Abstract
Programmed death (PD)-1 receptors and their ligands have been identified in the pathogenesis and development of systemic lupus erythematosus (SLE). Two key pathways, toll-like receptor and type I interferon, are significant to SLE pathogenesis and modulate the expression of PD-1 and the ligands (PD-L1, PD-L2) through activation of NF-κB and/or STAT1. These cell signals are regulated by tyrosine kinase (Tyro, Axl, Mer) receptors (TAMs) that are aberrantly activated in SLE. STAT1 and NF-κB also exhibit crosstalk with the aryl hydrocarbon receptor (AHR). Ligands to AHR are identified in SLE etiology and pathogenesis. These ligands also regulate the activity of the Epstein-Barr virus (EBV), which is an identified factor in SLE and PD-1 immunobiology. AHR is important in the maintenance of immune tolerance and the development of distinct immune subsets, highlighting a potential role of AHR in PD-1 immunobiology. Understanding the functions of AHR ligands as well as AHR crosstalk with STAT1, NF-κB, and EBV may provide insight into disease development, the PD-1 axis and immunotherapies that target PD-1 and its ligand, PD-L1.
Keywords: Systemic lupus erythematosus, PD-1, Aryl hydrocarbon receptor, Epstein-Barr virus
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disorder influenced by a complex etiology of both genetic and environmental factors which results in a clinically heterogeneous presentation [1]. The programmed death (PD)-1 receptor and its ligands (PD-L1, PD-L2) are immune regulatory molecules implicated in the development of SLE [2]. Targeting PD-1 receptors with antibodies to block their activation is an established therapeutic in the treatment of several cancers [3]. This antibody therapy may also lead to the development of immune-related adverse events that clinically present with symptoms similar to autoimmune diseases like SLE [1,3]. The complexities of the PD-1 axis are highlighted by the expression of PD-1 on myeloid and lymphoid subsets and the expression of PD-1 ligands on both immune and non-immune cells in the microenvironment. Understanding the regulatory signals involved in PD-1 receptor expression and function may be pivotal to the pathogenesis of disease and mechanisms of action for anti-PD-1 therapies and their adverse events and is therefore the purpose of this review.
2. The PD-1 axis
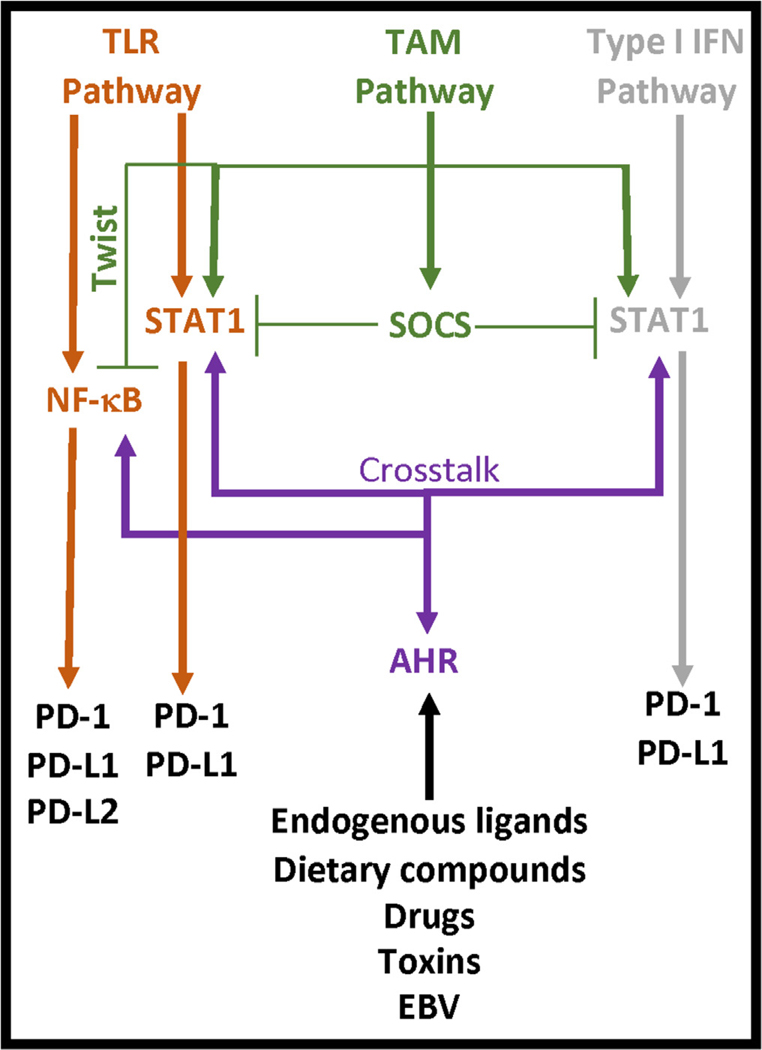
PD-1 and its ligands (PD-L1, PD-L2) provide negative signals that terminate and/or attenuate the immune response [4]. The most common interaction described involves CD4 T cell PD-1 with PD-L1 or PD-L2 on an antigen presenting cell. Ligation of these receptors induces the activation of an immunoreceptor tyrosine-based inhibitory motif (ITIM) in the PD-1 cytoplasmic tail which inhibits activation sequences contained in the immunological synapse [5]. However, PD-1 is also identified on B cells [6] and myeloid cells [7,8] and the ligands are identified on neutrophils [9], lymphocytes [10,11] and additional non-immune cells including tumors [12], epithelial cells [13], endothelial cells [14], and stromal cells [12]. PD-L1 and PD-L2 cross-compete for binding to PD-1 whereas PD-L1 also binds CD80 [4]. This latter interaction involves antigen presenting cell CD80 binding to CD4 T cell PD-L1 which reduces CD4 T cell activation [10]. Thus, cell signals from PD-1 or its ligands regulate the adaptive immune response. This highlights the rationale for PD-1 immunotherapies. However, immune-related adverse events, similar to various autoimmune diseases [1,3], are known to occur in response to PD-1 immunotherapies. Dysregulated cell signals in SLE may therefore identify pathways involved in controlling the PD-1 response. Two pathways of interest include the toll-like receptor (TLR) pathway and the type I interferon (IFN) pathway, which are highly active in the pathophysiology of SLE [15,16] and the regulation of the PD-1 axis [17–19]. TLR cell signals induce the activation of NF-κB and production of type I IFNs that subsequently activate STAT1 [20] and these transcription factors (NF-κB, STAT1) regulate the expression of PD-1 [21], PD-L1 [19,22] and PD-L2 [23]. Elevated expression levels of NF-κB [24] and STAT1 [25,26] in SLE indicate that regulatory signals, possibly associated with the activity of Tyro3, Axl and Mer (TAM) receptor tyrosine kinases or the aryl hydrocarbon receptor (AHR), are reduced or absent which may influence the PD-1 axis (Fig. 1).
Fig. 1. Common SLE cell signals that modulate the PD-1 axis.
