control group [8, 27, 28], or studied the changes in bone markers instead of actual BMD [10–13]. To overcome these limitations, we conducted this prospective, longitudinal, observational, real-world, controlled PSM study.
Despite the limitations of the previous studies, biologics therapy for RA either increased BMD [8, 30] or at least had a non-detrimental effect on BMD [31, 32] compared with baseline. Krieckaert et al. showed that after 1 year of adalimumab treatment, BMD of the hip and lumbar spine remained stable [33]. Marotte et al. also demonstrated that BMD at the spine and FN did not change after one year of infliximab treatment [34]. However, previous studies provided inconclusive evidence of the impact on BMD of b/tsDMARDs with different mechanisms of action.
Consistent with previous studies on anti-TNFs, our investigation revealed that participants in Group I demonstrated no substantial BMD loss from baseline after 3 years. However, Group II participants experienced substantial bone loss at the FN (P < 0.001), hip (total) (P = 0.004) and L1-4 (P = 0.04). This finding suggests that long-term b/tsDMARD therapy can protect against generalized osteoporosis in patients with RA better than csDMARD therapy.
In addition to strict control of disease activity by b/tsDMARD therapy, which might arrest generalized bone loss, bisphosphonate [35, 36] and denosumab [37] also demonstrated a protective effect against bone loss on patients with RA. As current study is a real-world investigation, we did not exclude participants who received AOT during the observation period to elucidate the interaction of b/tsDMARD therapy and AOT in terms of bone protective effects. As demonstrated in Fig. 3A, Group II participants who did not receive AOT had clear bone loss at all sites (P < 0.001), while, those in Group I who did not receive AOT experienced a protective effect against bone loss only at the hip (total) but not the FN (P = 0.046) or L1-4 (P = 0.004). On the other hand, AOT had a protective effect against bone loss in both groups at all sites (Fig. 3B). These results suggest that AOT plays the most important role in bone loss protection for patients with RA receiving either b/tsDMARD or csDMARD therapy.
In terms of combination therapy, although certain studies [33, 38] enrolled patients with RA who were treated with biologics and concomitant anti-osteoporosis medications, they did not discuss the interaction effect between b/tsDMARD and AOT on protection against bone loss. In the current investigation, the magnitude of percentage changes in BMD loss (ΔBMD%) at the FN by groups was as follows: b/tsDMARD+/AOT+ > csDMARD+/AOT+ > b/tsDMARD+/AOT- > csDMARD+/AOT- (P for trend = 0.018) (Fig. 4A). There was a similar trend at TH (P for trend = 0.06) and L1-4 (P for trend = 0.001) (Figs 4B and C) for these four therapy combinations. To the best of our knowledge, ours is the first study to reveal the additive effect of b/tsDMARD and AOT on the prevention of bone loss in patients with RA. Our results suggest that co-administration of b/tsDMARD and AOT has a better protective effect against generalized bone loss in patients with RA than either b/tsDMARD or AOT alone.
It has been proposed that the anti-inflammatory character of glucocorticoids and b/tsDMARD drugs underlies the protective effects against bone loss in patients with early RA [39, 40]. In the current study, the mean baseline DAS28-ESR and mean 3-year DAS 28-ESR were 3.7 (1.4) and 3.3 (1.0) (P < 0.001) in Group I participants, while it was 3.2 (1.1) and 3.1 (0.9) (P = 0.08) in Group II participants. Given Group I participants demonstrated less bone loss at all sites than those in Group II at the 3-year follow-up (Fig. 2), the anti-inflammatory character of b/tsDMARD likely played an essential role in protecting against bone loss not only in early RA but also in established RA after 3 years of therapy. Several cytokines, including TNFα, IL-1, IL-6 and IL-17 had activating effect on osteoclast differentiation and proliferation [41]. The b/tsDMARDs could counteract the effect of cytokines on bone through various mechanisms in patients with RA. Whether long-term b/tsDMARDs therapy has a direct inhibitory effect on osteoclastogenesis, which then mediated the bone loss in patients with RA, is not yet known.
