Abstract
PURPOSE
Prevention of xerostomia and stress is important to prolong healthy life expectancy and improve the quality of life. We aimed to investigate the effects of tongue rotation exercise for increasing salivary secretions and stabilizing salivary stress hormone levels.
MATERIALS AND METHODS
Twenty four participants without subjective oral dryness were enrolled. The exercises comprised tongue rotation exercise and empty chewing. The salivary stress hormone level was measured using a Salivary Amylase Monitor. Unstimulated whole saliva volume and salivary amylase activity were measured before tongue rotation exercise or empty chewing and subsequently 5, 10, and 15 minutes after these exercises. Differences in the rates of change of unstimulated whole saliva volume and salivary amylase activity were analyzed by repeated measure analysis of variance.
RESULTS
Statistically significant differences among the rates of change were not observed after empty chewing for unstimulated whole saliva volume and salivary amylase activity at the four measurement times. However, the rate of change of unstimulated whole saliva volume and salivary amylase activity were statistically significantly different among the four time points: before the tongue rotation exercise and 5, 10, and 15 minutes post-exercise (P< .05 and P<.01, respectively).
CONCLUSION
Tongue rotation is effective in increasing saliva secretion, reducing stress, improving oral function, and extending healthy life expectancy.
Keywords: Tongue rotation exercise, Salivary secretion, Salivary stress hormone
INTRODUCTION
In Japan's super-aging society, extending the healthy lifespan is desirable. Oral health is an essential component of a healthy life expectancy. Oral dysfunction can cause disorders such as xerostomia,1 mastication disorder, and dysphagia. Many elderly patients complain of xerostomia, with 50 – 88% of the elderly exhibiting salivary hypofunction status.2,3 Xerostomia can cause difficulties in speaking, chewing, tasting, and swallowing,4,5 may exacerbate dental caries, bacterial infection, and Candida infection,6 and typically results in the decrease of oral health-related quality of life in elderly patients. Thus, prevention of xerostomia is key to extending the healthy life expectancy and improving the quality of life. Additionally, stress status is important for extending healthy life expectancy, and research has indicated a clear relationship between stress and mortality.7,8
Recently, oral exercises such as lip stretching, tongue stretching, masticatory exercises, cheek stretching, speaking exercises, and swallowing exercises have been recommended to prevent or improve oral dysfunction. Oral function decreases with age, and the velocity of tongue movements and tongue motor skills are also affected by age.9 The tongue plays an important role as the major propulsive force during swallowing.10 Numerous studies have reported tongue strengthening and improvement of tongue pressure via the tongue stretching exercise using the Iowa Oral Performance Instrument (IOPI)10,11,12 or tongue depressor.13 Our previous study introduced tongue rotation exercise training and concluded that this method served to increase both maximum tongue pressure and labial closure strength of normal adults.14 Further, a recent report demonstrated that oral exercises can exert a beneficial effect on the peripheral and central nervous systems.15
Many individual muscles participate during the performance of the tongue exercise. The vagus nerve, which is a part of the parasympathetic nervous system, controls the muscles around the oral cavity. Therefore, tongue exercise may serve to stimulate the vagus nerve, altering the activation of the parasympathetic system. Because the parasympathetic nervous system competes with sympathetic nerve activity, it is possible to investigate one by assessing the activity of the other, and sympathetic nervous system stimulation can be evaluated by measuring the salivary amylase level, which increases with psychological stress.
The effects of the tongue stretching exercise have been reported in the contexts of increased tongue pressure and increased unstimulated whole saliva.16,17 However, the effects of a tongue rotation exercise that can be performed anywhere and anytime, without the need of special instruments, have been evaluated only for the purpose of increasing tongue pressure and labial closure strength. We hypothesized that tongue rotation exercise stimulates the muscle around the salivary gland and the vagus nerve, thus promoting salivary secretion and activating the parasympathetic nervous system. This study was designed to investigate the potential of the tongue rotation exercise toward increasing salivary secretion and reducing salivary stress hormone levels.
MATERIALS AND METHODS
Healthy adults without subjective oral dryness (17 men and 7 women; mean age, 25.6 ± 3.1 years) were selected as participants for this study. The sample size was determined by power analysis. The participants comprised volunteer students or staff members at The Nippon Dental University School of Life Dentistry at Niigata. The study conformed to recognized standards of the Declaration of Helsinki, and was approved by the ethics committee of The Nippon Dental University School of Life Dentistry at Niigata (ECNG-H-213). Informed consent was obtained from all participants before the initiation of the study.