PD-1 receptors are activated by TLR- and Type I IFN-induced NF-κB and/or STAT1 activation. TLR and Type I IFN cell signals are regulated by TAM receptor activity and the functions of suppressor of cytokine signaling (SOCS) and Twist transcriptional repressors. AHR is activated by ligands involved in the etiology, pathogenesis, and treatment of SLE. AHR also exhibits crosstalk with NF-κB and STAT1.
3. Systemic lupus erythematosus (SLE)
In the United States, the incidence and prevalence of SLE is approximately 5.5 and 73 per 100,000 people respectively [27]. The disease predominantly affects women of child-bearing age, with an increased prevalence in individuals with an African ancestry [27,28]. The etiology is a multifactorial process involving disruptions in innate and adaptive immunity that culminate into pronounced chronic immune dysregulation. Genetic, epigenetic and environmental factors are implicated in the initiation of the disease [29]. Genetic factors may include polymorphisms within TLR-7,8, and 9 [30], DNase I [31], and within the major histocompatibility (MHC) locus, including human leukocyte antigen (HLA) class I genes (MHCI), class II genes (MHCII), and class III genes encoding tumor necrosis factor (TNF)-α, complement C2 and C4 [32]. Identified environmental factors include agents that are infectious (e.g.: Epstein-Barr virus (EBV) [33–35], endogenous retroviruses [36]) and non-infectious (e.g.: ultraviolet light [37], smoking [38], drugs [39], stress [40], diet [41]). The exact combination of genetic and environmental factors that elicits the initiation of disease is yet to be determined. Disease heterogeneity coupled with a prolonged subclinical phase complicates and delays the diagnosis of SLE [42]. Diagnosis and classification is based on a combination of multiple clinical (butterfly rash, oral ulcers, non-scarring alopecia, synovitis, serositis, leukopenia, thrombocytopenia, renal and neurological functional deficits) and immunological (elevated anti-nuclear, phospholipid, dsDNA, β2 glycoprotein I, cardiolipin, or Smith antibodies; low levels of C3 and C4 complement) manifestations [1]. Treatment is dependent upon disease severity and may target TLR signaling (anti-malarials), cell proliferation (methotrexate, cyclophosphamide), NF-κB activity (corticosteroids), B cells (rituxumab) or cytokines such as B cell activating factor/BAFF (belimumab) [43].
Two key pathways in SLE immunogenesis are the TLR pathway [15] and the type I IFN pathway [16]. The TLR pathway is most commonly activated by components of pathogens, but in SLE, increased cell death, due to apoptosis, neutrophil extracellular trap (NET) activity and/or lack of clearance of the dying cells, results in the increased presence of nucleic autoantigens that activate TLRs (e.g. TLR3:dsRNA, TLR7/ 8:ssRNA, TLR9:DNA) and contribute to the pathology [44]. The TLR pathway can also induce the production of type I IFNs. Activation of dendritic cell (DC) TLR7 or TLR9 induces the extracellular release of the type I IFN, IFN-α [45], which is a cytokine elevated in SLE patients sera [46] (Fig. 2). In SLE patient peripheral blood B cells, another type I IFN, IFN-β, is elevated intracellularly compared to healthy controls, enhanced by TLR3 ligands, and strongly associated with increased auto-antibody production and renal disease [47]. TLR ligands also regulate myeloid production of complement proteins [48], which are notably reduced in SLE patients [1]. Lack of complement protein C3 also enhances TLR-induced type I IFN production [47,49], highlighting the strong association between the TLR and type I IFN pathways in SLE.
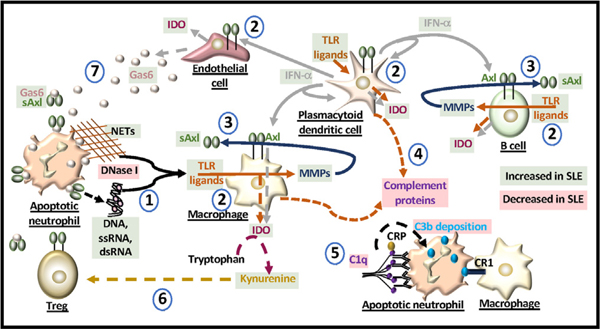
Fig. 2. Molecules dysregulated in SLE.
(1) Mutations in DNase I can limit the degradation of DNA from apoptotic cells and/or from neutrophil extracellular traps (NETs) and induce the release of nucleic acids that can act as TLR ligands. (2) IFN-α and IDO production are elevated in SLE. TLR ligands induce the production of IFN-α and IDO from antigen presenting cells and endothelial cells. IFN-α also induces the production of IDO and cell surface expression of Axl. (3) TLRs can generate matrix metalloproteinases (MMPs) that cleave Axl from the cell surface. In SLE, soluble Axl (sAxl) is elevated and generated mainly from macrophages and B cells. (4) The production of complement proteins is modulated by TLR ligands. (5) C1q binds antibodies that opsonize the apoptotic cell. C reactive protein (CRP) binds to C1q in activating the complement cascade involving C3b deposition which binds to macrophage complement receptor 1 (CR1). Reduced levels of C1q alter macrophage uptake of apoptotic cells. (6) IDO generates kynurenine from tryptophan that can enhance the formation of Tregs. Kynurenine production is elevated in SLE but the levels of Tregs do not increase. Ligation of Treg Axl with Gas6 enhances their suppressor activity which may be blocked by sAxl. (7) Gas6 sourced from activated endothelial cells is elevated in SLE and binds apoptotic cells and Axl. In SLE, the levels of sAxl may block immune cell recognition of apoptotic cells and alter the function of immune and non-immune cells in the microenvironment.
TAMs are identified in the etiopathogenesis of SLE [50–52] and are known to regulate the activation of TLR and Type I IFN pathways [53] (Fig. 1). In SLE patients, soluble Axl (sAxl) production is elevated [54]. This may occur in response to IFN-α-induced Axl expression [55,56] that is cleaved by TLR-induced matrix metalloproteinases (e.g. ADAM 10, ADAM 17) [54,57] (Fig. 2). Because expression of PD-L1 can be similarly induced by IFN-α [58], TLR-induced sheddases may also be responsible for the production of soluble PD-L1 (sPD-L1) in some cases of SLE (Table 1). TAM ligation is pivotal to cell migration, survival, and efferocytosis [53]. The ligands alone or bound to an apoptotic cell interact with TAMs and include vitamin K-dependent growth arrest-specific protein 6 (GAS6), which has the highest affinity for Axl yet binds all three receptors, or protein S, which only binds Tyro3 and Mer [53,59]. Elevated levels of Gas6 in active SLE [52,60] would be anticipated to bind Axl on T regulatory cells (Tregs) for enhanced suppressor activity [61] and inhibit antigen presenting cell type I IFN and TLR signals through the activation of suppressor of cytokine signaling (SOCS) and Twist transcriptional repressors [59] (Figs. 1 and 2). Dysregulation of TAMs or production of sAxl, which are known to occur in SLE [50–52,54], may thwart these responses and the downstream activation of PD-1 and the PD-1 ligands.
Table 1.