Several studies have suggested that low-dose glucocorticoids have a negative on bone metabolism, but this effect might be nullified by the assistances of the suppression of inflammatory cytokines, particularly in early RA [42]. On the other hand, glucocorticoids have been proposed as a risk factor for bone loss in patients with RA who are receiving biologics [38]. Therefore, to investigate the effect of b/tsDMARD on bone loss protection, we must consider the effect of glucocorticoids. In the current study, when considering possible confounding factors, we made use of PSM to retrieve the control group (Group II) to eliminate the confounding factors, including use and dosage of glucocorticoids. However, although the use and dosage of glucocorticoids was considered, it did not show a substantial effect on bone loss in either group (data not shown).
As mentioned, the observation period of most studies investigating the effect of biologics on bone loss in RA was 1–2 years, and most reassessed BMD after a 1-year follow-up [8, 11, 29, 31]. As suggested by Orsolini et al. [43] the time interval of the follow-up assessment must be long enough to determine whether any change is real and to exclude precision errors of repeated measurements. In the case of RA, a 1-year observation period might not be long enough to draw any solid conclusion. Hence, a 3-year follow-up period, as with our study, seems more adequate to assess BMD changes and has been performed in several pivotal anti-osteoporosis medication studies [44, 45].
A strength of our study is that we measured and recorded many variables at baseline, including levels of 25(OH) Vitamin D intact parathyroid hormone (iPTH), mean DAS28-ESR and lifestyle, among others that could potentially influence BMD changes during the study period. In addition, most previous studies were single-arm studies without a control group or with a control group of patients without RA, which might not provide confirmative evidence regarding whether b/tsDMARD has a better bone loss protective effect than csDMARD. This was our rationale for enrolling an adequate control group by PSM. After PSM, the major contributing factors, such as age, gender, body weight and BMI were adjusted between the groups. This study design is a novel investigation model for researching the effect of DMARDs on BMD in patients with RA. Furthermore, we included participants who received AOT before enrolment or during the observation period. In this way, we were able to analyse the synergistic effect of b/tsDMARD and AOT on bone.
The current study has some limitations. We did not measure bone markers to support our observations. However, the results of previous studies on biologics in terms of bone marker changes were consistent with our results [8, 27, 46–48], showing an increase in bone formation markers and a decrease in bone resorption markers. In addition, bone markers are only a surrogate index of BMD changes and are subject to significant day-to-day variations, which is why we did not measure bone markers in this study. Fracture prevention is a difficult outcome of our study instead of BMD changes only. However, data concerning the effects of b/tsDMARD on fracture risk are scarce and reveal conflicting results [49, 50]. Our data did not find a significantly lower new fracture rate in Group I than Group II (data not shown). In the future, studies must enrol more participants and have a longer follow-up to be able to elucidate whether b/tsDMARD therapy can reduce fracture risk compared with csDMARD therapy. In the current study, we enrolled participants who received either biologics or target therapy (JAK inhibitors). In these groups, we could not differentiate between the extent of bone loss protection effect.
Further studies are warranted to explore the specific influence of each biologic or target therapy on bone health and metabolism, and future research should focus on better profiling of patients with RA, targeting various therapeutic choices and more extended observation periods.
Conclusion
We conclude that long-term b/tsDMARDs therapy for patients with RA had a protective effect on bone loss at all sites measured. On the other hand, patients on conventional therapy experienced substantial bone density loss. Patients with RA who received AOT experienced a protective effect on bone loss, irrespective of b/tsDMARDs or csDMARDs therapy.
Supplementary Material
Acknowledgement
We are grateful for the support and instruction we have received from Taiwan Bone Muscle Joint Total Care Association (TBMJ).