The exercises performed in this study were tongue rotation and empty chewing. The tongue rotation exercise procedure involved rotation of tongue every 2 s with the mouth closed. Participants were instructed to perform the exercise by pressing the apex of the tongue against the gingivobuccal fold while watching the clock to employ a speed of 2 s per rotation. For rotation to the right, the tongue was moved toward the right cheek through the back side of the upper lip from the left cheek, then through the back side of the lower lip from the right cheek. This procedure was repeated 20 times, followed by a reverse rotation to the left, also repeated 20 times.14 Empty chewing without a bolus was performed to provide a comparison with the tongue rotation exercise.18 Participants were instructed to perform empty chewing 40 times, with a speed of 2 s per chewing movement. Tongue rotation and empty chewing exercises were performed at the same time of day on different days.
The participants were instructed to abstain from eating and exercising 2 h before the experiment and were seated in relaxed position. The experimental schedule is depicted in Figure 1. First, the volume of unstimulated whole saliva was measured by ejecting gathered saliva from the mouth into a test tube (SARSTEDT AG & Co., Nümbrecht, Germany) for 5 min. Salivary amylase activity was measured automatically using the Salivary Amylase Monitor (Nipro Co., Osaka, Japan) (Fig. 2) for 1 min.19 This hand-held monitor comprises a disposable test strip and a monitor, which is equipped with a saliva-transfer device and an optical device. The collecting paper on the test strip was inserted directly into the oral cavity, and approximately 20 – 30 µL of whole saliva was collected from under the tongue in approximately 30 s. Following this, the test strip was immediately loaded into the automatic saliva-transfer device.19
Fig. 1. Experiment schedule.
Fig. 2. Salivary Amylase Monitor.
Unstimulated whole saliva volume and salivary amylase activity were measured one time at each measurement time: before participants performed the tongue rotation exercise or empty chewing, with subsequent measurements at 5, 10, and 15 min after the exercises. The volume of stimulated whole saliva during the tongue rotation exercise and empty chewing was also measured one time by ejecting gathered saliva from the mouth into a test tube.
The rates of change of unstimulated whole saliva volume and salivary amylase activity were calculated according to the formula (AE − BE) / BE × 100 (%) (AE, the value after the tongue rotation exercise or empty chewing; BE, the value before the tongue rotation exercise or empty chewing) and used for analysis. The ratio of stimulated whole saliva volume during the tongue rotation exercise or empty chewing to unstimulated whole saliva volume before the exercises was calculating according to the formula DE / BE (DE, the volume of stimulated whole saliva during the exercises; BE, the volume of unstimulated whole saliva before the exercises), and used for analysis.
The differences in the rates of change of unstimulated whole saliva volume following tongue rotation or empty chewing exercises at the four measurement times were analyzed by repeated measure ANOVA. Similarly, the differences in the rates of change of salivary amylase activity following the tongue rotation exercise or empty chewing at the four measurement times were analyzed by repeated measure ANOVA. A paired t-test was performed to evaluate the difference in the ratio of stimulated whole saliva volume during the tongue rotation exercise or empty chewing to unstimulated whole saliva volume before the exercises, comparing the tongue rotation exercise with empty chewing. Statistical analysis was performed using statistical analysis software (SPSS 17.0, SPSS Japan, Tokyo, Japan), and differences of α < .05 were considered significant.
RESULTS
Fig. 3 shows the mean rate of change of unstimulated whole saliva volume obtained before and after the tongue rotation exercise and empty chewing at four time points: before exercise and 5, 10, and 15 minutes post-exercise. There were no statistically significant differences in the rates of change of unstimulated whole saliva volume following empty chewing at the four measurement times (P = .727). The rate of change of unstimulated whole saliva volume for the tongue rotation exercise was statistically significantly different among the four time points: before the tongue rotation exercise and 5, 10, and 15 minutes post-exercise (P < .05).
Fig. 3. Rate of change of unstimulated whole saliva volume in the tongue rotation exercise vs. empty chewing.