Clinical PD-1 phenotypes identified in SLE.
| Cell type | Patients | Source | PD-1 marker |
|---|---|---|---|
| Neutrophils [9] (CD15+CD3−) | Chinese patients diagnosed according to the revised criteria for SLE from the American College of Rheumatology (ACR) 1997 | Whole blood | PD-L1hi is compared to healthy controls, correlated with the SLEDAI score, and can be reduced with immunosuppressive drugs |
| DCs (CD14lo) and monocytes (CD14hi) [75] | U.S. pediatric patients diagnosed according to ACR 1997 | Peripheral blood mononuclear cells | PD-L1reduced is compared to healthy controls |
| Mucosal-Associated Invariant T (MAIT) cells [84] (CD3+TCRγδ−Vα7.2+CD161high) | Korean SLE patients diagnosed according to ACR 1997 | (PBMC) | PD-1elevated is identified on MAIT cells that are reduced in percentages compared to healthy controls |
| CD4 T follicular helper (Tfh) [85] (CD4+CXCR5+) | Chinese SLE patients diagnosed according to ACR 1997 | PBMC | PD-1+ Tfh cell expansion is positively associated with SLEDAI score and B regulatory cell (CD19+CD5+CD1dhigh) expansion |
| CD4 Tfh cell [86] (CXCR5+/−CXCR3−) | Chinese SLE patients diagnosed according to ACR 1997 | PBMC | PD-1+ Tfh cell percentages are positively associated with SLEDAI score |
| CD4 T cell [87] (CD25+) | Icelandic and Swedish patients with at least 4 of ACR 1982 classification criteria for SLE | PBMC | PD-1reduced is compared to controls and associated with PD-1.3A polymorphism |
| CD8 T cell [88] (IL-7 receptorlo) | United Kingdom SLE patients presenting with untreated, active (at least 4 ACR 1982 criteria) disease | Anti-CD3/anti-CD28 treated PBMCs | PD-1reduced is compared to PD-1 on characterized “exhausted” CD8 T cells highlighting a defect in these cells that is also linked to poor SLE patient outcomes |
| B cell [89] (IgG antibodies) | Chinese SLE patients diagnosed according to ACR 1997 | PBMC | Anti-PD-1 antibodies are elevated and positively associated with SLEDAI score |
| B cell [90] (CD11chi T-bet+ CD27lo CD38lo) | U.S. SLE patients | Sera | PD-1 and PD-L1 transcripts are elevated compared to healthy controls and the frequency of these cells is associated with SLEDAI score |
| DN2 effector B cell [91] (CD27− IgD− CXCR5−CD11c+T-bet+) | U.S. African American SLE patients diagnosed according to ACR 1997 | PBMC | PD-1 transcripts are elevated compared to healthy controls and the frequency of these cells is associated with SLEDAI scor |
| Unknown [92] | Chinese SLE patients | Sera | Soluble PD-L1 is elevated compared to healthy controls |
AHR regulates the type I IFN and TLR pathways by blocking cell signals involved in type I IFN production [62] and exhibiting crosstalk with the transcription factors NF-kB [63] and STAT1 [64] (Fig. 1). This nuclear receptor responds to EBV activity, endogenous ligands in tryptophan catabolism (e.g. kynurenine, 6-formylindolo[3,2-b]carbazole (FICZ)), exogenous ligands found in the diet (e.g. flavonoids, indoles), cigarette smoke (e.g. benzo(a)pyrene), and certain drugs (e.g. omeprazole) [65–67]. Type I IFN or TLR cell signals induce the production of indoleamine 2,3-dioxygenase (IDO) [63,68], which is responsible for the catabolism of tryptophan into the AHR ligand kynurenine [69]. In SLE patients, lower serum levels of tryptophan and higher levels of kynurenine compared to healthy controls, identify increased IDO activity [70]. In myeloid cells, AHR is required for IDO production [71] but IDO is not a relevant factor in myeloid AHR-mediated IL-10 production involving apoptotic thymic cell co-cultures [72]. Moreover, the absence of AHR in myeloid cells promotes TLR-induced hypersensitivity [73] and the development of SLE [72], suggesting that AHR activation is required for myeloid immune tolerance. This is supported by experiments with human DCs that demonstrate increased TLR4-induced AHR, PD-L1, and PD-L2 expression in association with enhanced IDO and IL-10 production upon secondary TLR4 stimulation [63]. These immune-tolerant DCs are immunotherapeutic targets in cancer [74] that could also be targeted in SLE. The lack of identified PD-L1 expression on SLE patient DCs [75] may indicate a disruption in AHR cell signals or cleavage of PD-1 ligands from the cell surface. Because B cells are also able to produce IDO and express PD-L1 and PD-L2 [11,76] that bind PD-1+ follicular T helper cells (Tfh) [77], AHR expression in B cells may be a therapeutic target in regulating germinal center reactions in SLE.
Moreover, endothelial cells are another source of IDO and these cells also express PD-L1 and PD-L2 [14,78], possibly via AHR, type I IFN, and/or TLR signals [71,79,80]. Endothelial dysfunction is a characteristic feature of SLE that increases the risk of atherosclerosis and cardiovascular diseases in these patients [81]. Aberrant endothelial activity may alter PD-L1 and PD-L2 expression on these cells that are known to suppress CD8 T cell activation by binding CD8 T cell PD-1 [14]. Endothelial Gas6 and AHR ligands such as the uremic toxin indoxyl sulfate can each independently promote leukocyte adhesion to the endothelium [82,83]. Because ligation of TAMs and AHR regulate signals involved in the expression of PD-L1 and PD-L2 (Fig. 1), elevated production of Gas6 [52,60] and/or kynurenine [71] in SLE may alter endothelial PD-L1 and PD-L2 expression. Additional examination of the regulatory cell signals in the PD-1 axis is needed to understand the relevance of the various cell types and biomarkers in SLE (e.g. PD-1 antibodies, sPD-L1) (Table 1).
4. The PD-1 axis and SLE
In SLE, the number of PD-1+Tfh cells increase with disease severity and their development is regulated by B cell PD-L1 ligation [77,85,86]. B cell PD-1 ligation inhibits tyrosine phosphorylation of effector molecules (e.g. SYK, SHP-2) effectively blocking B cell receptor signaling [6] (Fig. 3). The expansion of B cells in SLE [90] may suggest that B cell PD-1 is not effectively expressed or ligated in SLE despite increases in transcript levels [90,91]. The lack of significant PD-1 expression on SLE patient CD8 T cells is an identified defect in regulatory cell signals for this cell type [88]. In tumor models, macrophage PD-1 expression correlates negatively with their phagocytic potency [7]. Possibly, macrophages in SLE also express PD-1 as a biomarker of their reduced ability to clear apoptotic cells.