Funding: This work was supported with grant CMRPG8F1111 from Chang Gung Memorial Hospital (https://www.cgmh.org.tw/), which sponsored the cost of data collecting, inputting and processing as well as the publication. The funder had no role in the study design, data analysis, decision to publish or manuscript preparation.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Gough AK, Lilley J, Eyre S, Holder RL, Emery P.. Generalised bone loss in patients with early rheumatoid arthritis. Lancet 1994;344:23–7. [DOI] [PubMed] [Google Scholar]
- 2. Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK.. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum 2000;43:522–30. [DOI] [PubMed] [Google Scholar]
- 3. Guler-Yuksel M, Bijsterbosch J, Goekoop-Ruiterman YP. et al. Bone mineral density in patients with recently diagnosed, active rheumatoid arthritis. Ann Rheum Dis 2007;66:1508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lodder MC, de Jong Z, Kostense PJ. et al. Bone mineral density in patients with rheumatoid arthritis: relation between disease severity and low bone mineral density. Ann Rheum Dis 2004;63:1576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sinigaglia L, Nervetti A, Mela Q. et al. A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian Study Group on Bone Mass in Rheumatoid Arthritis. J Rheumatol 2000;27:2582–9. [PubMed] [Google Scholar]
- 6. Xue AL, Wu SY, Jiang L. et al. Bone fracture risk in patients with rheumatoid arthritis: a meta-analysis. Medicine 2017;96:e6983.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Vollenhoven R. Treat-to-target in rheumatoid arthritis - are we there yet?. Nat Rev Rheumatol 2019;15:180–6. [DOI] [PubMed] [Google Scholar]
- 8. Lange U, Teichmann J, Müller-Ladner U, Strunk J.. Increase in bone mineral density of patients with rheumatoid arthritis treated with anti-TNF-alpha antibody: a prospective open-label pilot study. Rheumatology 2005;44:1546–8. [DOI] [PubMed] [Google Scholar]
- 9. Eekman DA, Vis M, Bultink IEM. et al. Stable bone mineral density in lumbar spine and hip in contrast to bone loss in the hands during long-term treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis 2011;70:389–90. [DOI] [PubMed] [Google Scholar]
- 10. Vis M, Wolbink GJ, Lodder MC. et al. Early changes in bone metabolism in rheumatoid arthritis patients treated with infliximab. Arthritis Rheum 2003;48:2996–7. [DOI] [PubMed] [Google Scholar]
- 11. Chopin F, Garnero P, le Henanff A. et al. Long-term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann Rheum Dis 2007;67:353–7. [DOI] [PubMed] [Google Scholar]
- 12. Ziolkowska M, Kurowska M, Radzikowska A. et al. High levels of osteoprotegerin and soluble receptor activator of nuclear factor kappa B ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor alpha treatment. Arthritis Rheum 2002;46:1744–53. [DOI] [PubMed] [Google Scholar]
- 13. Torikai E, Kageyama Y, Takahashi M. et al. The effect of infliximab on bone metabolism markers in patients with rheumatoid arthritis. Rheumatology 2006;45:761–4. [DOI] [PubMed] [Google Scholar]
- 14. Zerbini CAF, Clark P, Mendez-Sanchez L. et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int 2017;28:429–46. [DOI] [PubMed] [Google Scholar]
- 15. Kume K, Amano K, Yamada S. et al. The effect of tocilizumab on bone mineral density in patients with methotrexate-resistant active rheumatoid arthritis. Rheumatology 2014;53:900–3. [DOI] [PubMed] [Google Scholar]
- 16. Briot K, Rouanet S, Schaeverbeke T. et al. The effect of tocilizumab on bone mineral density, serum levels of Dickkopf-1 and bone remodeling markers in patients with rheumatoid arthritis. Joint Bone Spine 2015;82:109–15. [DOI] [PubMed] [Google Scholar]