**: P < .01
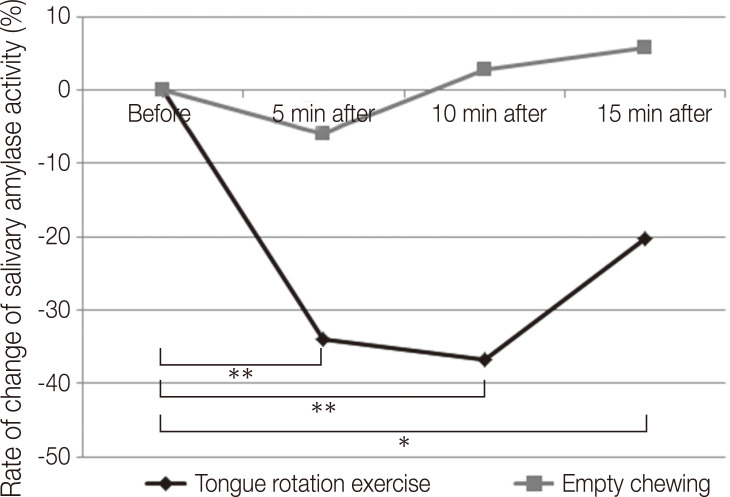
Fig. 4 shows the mean rate of change of salivary amylase activity before and 5, 10, and 15 min after the tongue rotation exercise and empty chewing. There were no statistically significant differences in the rates of change of salivary amylase activity before and after empty chewing (P = .734). Furthermore, the rate of change of salivary amylase activity for the tongue rotation exercise was statistically significantly different among the four time points: before the tongue rotation exercise and 5, 10, and 15 min post-exercise (P < .01).
Fig. 4. Rate of change of salivary amylase activity in the tongue rotation exercise vs. empty chewing.
*: P < .05, **: P < .01
A statistically significant difference in the ratio of stimulated whole saliva volume during the tongue rotation exercise or empty chewing to unstimulated whole saliva volume before the exercises was observed on comparing the tongue rotation exercise with empty chewing (P < .01). The stimulated whole saliva volume obtained during the tongue rotation exercise was approximately three times the volume obtained after empty chewing (Fig. 5).
Fig. 5. Ratio of stimulated whole saliva volume during the tongue rotation exercise and empty chewing to unstimulated whole saliva volume before the exercises.
**: P < .01
DISCUSSION
Oral dysfunction results in oral health disorders such as xerostomia, resulting in decreased oral health-related quality of life among the elderly. In Japan's super-aging society, extending the healthy lifespan is an important goal. Oral exercises such as lip stretching, tongue stretching, masticatory exercises, cheek stretching, speaking exercises, and swallowing exercises have been reported to improve tongue pressure, swallowing function, and xerostomia.10,11,16,20,21
Recently, the tongue rotation exercise was established as program by determining the optimal speed and number of tongue rotations to be performed.14 Practicing this exercise involves the participation of many stomatognathic muscles, including the longitudinal, transverse, genioglossus, hyoglossus, styloglossus, palatoglossus, lateral pterygoid, and geniohyoid muscles.22,23,24 Additionally, tongue rotation exercises performed with the mouth closed involve other stomatognathic muscles, including the masseter, medial pterygoid, anterior cervical, and orbicularis muscles.14
In previous studies, tongue strengthening exercises using the IOPI have been performed by pressing the bulb toward the hard palate with the tongue, using as much force as possible.10,11,12 Another study employed a tongue depressor for tongue directional exercises instead of IOPI.13 Previous tongue strengthening exercises required the use of devices, and the exercise movement was limited to a single direction. In contrast, the tongue rotation exercise used in this study can be performed anytime and anywhere, without the requirement of any device. Furthermore, the tongue rotation exercise works a full spectrum of stomatognathic muscles in comparison with previously published tongue strengthening exercises. Therefore, the tongue rotation exercise can be considered to be a more effective and comprehensive tongue exercise than those reported in previous studies.
This study aimed to investigate the potential of the tongue rotation exercise toward increasing salivary secretion and reducing salivary stress hormone levels in comparison with empty chewing. Empty chewing was set as the baseline condition for comparison because chewing stimulation increases salivary secretion volume,25 and chewing is known to be an effective stress-coping behavior.26
The rates of change of unstimulated whole saliva volume did not vary before or after empty chewing; conversely, the rate of change of unstimulated whole saliva volume increased approximately 25% within 5 min following the tongue rotation exercise. These findings indicate that the tongue rotation exercise serves to increase the unstimulated whole saliva volume. Hakura recently reported that the quantity of saliva increased following an oral function promotion program comprising lip stretching, tongue stretching, masticatory exercises, cheek stretching, speaking exercises, and swallowing exercises.24 Cho et al. demonstrated that the volume of unstimulated saliva doubled after an oral exercise program.16 Ohara et al. reported the effectiveness of an oral health educational program that included lecture, oral hygiene instruction, oral functional exercise, and salivary gland massages for improving the unstimulated salivary flow rate.17 Our study evaluated the short-range effectiveness of the tongue rotation exercise, and the results are consistent with the previous studies. The volume of stimulated whole saliva obtained during the tongue rotation exercise was approximately three times higher than that obtained following empty chewing. Tongue rotation exercise can effectively increase the amount of stimulated saliva compared with empty chewing. The reason that unstimulated and stimulated saliva volumes increased following the tongue rotation exercise is likely to be related to the participation of numerous stomatognathic muscles, which stimulated the salivary glands to secrete saliva. These results indicate that the tongue rotation exercise increases the volume of unstimulated and stimulated saliva and holds promise for patients with oral dryness. In the future, the effectiveness of the tongue rotation exercise should be investigated in patients with oral dryness.