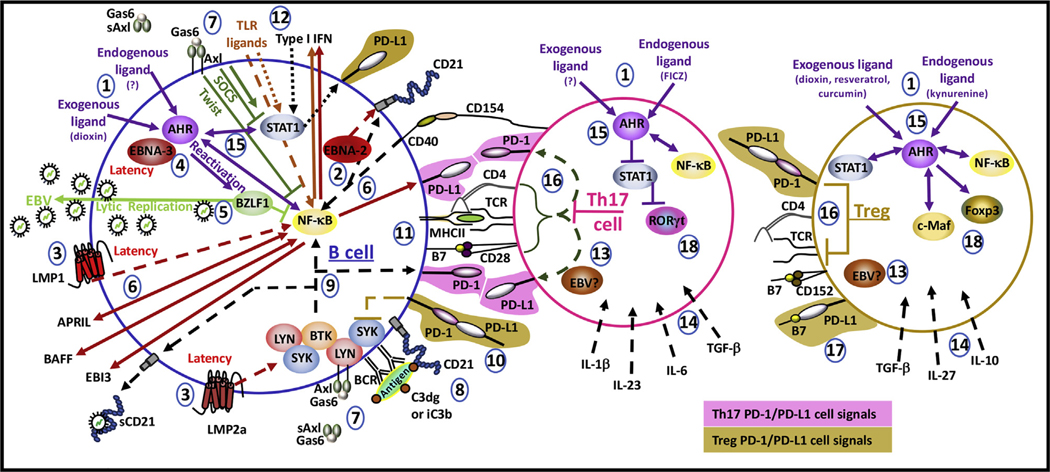
Fig. 3. Possible cell signals in lymphocytes.
(1) Distinct endogenous and exogenous AHR ligands regulate the differentiation of lymphocytes. (2) Latent EBV protein EBNA-2 and CD40 ligation induce cell surface expression of CD21. (3) Latent EBV proteins LMP1 and LMP2a mimic respective cell signals induced by CD40 and the BCR. (4) Latent EBV protein EBNA-3 enhances dioxin-induced AHR transcriptional activity. (5) Dioxin-induced AHR activates EBV protein BZLF1 involved in lytic replication and antagonizing NF-κB. (6) LMP1 induces the production of APRIL and the NF-kB-induced expression of PD-L1 and production of BAFF and EBI3. Autocrine APRIL and BAFF activate NF-κB. (7) Axl, activated by Gas6, can complex with kinases (LYN, SYK), activate the PI3K/AKT pathway, or in the presence of type I IFN, activate JAK/STAT signals. In SLE, soluble Axl (sAxl) is produced. (8) CD21 is an EBV receptor that also assists the BCR in the recognition of complement (C3dg, iC3b) bound to antigens. (9) BCR activation induces NF-κB cell signals, CD21 shedding, and the expression of PD-1. (10) B cell PD-1 ligation to PD-L1 inhibits SYK activity. (11) The immunological synapse involves CD40 and B7 ligation associated with MHC:peptide interaction with the TCR for full activation of the B and T cell. (12) LMP1 and TLR NF-κB -induced production of type I IFN activates STAT1. (13) The functions of EBV in T cells are not clearly known. (14) Distinct cytokines regulate the development of T cell subsets. (15) AHR regulates the activity of NF-κB and STAT1 in lymphocytes. (16) In Th17 cells, PD-1 and PD-L1 are repressed whereas in Tregs, these receptors are expressed. T cell PD-1 ligation inhibits activation sequences contained in the immunological synapse. (17) T cell PD-L1 ligation with the B7 molecule, CD80, also suppresses T cell activation. (18) STAT1 antagonizes RORγt which is a transcription factor required for Th17 cell differentiation. Foxp3 and c-Maf are associated with subsets of Tregs. AHR induces the expression of Foxp3 and c-Maf exhibits crosstalk with AHR.
PD-1 polymorphisms have been identified in SLE. The susceptibility to lupus nephritis and SLE is associated with PD1.3 and PD1.5 polymorphisms, respectively [2,93]. PD1.6 polymorphisms may be a protective factor to SLE [94]. Both TLR ligands and IFN-α induce PD-1 expression on myeloid [17,95] and lymphoid subsets [96,97]. TAM cell signals, which are disrupted in SLE [50–52] and regulate TLR and IFN-α pathways [53], likely influence PD-1 activation in SLE immune subsets. This is supported by independent models that display glomerulonephritis in PD-1-deficient [98] and the Mer-deficient mice [99]. However, Axl deficiency or blockade exhibits a protective effect against glomerulonephritis [99,100] and in tumor models, Axl blockade improves PD-1 immunotherapy [101]. This suggests that Axl exhibits a distinct mechanism in regulating the PD-1 axis. Identified links in tumor cells between Axl and PD-L1 expression [102] indicate that Axl inhibitors could block B cell PD-L1 interactions with proliferating PD-1+Tfh cells in SLE [77] (Table 1).
The production of PD-1 antibodies in SLE [89] may break immune tolerance established by the expression of PD-L1 and PD-L2 on epithelial and endothelial cells [13,14], resulting in nephritis, similar to cases identified with PD-1 therapies [103]. Polymorphisms in PD-L1 or PD-L2 have not revealed associated risks in developing SLE [104]. PD-L1 expression is upregulated on SLE patient peripheral blood neutrophils but reduced on DCs and monocytes (Table 1). These responses may be partly explained by a lack of C3 and C1q complement proteins in SLE [28] since neutrophil PD-L1 expression is negatively correlated with C3 [9] and C1q opsonized apoptotic cells induce PD-L1 and PD-L2 expression on human macrophages and DCs [105]. The complexity is further displayed in lupus-prone murine models that demonstrate improved kidney function in response to PD-1 activation [106] or blockade [107].
5. AHR and the PD-1 axis
The transcription factor and nuclear receptor, AHR, is integral to hematopoiesis, xenobiotic metabolism, adhesion, and migration [108,109]. The expression of AHR is induced by TLR cell signals that activate the NF-κB p65(RelA)/p50 heterodimer in the AHR promoter [110,111]. AHR ligands are highly linked to the amino acid tryptophan. FICZ, which is a product of UV-B irradiated tryptophan [112], is an AHR ligand that may mediate SLE patient sunlight-induced flares [112]. IDO oxidation of tryptophan forms the AHR ligand kynurenine, which is elevated in SLE patient sera [70]. FICZ and newly identified trace derivatives of kynurenine exhibit greater affinity to AHR compared to kynurenine [108,113]. Full degradation of kynurenine generates nicotinamide adenine dinucleotide (NAD) [114], which is a molecule essential for life that is reduced in cells treated with another high affinity AHR ligand, dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin, TCDD) [115]. Thus, the type of ligand may affect AHR activation and downstream cell signals in immunity.