- 17. Roussy JP, Bessette L, Bernatsky S, Rahme E, Lachaine J.. Biologic disease-modifying anti-rheumatic drugs and the risk of non-vertebral osteoporotic fractures in patients with rheumatoid arthritis aged 50 years and over. Osteoporos Int 2013;24:2483–92. [DOI] [PubMed] [Google Scholar]
- 18. Deodhar A, Dore RK, Mandel D. et al. Denosumab-mediated increase in hand bone mineral density associated with decreased progression of bone erosion in rheumatoid arthritis patients. Arthritis Care Res 2010;62:569–74. [DOI] [PubMed] [Google Scholar]
- 19. Kaneko T, Okamura K, Yonemoto Y. et al. Short-term daily teriparatide in patients with rheumatoid arthritis. Mod Rheumatol 2018;28:468–73. [DOI] [PubMed] [Google Scholar]
- 20. Shin K, Park SH, Park W. et al. Monthly oral ibandronate reduces bone loss in Korean women with rheumatoid arthritis and osteopenia receiving long-term glucocorticoids: a 48-week double-blinded randomized placebo-controlled investigator-initiated trial. Clin Ther 2017;39:268–78.e2. [DOI] [PubMed] [Google Scholar]
- 21. Cheng TT, Yu SF, Su FM. et al. Anti-CCP-positive patients with RA have a higher 10-year probability of fracture evaluated by FRAX(R): a registry study of RA with osteoporosis/fracture. Arthritis Res Ther 2018;20:16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 23. Aletaha D, Neogi T, Silman AJ. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 24. Genant HK, Wu CY, van Kuijk C, Nevitt MC.. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 2009;8:1137–48. [DOI] [PubMed] [Google Scholar]
- 25. Smolen JS, Landewe R, Breedveld FC. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smolen JS, Landewe R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 27. Dischereit G, Tarner IH, Muller-Ladner U, Lange U.. Infliximab improves bone metabolism and bone mineral density in rheumatoid arthritis and ankylosing spondylitis: a prospective 2-year study. Clin Rheumatol 2013;32:377–81. [DOI] [PubMed] [Google Scholar]
- 28. Yasunori K, Masaaki T, Tetsuyuki N, Hayato K, Akira N.. Reduction of urinary levels of pyridinoline and deoxypyridinoline and serum levels of soluble receptor activator of NF-kappaB ligand by etanercept in patients with rheumatoid arthritis. Clin Rheumatol 2008;27:1093–101. [DOI] [PubMed] [Google Scholar]
- 29. Vis M, Voskuyl AE, Wolbink GJ, Dijkmans BA, Lems WF; OSTRA Study Group. Bone mineral density in patients with rheumatoid arthritis treated with infliximab. Ann Rheum Dis 2005;64:336–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suzuki T, Nakamura Y, Kato H.. Effects of denosumab on bone metabolism and bone mineral density with anti-TNF inhibitors, tocilizumab, or abatacept in osteoporosis with rheumatoid arthritis. Ther Clin Risk Manag 2018;14:453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vis M, Havaardsholm EA, Haugeberg G. et al. Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFkappaB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis 2006;65:1495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tada M, Inui K, Sugioka Y. et al. Abatacept might increase bone mineral density at femoral neck for patients with rheumatoid arthritis in clinical practice: aIRTIGHT study. Rheumatol Int 2018;38:777–84. [DOI] [PubMed] [Google Scholar]
- 33. Krieckaert CL, Nurmohamed MT, Wolbink G, Lems WF.. Changes in bone mineral density during long-term treatment with adalimumab in patients with rheumatoid arthritis: a cohort study. Rheumatology 2013;52:547–53. [DOI] [PubMed] [Google Scholar]
- 34. Marotte H, Pallot-Prades B, Grange L. et al. A 1-year case-control study in patients with rheumatoid arthritis indicates prevention of loss of bone mineral density in both responders and nonresponders to infliximab. Arthritis Res Ther 2007;9:R61.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee JS, Lim DH, Oh JS. et al. Effect of TNF inhibitors on bone mineral density in rheumatoid arthritis patients receiving bisphosphonate: a retrospective cohort study. Rheumatol Int 2019, doi: 10.1007/s00296-019-04418-1. [DOI] [PubMed] [Google Scholar]