Regarding the rate of change of salivary amylase activities, no significant effect was observed before and after empty chewing at the four measurement times. Mastication during stressful conditions induces the inhibition of stress-induced activation of the autonomic nervous system.27 In this study, the participants were not exposed to stressful conditions; thus, the salivary amylase activity could be expected to remain constant. Further, it was considered that empty chewing may not inhibit stress activation. In contrast, the rate of change of the salivary amylase activity was reduced after performing the tongue rotation exercise. This result suggests that the tongue rotation exercise may inhibit stress activation. Notably, the tongue rotation exercise employs many stomatognathic muscles, which in turn stimulate the vagus nerve and the parasympathetic nervous system such that the salivary amylase activity would be inhibited. The vagus nerve is one of the cranial nerves, and it runs from the medulla oblongata to the cranium, neck, chest, and abdomen.
This study demonstrates that the tongue rotation exercise increases the unstimulated and stimulated saliva volumes and decreases the salivary amylase activity. It is effective in increasing the secretion of saliva and reducing stress. Thus, the tongue rotation exercise holds promise as an effective oral exercise that improves oral function and helps extend the healthy life expectancy.
CONCLUSION
This study investigated the effects of the tongue rotation exercise for increasing salivary secretion and reducing the salivary stress hormone level. The present findings revealed that the tongue rotation exercise effectively increases the volume of saliva and reduces the salivary amylase activity, an indicator of stress level.
References
- 1.Sreebny LM, Valdini A. Xerostomia. A neglected symptom. Arch Intern Med. 1987;147:1333–1337. doi: 10.1001/archinte.147.7.1333. [DOI] [PubMed] [Google Scholar]
- 2.Osterberg T, Landahl S, Hedegård B. Salivary flow, saliva, pH and buffering capacity in 70-year-old men and women. Correlation to dental health, dryness in the mouth, disease and drug treatment. J Oral Rehabil. 1984;11:157–170. doi: 10.1111/j.1365-2842.1984.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 3.Närhi TO, Meurman JH, Ainamo A, Nevalainen JM, Schmidt-Kaunisaho KG, Siukosaari P, Valvanne J, Erkinjuntti T, Tilvis R, Mäkilä E. Association between salivary flow rate and the use of systemic medication among 76-, 81-, and 86-year-old inhabitants in Helsinki, Finland. J Dent Res. 1992;71:1875–1880. doi: 10.1177/00220345920710120401. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo R. Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med. 2000;11:216–229. doi: 10.1177/10454411000110020501. [DOI] [PubMed] [Google Scholar]
- 5.Ikebe K, Nokubi T, Sajima H, Kobayashi S, Hata K, Ono T, Ettinger R. Perception of dry mouth in a sample of community-dwelling older adults in Japan. Spec Care Dentist. 2001;21:52–59. doi: 10.1111/j.1754-4505.2001.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 6.Torres SR, Peixoto CB, Caldas DM, Silva EB, Akiti T, Nucci M, de Uzeda M. Relationship between slivary flow rates and Candida counts in subjects with xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:149–154. doi: 10.1067/moe.2002.119738. [DOI] [PubMed] [Google Scholar]
- 7.Takeshita J, Masaki K, Ahmed I, Foley DJ, Li YQ, Chen R, Fujii D, G Ross W, Petrovitch H, White L. Are depressive symptoms a risk factor for mortality in elderly Japanese American men?: the Honolulu-Asia aging study. Am J Psychiatry. 2002;159:1127–1132. doi: 10.1176/appi.ajp.159.7.1127. [DOI] [PubMed] [Google Scholar]