5.1. AHR in T cells
The AHR ligands dioxin and kynurenine induce the formation of T regulatory cells (Tregs) [112] and the expression of PD-1 on CD8 T cells [116]. FICZ, which has higher binding affinity to AHR than kynurenine, induces the formation of the proinflammatory interleukin-17 producing T cells (Th17) [108]. Despite elevated levels of kynurenine in SLE patients [70] the ratio of Tregs to Th17 cells is reduced in SLE patient peripheral blood compared to healthy controls [117] and the levels of PD-1 on SLE patient CD8 T cells is also reduced compared to healthy controls [88]. The expression of PD-1 on CD4 and CD8 T cells is mediated by the NF-κB p50 homodimer [118]. Because AHR is able to form a complex with NF-κB p50 [64], the activity of AHR in these cells may direct PD-1 function. NF-κB and AHR activity are involved in the generation of both Treg and Th17 cells [119,120]. AHR is also characterized in regulating STAT1 activity during Th17 differentiation [121]. STAT1 promotes Foxp3 [122] but represses IL-17 [123] transcriptional activity. The ability of AHR to complex with NF-κB p50 and STAT1 in myeloid cells [64] suggests that similar interactions occur in Th17 cells to promote their activity and suppress PD-1 receptors. A lack of STAT1 activation in T cells may also explain the increased levels of STAT1 protein in SLE patient CD4 T cells compared to healthy controls [26]. Dietary compounds (e.g. curcumin and resveratrol) can bind AHR, ameliorate SLE symptoms [124,125], and regulate the activation of NF-κB [126,127] and STAT1 [128,129]. These compounds antagonize the AHR ligand FICZ, inhibit the formation of Th17 cells and enhance the generation of Foxp3+Tregs [130–132]. AHR-induced repression of STAT1 [123] may promote the activation of the nuclear receptor retinoic acid receptor-related orphan receptor (ROR)-γt, which is required for the differentiation of Th17 cells [123,133]. Exogenous synthetic RORγt ligands but not currently known endogenous RORγt ligands reduce PD-1 expression on T cell lines and murine primary T cells [134] (Fig. 3). Whether certain RORγt ligands, additional dietary ligands or rapidly metabolized ligands, which do not accumulate in the same manner as toxins, regulate PD-1 expression and tolerance continues to be explored [135,136].
5.2. AHR in B cells
Similar properties of AHR exist in B cells. STAT1 and TLR7 signaling are required for the formation of germinal centers that are common in SLE [137]. This process is regulated by TLR9 signals [137] and the various heterodimers or homodimers of NF-κB [138]. Distinct findings of AHR activity in B cells suggests that AHR may interact with STAT1 and NF-κB signals in these cells. For example, cross-linking the B cell receptor significantly induces the expression of AHR and activation of AHR with dioxin negatively affects the processes of class-switch re-combination and plasma cell differentiation [139]. Dioxin, like kynurenine, encourages the production of Tregs via AHR activation [140] which suggests that ligands such as FICZ, which induce Th17 formation [108], may also distinctly affect the activity of B cells. AHR is an identified as a factor required for murine B cell proliferation [141]. Because B cells exhibit increased proliferation in the absence of PD-1 [142], AHR may potentially modulate PD-1 expression in B cells as well as BAFF cell signals, which have established functions in B cell survival, class-switch recombination, and plasma cell differentiation [143].
5.3. AHR in myeloid cells
In myeloid cells, AHR is established in the regulation of immune tolerance and the expression of PD-1 ligands [63,72], which may be in response to AHR complexing with STAT1 and NF-κB p50 [64]. Tolerance and the formation of M2 alternative macrophages is dependent upon NF-κB p50 in myeloid cells [144], further implicating AHR ligands and cell signals in the SLE response. In mouse embryonic fibroblasts, viral induced IFN-β is regulated by AHR activation of 2,3,7,8-tetrachlorodibenzo p-dioxin-inducible poly(ADP-ribose)polymerase (TIPARP), which antagonizes cell signals involved in type I IFN production [62]. Whether this cell signaling mechanism in response to various AHR ligands also exists in DCs that generate IFN-α in SLE is not known.
6. EBV and the PD-1 axis
EBV infection and reactivation are associated with SLE etiology [33–35]. The seroprevalence of EBV progressively increases during childhood with greater than 90% of U.S. adults exhibiting antibody positive titers by the age of 35 [145]. EBV can infect neutrophils [146], T cells [147], epithelial cells [148], but preferentially infects B cells [149]. Two common EBV proteins are latent membrane protein 1 (LMP1) and LMP2a which mimic cell signals induced by CD40 ligation and BCR activation respectively [149]. LMP1-induced NF-κB activation generates various cytokines in B cells (e.g. the IL-27 subunit EBV-induced gene 3 (EBI3), A proliferation-inducing ligand (APRIL), BAFF, IFN-α, IFN-γ) that are elevated in SLE [150–152]. The production of IFNs by LMP1 subsequently induces STAT1 activity [152] and these cell signals may play role in LMP1-induced PD-L1 expression in infected cell lines [153]. Possibly, these same signals regulate peripheral blood neutrophil PD-L1 over-expression in SLE [9].
In various cell lines, the EBV immediate early viral transactivator, BRLF1, has been shown to induce the expression of Mer [154], indicating that TAM receptors are regulated by EBV activity. Either BRLF1 or another EBV immediate early viral transactivator, BZLF1, is sufficient to induce lytic replication in both latently infected epithelial cells and B cells [155]. Research has identified AHR complexed with the latent protein, EBV nuclear antigen-3 (EBNA-3) [66], and dioxin-induced AHR is directly involved in the reactivation of the EBV immediate early viral transactivator, BZLF1 [67]. This initiating factor in lytic viral replication antagonizes latent viral signals, in part, by blocking NF-κB activity [156] (Fig. 3).
T cell AHR and PD-L1 expression are also induced by the EBV cytokine IL-27. In these cells, AHR interacts with c-Maf, a transcription factor identified in a subset of Tregs [157,158]. These CD4+CD25+Foxp3−c-Maf+ Treg cells, termed Treg-of-B cells, are formed in response to repeated interactions with B cells [159]. Treg-of-B cells express PD-1 and additional checkpoints in regulating Th2, Th1, and Th17 responses under physiological cues that have yet to be fully elucidated (Fig. 3).
Moreover, EBV binds to B cells via complement receptor-2 (CR2, CD21) and the levels of soluble CD21 (sCD21), released subsequent to BCR activation, are indicated to block EBV infection [160–162]. CD40 ligation and the latent EBV molecule, EBNA-2, induce CD21 expression [163,164] which then cooperates with the BCR to recognize and respond to complement (C3dg or iC3b)-bound antigens [165] (Fig. 3). In SLE patients, low levels of CD21 are identified on activated naïve and memory B cell subsets and the levels of serum sCD21 are reduced compared to healthy controls [166,167]. In mice deficient of CD21 and the complement receptor 1 (CR1, CD35), B cells express increased levels of PD-1 [168] and increased levels of PD-1 transcripts are expressed by activated B cells in human SLE [90,91]. Interestingly, the expansion of PD-1+ B cell subsets similar to the ones expanded in active SLE have been reported in association with the development of autoimmune complications of checkpoint inhibitors in melanoma patients [169]. Because PD-1 ligation antagonizes TCR and BCR cell signals [5,6], the presence of sPD-L1 in SLE [92] may directly affect the activity of lymphocytes. The production of sPD-L1 is also linked to an SLE associated disease, non-Hodgkin lymphoma (NHL) [170,171].