- 36. Tada M, Inui K, Sugioka Y. et al. Use of bisphosphonate might be important to improve bone mineral density in patients with rheumatoid arthritis even under tight control: the TOMORROW study. Rheumatol Int 2017;37:999–1005. [DOI] [PubMed] [Google Scholar]
- 37. Nakamura Y, Suzuki T, Kato H.. Denosumab significantly improves bone mineral density with or without bisphosphonate pre-treatment in osteoporosis with rheumatoid arthritis: denosumab improves bone mineral density in osteoporosis with rheumatoid arthritis. Arch Osteoporos 2017;12:80.. [DOI] [PubMed] [Google Scholar]
- 38. Tawaratsumida H, Setoguchi T, Arishima Y. et al. Risk factors for bone loss in patients with rheumatoid arthritis treated with biologic disease-modifying anti-rheumatic drugs. BMC Res Notes 2017;10:765.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Finzel S, Kraus S, Figueiredo CP. et al. Comparison of the effects of tocilizumab monotherapy and adalimumab in combination with methotrexate on bone erosion repair in rheumatoid arthritis. Ann Rheum Dis 2019;78:1186–91. [DOI] [PubMed] [Google Scholar]
- 40. Haugeberg G, Strand A, Kvien TK, Kirwan JR.. Reduced loss of hand bone density with prednisolone in early rheumatoid arthritis: results from a randomized placebo-controlled trial. Arch Intern Med 2005;165:1293–7. [DOI] [PubMed] [Google Scholar]
- 41. Orsolini G, Fassio A, Rossini M. et al. Effects of biological and targeted synthetic DMARDs on bone loss in rheumatoid arthritis. Pharmacol Res 2019;147:104354.. [DOI] [PubMed] [Google Scholar]
- 42. Blavnsfeldt AG, de Thurah A, Thomsen MD. et al. The effect of glucocorticoids on bone mineral density in patients with rheumatoid arthritis: a systematic review and meta-analysis of randomized, controlled trials. Bone 2018;114:172–80. [DOI] [PubMed] [Google Scholar]
- 43. Orsolini G, Adami G, Adami S. et al. Short-term effects of TNF inhibitors on bone turnover markers and bone mineral density in rheumatoid arthritis. Calcif Tissue Int 2016;98:580–5. [DOI] [PubMed] [Google Scholar]
- 44. Black DM, Delmas PD, Eastell R. et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356:1809–22. [DOI] [PubMed] [Google Scholar]
- 45. Cummings SR, San Martin J, McClung MR. et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756–65. [DOI] [PubMed] [Google Scholar]
- 46. Rossini M, Adami G, Viapiana O, Idolazzi L, Gatti D.. Denosumab, cortical bone and bone erosions in rheumatoid arthritis. Ann Rheum Dis 2016;75:e70.. [DOI] [PubMed] [Google Scholar]
- 47. Wheater G, Hogan VE, Teng YK. et al. Suppression of bone turnover by B-cell depletion in patients with rheumatoid arthritis. Osteoporos Int 2011;22:3067–72. [DOI] [PubMed] [Google Scholar]
- 48. Garnero P, Thompson E, Woodworth T, Smolen JS.. Rapid and sustained improvement in bone and cartilage turnover markers with the anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a substudy of the multicenter double-blind, placebo-controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum 2010;62:33–43. [DOI] [PubMed] [Google Scholar]
- 49. Kawai VK, Stein CM, Perrien DS, Griffin MR.. Effects of anti-tumor necrosis factor alpha agents on bone. Curr Opin Rheumatol 2012;24:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim SY, Schneeweiss S, Liu J, Solomon DH.. Effects of disease-modifying antirheumatic drugs on nonvertebral fracture risk in rheumatoid arthritis: a population-based cohort study. J Bone Miner Res 2012;27:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.