- 8.Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimäki M, Batty GD. Association between psychological distress and mortality: individual participant pooled analysis of 10 prospective Cohort studies. BMJ. 2012;345:e4933. doi: 10.1136/bmj.e4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikutani T, Enomoto R, Tamura F, Oyaizu K, Suzuki A, Inaba S. Effects of oral functional training for nutritional improvement in Japanese older people requiring long-term care. Gerodontology. 2006;23:93–98. doi: 10.1111/j.1741-2358.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- 10.Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. 2005;53:1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 11.Lazarus C, Logemann JA, Huang CF, Rademaker AW. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55:199–205. doi: 10.1159/000071019. [DOI] [PubMed] [Google Scholar]
- 12.Park JS, Kim HJ, Oh DH. Effect of tongue strength training using the Iowa Oral Performance Instrument in stroke patients with dysphagia. J Phys Ther Sci. 2015;27:3631–3634. doi: 10.1589/jpts.27.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark HM, O'Brien K, Calleja A, Corrie SN. Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52:1034–1047. doi: 10.1044/1092-4388(2009/08-0062). [DOI] [PubMed] [Google Scholar]
- 14.Arakawa I, Koide K, Takahashi M, Mizuhashi F. Effect of the tongue rotation exercise training on the oral functions in normal adults - Part 1 Investigation of tongue pressure and labial closure strength. J Oral Rehabil. 2015;42:407–413. doi: 10.1111/joor.12271. [DOI] [PubMed] [Google Scholar]
- 15.Fujiu M, Logemann JA. Effect of a tongue-holding maneuver on posterior pharyngeal wall movement during deglutition. Am J Speech Lang Pathol. 1996;5:23–30. [Google Scholar]
- 16.Cho EP, Hwang SJ, Clovis JB, Lee TY, Paik DI, Hwang YS. Enhancing the quality of life in elderly women through a programme to improve the condition of salivary hypofunction. Gerodontology. 2012;29:e972–e980. doi: 10.1111/j.1741-2358.2011.00594.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohara Y, Yoshida N, Kono Y, Hirano H, Yoshida H, Mataki S, Sugimoto K. Effectiveness of an oral health educational program on community-dwelling older people with xerostomia. Geriatr Gerontol Int. 2015;15:481–489. doi: 10.1111/ggi.12301. [DOI] [PubMed] [Google Scholar]
- 18.Minami I, Akhter R, Luraschi J, Oogai K, Nemoto T, Peck CC, Murray GM. Jaw-movement smoothness during empty chewing and gum chewing. Eur J Oral Sci. 2012;120:195–200. doi: 10.1111/j.1600-0722.2012.00963.x. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki T, Miyaoka T, Okazaki S, Yasuda H, Kawamukai T, Utani E, Wake R, Hayashida M, Horiguchi J, Tsuji S. High salivary alpha-amylase levels in patients with schizophrenia: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:688–691. doi: 10.1016/j.pnpbp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Oh JC. Effects of tongue strength training and detraining on tongue pressures in healthy adults. Dysphagia. 2015;30:315–320. doi: 10.1007/s00455-015-9601-x. [DOI] [PubMed] [Google Scholar]
- 21.Izuno H, Hori K, Sawada M, Fukuda M, Hatayama C, Ito K, Nomura Y, Inoue M. Physical fitness and oral function in community-dwelling older people: a pilot study. Gerodontology. 2016;33:470–479. doi: 10.1111/ger.12186. [DOI] [PubMed] [Google Scholar]
- 22.Irie K. Chapter 5. Neck. In: Yajima T, Takano Y, editors. Liebgott the anatomical basis of dentistry. 2nd ed. Tokyo, Japan: Nishimurashoten; 2006. pp. 121–123. (in Japanese) [Google Scholar]
- 23.Nakamura M. Chapter 7. Each part of the head. In: Yajima T, Takano Y, editors. Liebgott the anatomical basis of dentistry. 2nd ed. Tokyo, Japan: Nishimurashoten; 2006. pp. 264–265. (in Japanese) [Google Scholar]
- 24.Hakuta C, Mori C, Ueno M, Shinada K, Kawaguchi Y. Evaluation of an oral function promotion programme for the independent elderly in Japan. Gerodontology. 2009;26:250–258. doi: 10.1111/j.1741-2358.2008.00269.x. [DOI] [PubMed] [Google Scholar]
- 25.Okuma N, Saita M, Hoshi N, Soga T, Tomita M, Sugimoto M, Kimoto K. Effect of masticatory stimulation on the quantity and quality of saliva and the salivary metabolomic profile. PLoS One. 2017;12:e0183109. doi: 10.1371/journal.pone.0183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubo KY, Iinuma M, Chen H. Mastication as a stress-coping behavior. Biomed Res Int. 2015;2015:876409. doi: 10.1155/2015/876409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]