7. SLE and non-Hodgkin lymphoma (NHL)
The diagnosis of SLE is independently and significantly associated with higher proportions of blood cancers compared to age and sex-matched controls [171]. NHL is the most common SLE associated blood cancer, which accounts for 4.3% of all cancers, affects both men and women, and originates from oncogenic B- or T-cells [171,172]. The highest NHL incidence occurs in diffuse large-B-cell lymphoma (DLBCL) and B-cell chronic lymphoid leukemia (B-CLL) affecting 4–7 and 4–8 per 100,000 people respectively [172]. DLBCL is the most common type of NHL in SLE patients whereas B-CLL is a rare occurrence [172–174]. In DLBCL, sPD-L1 is a marker of poor prognosis [170], which may indicate that sPD-L1 in SLE is a risk factor for this disease. In B-CLL, membrane Axl is a contributing factor in disease progression [175] by complexing with kinases, such as LYN and SYK [176]. The lower risk of B-CLL may reflect the increased levels of sAxl in SLE that potentially block membrane Axl cell signals. Similar to SLE, DLBCL and B-CLL are driven by EBV infection [177], polymorphisms in complement [178] and TLR [179,180] genes. Continued evaluation of the cell signal similarities and differences between SLE and NHL may allow for the identification of biomarkers for SLE patients at risk of developing NHL and possibly better therapies for both diseases.
8. Summary
SLE is the result of complex genetic and environmental factors that alter innate and adaptive immunity. The PD-1 axis is involved in the regulation of innate and adaptive immune subsets in SLE and certain cancers. This axis is also a target in the treatment of various diseases where the mechanisms that regulate immune checkpoints have yet to be fully revealed. The complexities of PD-1 immunity are embedded in the multiple cell signaling pathways that regulate the expression and activation of the receptors on both immune and parenchymal cells in the microenvironment.
Dysregulation of SLE TAMs, such as Axl, may affect TAM-induced regulation of type I IFN and TLR cell signals and their downstream responses involving the PD-1 axis. These cell signal networks are also influenced by AHR (Figs. 1 and 3). The ability of AHR to induce PD-1 coupled with noted deficits in the phagocytic potency of PD-1+ or TAM deficient macrophages in various models may indicate that AHR ligands are involved in TAM expression and/or the clearance of apoptotic cells. Because STAT1 and NF-κB are integral to myeloid, B cell, Treg and Th17 development, further exploration of these transcription factors in the AHR response is needed. The involvement of AHR in the generation of Tregs, that can express PD-1 receptors, and Th17 cells, that lack these checkpoints, suggests that AHR cell signals participate in PD-1 immunity, highlighting AHR as a therapeutic target. Mechanisms to block FICZ interactions with AHR but promote AHR ligation with kynurenine or kynurenine trace derivatives may promote tolerance. Moreover, because kynurenine is a precursory step in the production of NAD, metabolic enzymes and metabolites in kynurenine catabolism may be pivotal to the PD-1 axis and the function of immune subsets in SLE.
Endogenous or exogenous AHR ligands can also regulate EBV latent or lytic activity, suggesting that EBV-induced TAM receptor expression or LMP1-induced PD-L1 expression may involve AHR. Evidence that the EBV receptor, CD21, regulates PD-1 expression needs further exploration. The involvement of the EBV associated cytokine, IL-27, in the expression of AHR and PD-L1 further highlights the complex interactions involved in the PD-1 response. These integrated functions of AHR in SLE and the PD-1 axis in various cell types suggest that interventions that prevent or induce PD-1 ligation modulate AHR expression and/or responses. The identification of sPD-L1, PD-1 antibodies, and kynurenine in SLE highlights possible dysfunction between AHR and the PD-1 axis. Understanding how AHR or additional AHR associated transcription factors influence the development and activation of distinct cell types in a multi-cellular microenvironment may aid in identifying factors in the progression and treatment of SLE and NHL. Identification of AHR ligands that enhance or inhibit cell surface PD-1 and PD-L1 may also be beneficial in immunotherapies that target these receptors.
Support
National Institutes of Health, Intramural Research Funds.
References
- [1].Kuhn A, Bonsmann G, Anders HJ, Herzer P, Tenbrock K, Schneider M, The diagnosis and treatment of systemic lupus erythematosus, Dtsch. Arztebl. Int 112 (2015) 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lee YH, Woo JH, Choi SJ, Ji JD, Song GG, Association of programmed cell death 1 polymorphisms and systemic lupus erythematosus: a meta-analysis, Lupus 18 (2009) 9–15. [DOI] [PubMed] [Google Scholar]
- [3].Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. , Immune-related adverse events with immune checkpoint blockade: a comprehensive review, Eur. J. Cancer 54 (2016) 139–148. [DOI] [PubMed] [Google Scholar]
- [4].Ghiotto M, Gauthier L, Serriari N, Pastor S, Truneh A, Nunes JA, et al. , PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1, Int. Immunol 22 (2010) 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T, Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2, J. Exp. Med 209 (2012) 1201–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T, PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine, Proc. Natl. Acad. Sci. U. S. A 98 (2001) 13866–13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. , PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity, Nature 545 (2017) 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lim TS, Chew V, Sieow JL, Goh S, Yeong JP, Soon AL, et al. , PD-1 expression on dendritic cells suppresses CD8+ T cell function and antitumor immunity, Oncoimmunology 5 (2016) e1085146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Luo Q, Huang Z, Ye J, Deng Y, Fang L, Li X, et al. , PD-L1-expressing neutrophils as a novel indicator to assess disease activity and severity of systemic lupus erythematosus, Arthritis Res. Ther. 18 (2016) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ, Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses, Immunity 27 (2007) 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ, PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells, Nat. Immunol 11 (2010) 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, et al. , PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer, Clin. Cancer Res. 23 (2017) 3158–3167. [DOI] [PubMed] [Google Scholar]
- [13].Dimitrov V, Bouttier M, Boukhaled G, Salehi-Tabar R, Avramescu RG, Memari B, et al. , Hormonal vitamin D up-regulates tissue-specific PD-L1 and PD-L2 surface glycoprotein expression in humans but not mice, J. Biol. Chem 292 (2017) 20657–20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, et al. , Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis, Eur. J. Immunol 33 (2003) 3117–3126. [DOI] [PubMed] [Google Scholar]
- [15].Celhar T, Fairhurst AM, Toll-like receptors in systemic lupus erythematosus: potential for personalized treatment, Front. Pharmacol 5 (2014) 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Crow MK, Type I interferon in the pathogenesis of lupus, J. Immunol 192 (2014) 5459–5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bally AP, Lu P, Tang Y, Austin JW, Scharer CD, Ahmed R, et al. , NF-kappaB regulates PD-1 expression in macrophages, J. Immunol 194 (2015) 4545–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rodriguez-Garcia M, Porichis F, de Jong OG, Levi K, Diefenbach TJ, Lifson JD, et al. , Expression of PD-L1 and PD-L2 on human macrophages is upregulated by HIV-1 and differentially modulated by IL-10, J. Leukoc. Biol 89 (2011) 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, et al. , Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression, Cell Rep. 19 (2017) 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Uematsu S, Akira S, Toll-like receptors J Biol. Chem 282 (2007) 15319–15323. [DOI] [PubMed] [Google Scholar]
- [21].Bally AP, Austin JW, Boss JM, Genetic and epigenetic regulation of PD-1 expression, J. Immunol 196 (2016) 2431–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ritprajak P, Azuma M, Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma, Oral Oncol. 51 (2015) 221–228. [DOI] [PubMed] [Google Scholar]
- [23].Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ, Programmed death ligand 2 in cancer-induced immune suppression, Clin. Dev. Immunol 2012 (2012) 656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sun F, Teng J, Yu P, Li W, Chang J, Xu H, Involvement of TWEAK and the NF-kappaB signaling pathway in lupus nephritis, Exp. Ther. Med 15 (2018) 2611–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dong J, Wang QX, Zhou CY, Ma XF, Zhang YC, Activation of the STAT1 signalling pathway in lupus nephritis in MRL/lpr mice, Lupus 16 (2007) 101–109. [DOI] [PubMed] [Google Scholar]
- [26].Goropevsek A, Gorenjak M, Gradisnik S, Dai K, Holc I, Hojs R, et al. , Increased levels of STAT1 protein in blood CD4 T cells from systemic lupus erythematosus patients are associated with perturbed homeostasis of activated CD45RA(−) FOXP3(hi) regulatory subset and follow-up disease severity, J. Interferon Cytokine Res. 37 (2017) 254–268. [DOI] [PubMed] [Google Scholar]
- [27].Lewis MJ, Jawad AS, The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus, Rheumatology (Oxford) 56 (2017) i67–i77. [DOI] [PubMed] [Google Scholar]
- [28].Macedo AC, Isaac L, Systemic lupus erythematosus and deficiencies of early components of the complement classical pathway, Front. Immunol 7 (2016) 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tsokos GC, Systemic lupus erythematosus N. Engl. J. Med 365 (2011) 2110–2121. [DOI] [PubMed] [Google Scholar]
- [30].Lee YH, Choi SJ, Ji JD, Song GG, Association between toll-like receptor polymorphisms and systemic lupus erythematosus: a meta-analysis update, Lupus 25 (2016) 593–601. [DOI] [PubMed] [Google Scholar]
- [31].Gupta S, Kaplan MJ, The role of neutrophils and NETosis in autoimmune and renal diseases, Nat. Rev. Nephrol 12 (2016) 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Relle M, Schwarting A, Role of MHC-linked susceptibility genes in the pathogenesis of human and murine lupus, Clin. Dev. Immunol 2012 (2012) 584374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Parks CG, Cooper GS, Hudson LL, Dooley MA, Treadwell EL, St Clair EW, et al. , Association of Epstein-Barr virus with systemic lupus erythematosus: effect modification by race, age, and cytotoxic T lymphocyte-associated antigen 4 genotype, Arthritis Rheum. 52 (2005) 1148–1159. [DOI] [PubMed] [Google Scholar]
- [34].James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB, An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus, J. Clin. Investig 100 (1997) 3019–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Han L, Zhang Y, Wang Q, Xin M, Yang K, Lei K, et al. , Epstein-Barr virus infection and type I interferon signature in patients with systemic lupus erythematosus, Lupus 27 (6) (2018) 961203317753069. [DOI] [PubMed] [Google Scholar]
- [36].Perl A, Fernandez D, Telarico T, Phillips PE, Endogenous retroviral pathogenesis in lupus, Curr. Opin. Rheumatol 22 (2010) 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lehmann P, Homey B, Clinic and pathophysiology of photosensitivity in lupus erythematosus, Autoimmun. Rev 8 (2009) 456–461. [DOI] [PubMed] [Google Scholar]
- [38].Montes RA, Mocarzel LO, Lanzieri PG, Lopes LM, Carvalho A, Almeida JR, Smoking and its association with morbidity in systemic lupus erythematosus evaluated by the systemic lupus international collaborating clinics/american college of rheumatology damage index: preliminary data and systematic review, Arthr. Rheumatol 68 (2016) 441–448. [DOI] [PubMed] [Google Scholar]
- [39].Rubin RL, Drug-induced lupus, Expet Opin. Drug Saf. 14 (2015) 361–378. [DOI] [PubMed] [Google Scholar]
- [40].Roberts AL, Malspeis S, Kubzansky LD, Feldman CH, Chang SC, Koenen KC, et al. , Association of trauma and posttraumatic stress disorder with incident systemic lupus erythematosus in a longitudinal cohort of women, Arthr. Rheumatol 69 (2017) 2162–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rosser EC, Mauri C, A clinical update on the significance of the gut microbiota in systemic autoimmunity, J. Autoimmun 74 (2016) 85–93. [DOI] [PubMed] [Google Scholar]
- [42].Robertson JM, James JA, Preclinical systemic lupus erythematosus, Rheum. Dis. Clin. N. Am 40 (2014) 621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yildirim-Toruner C, Diamond B, Current and novel therapeutics in the treatment of systemic lupus erythematosus, J. Allergy Clin. Immunol. 127 (2011) 303–312 quiz 13–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kono DH, Baccala R, Theofilopoulos AN, TLRs and interferons: a central paradigm in autoimmunity, Curr. Opin. Immunol 25 (2013) 720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bao M, Liu YJ, Regulation of TLR7/9 signaling in plasmacytoid dendritic cells, Prot. Cell 4 (2013) 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bauer JW, Baechler EC, Petri M, Batliwalla FM, Crawford D, Ortmann WA, et al. , Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus, PLoS Med. 3 (2006) e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hamilton JA, Wu Q, Yang P, Luo B, Liu S, Li J, et al. , Cutting edge: intracellular IFN-beta and distinct type I IFN expression patterns in circulating systemic lupus erythematosus B cells, J. Immunol 201 (8) (2018. October 15) 2203–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lubbers R, van Essen MF, van Kooten C, Trouw LA, Production of complement components by cells of the immune system, Clin. Exp. Immunol 188 (2017) 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lebel ME, Langlois MP, Daudelin JF, Tarrab E, Savard P, Leclerc D, et al. , Complement component 3 regulates IFN-alpha production by plasmacytoid dendritic cells following TLR7 activation by a plant virus-like nanoparticle, J. Immunol 198 (2017) 292–299. [DOI] [PubMed] [Google Scholar]
- [50].Ekman C, Jonsen A, Sturfelt G, Bengtsson AA, Dahlback B, Plasma concentrations of Gas6 and sAxl correlate with disease activity in systemic lupus erythematosus, Rheumatology (Oxford) 50 (2011) 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu J, Ekman C, Jonsen A, Sturfelt G, Bengtsson AA, Gottsater A, et al. , Increased plasma levels of the soluble Mer tyrosine kinase receptor in systemic lupus erythematosus relate to disease activity and nephritis, Arthritis Res. Ther. 13 (2011) R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Recarte-Pelz P, Tassies D, Espinosa G, Hurtado B, Sala N, Cervera R, et al. , Vitamin K-dependent proteins GAS6 and Protein S and TAM receptors in patients of systemic lupus erythematosus: correlation with common genetic variants and disease activity, Arthritis Res. Ther. 15 (2013) R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lemke G, Biology of the TAM receptors, Cold Spring Harb. Perspect. Biol. 5 (2013) a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Orme JJ, Du Y, Vanarsa K, Mayeux J, Li L, Mutwally A, et al. , Heightened cleavage of Axl receptor tyrosine kinase by ADAM metalloproteases may contribute to disease pathogenesis in SLE, Clin. Immunol 169 (2016) 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sharif MN, Sosic D, Rothlin CV, Kelly E, Lemke G, Olson EN, et al. , Twist mediates suppression of inflammation by type I IFNs and Axl, J. Exp. Med 203 (2006) 1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Scutera S, Fraone T, Musso T, Cappello P, Rossi S, Pierobon D, et al. , Survival and migration of human dendritic cells are regulated by an IFN-alpha-inducible Axl/Gas6 pathway, J. Immunol 183 (2009) 3004–3013. [DOI] [PubMed] [Google Scholar]
- [57].Moller-Hackbarth K, Dewitz C, Schweigert O, Trad A, Garbers C, Rose-John S, et al. , A disintegrin and metalloprotease (ADAM) 10 and ADAM17 are major sheddases of T cell immunoglobulin and mucin domain 3 (Tim-3), J. Biol. Chem 288 (2013) 34529–34544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Xiao W, Klement JD, Lu C, Ibrahim ML, Liu K, IFNAR1 controls autocrine type I IFN regulation of PD-L1 expression in myeloid-derived suppressor cells, J. Immunol 201 (2018) 264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].van der Meer JH, van der Poll T, van ‘t Veer C, TAM receptors, Gas6, and protein S: roles in inflammation and hemostasis, Blood 123 (2014) 2460–2469. [DOI] [PubMed] [Google Scholar]
- [60].Wu CS, Hu CY, Tsai HF, Chyuan IT, Chan CJ, Chang SK, et al. , Elevated serum level of growth arrest-specific protein 6 (Gas6) in systemic lupus erythematosus patients is associated with nephritis and cutaneous vasculitis, Rheumatol. Int 34 (2014) 625–629. [DOI] [PubMed] [Google Scholar]
- [61].Zhao GJ, Zheng JY, Bian JL, Chen LW, Dong N, Yu Y, et al. , Growth arrest-specific 6 enhances the suppressive function of CD4(+)CD25(+) regulatory T cells mainly through Axl receptor, Mediat. Inflamm 2017 (2017) 6848430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yamada T, Horimoto H, Kameyama T, Hayakawa S, Yamato H, Dazai M, et al. , Constitutive aryl hydrocarbon receptor signaling constrains type I interferon-mediated antiviral innate defense, Nat. Immunol 17 (2016) 687–694. [DOI] [PubMed] [Google Scholar]
- [63].Salazar F, Awuah D, Negm OH, Shakib F, Ghaemmaghami AM, The role of indoleamine 2,3-dioxygenase-aryl hydrocarbon receptor pathway in the TLR4-induced tolerogenic phenotype in human DCs, Sci. Rep 7 (2017) 43337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, et al. , Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses, J. Exp. Med 206 (2009) 2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Murray IA, Patterson AD, Perdew GH, Aryl hydrocarbon receptor ligands in cancer: friend and foe, Nat. Rev. Cancer 14 (2014) 801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kashuba EV, Gradin K, Isaguliants M, Szekely L, Poellinger L, Klein G, et al. , Regulation of transactivation function of the aryl hydrocarbon receptor by the Epstein-Barr virus-encoded EBNA-3 protein, J. Biol. Chem 281 (2006) 1215–1223. [DOI] [PubMed] [Google Scholar]
- [67].Inoue H, Mishima K, Yamamoto-Yoshida S, Ushikoshi-Nakayama R, Nakagawa Y, Yamamoto K, et al. , Aryl hydrocarbon receptor-mediated induction of EBV reactivation as a risk factor for Sjogren’s syndrome, J. Immunol 188 (2012) 4654–4662. [DOI] [PubMed] [Google Scholar]
- [68].Dai W, Gupta SL, Regulation of indoleamine 2,3-dioxygenase gene expression in human fibroblasts by interferon-gamma. Upstream control region discriminates between interferon-gamma and interferon-alpha, J. Biol. Chem 265 (1990) 19871–19877. [PubMed] [Google Scholar]
- [69].Moon YW, Hajjar J, Hwu P, Naing A, Targeting the indoleamine 2,3-dioxy-genase pathway in cancer, J. Immunother. Cancer 3 (2015) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Widner B, Sepp N, Kowald E, Ortner U, Wirleitner B, Fritsch P, et al. , Enhanced tryptophan degradation in systemic lupus erythematosus, Immunobiology 201 (2000) 621–630. [DOI] [PubMed] [Google Scholar]
- [71].Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. , Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 19961–19966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Shinde R, Hezaveh K, Halaby MJ, Kloetgen A, Chakravarthy A, da Silva Medina T, et al. , Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans, Nat. Immunol 19 (2018) 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K, et al. , Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopoly-saccharide-induced septic shock, Mol. Cell Biol. 29 (2009) 6391–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cheong JE, Sun L, Targeting the Ido1/TDO2-KYN-AhR pathway for cancer immunotherapy - challenges and opportunities, Trends Pharmacol. Sci. 39 (2018) 307–325. [DOI] [PubMed] [Google Scholar]
- [75].Mozaffarian N, Wiedeman AE, Stevens AM, Active systemic lupus erythematosus is associated with failure of antigen-presenting cells to express programmed death ligand-1, Rheumatology (Oxford) 47 (2008) 1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Scott GN, DuHadaway J, Pigott E, Ridge N, Prendergast GC, Muller AJ, et al. , The immunoregulatory enzyme Ido paradoxically drives B cell-mediated autoimmunity, J. Immunol 182 (2009) 7509–7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H, PD-1 controls follicular T helper cell positioning and function, Immunity 49 (2018) 264–274 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].King NJ, Thomas SR, Molecules in focus: indoleamine 2,3-dioxygenase, Int. J. Biochem. Cell Biol. 39 (2007) 2167–2172. [DOI] [PubMed] [Google Scholar]
- [79].Agbor LN, Elased KM, Walker MK, Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness, Biochem. Pharmacol 82 (2011) 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lucas ED, Finlon JM, Burchill MA, McCarthy MK, Morrison TE, Colpitts TM, et al. , Type 1 IFN and PD-L1 coordinate lymphatic endothelial cell expansion and contraction during an inflammatory immune response, J. Immunol 201 (2018) 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Atehortua L, Rojas M, Vasquez GM, Castano D, Endothelial alterations in systemic lupus erythematosus and rheumatoid arthritis: potential effect of monocyte interaction, Mediat. Inflamm 2017 (2017) 9680729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tjwa M, Bellido-Martin L, Lin Y, Lutgens E, Plaisance S, Bono F, et al. , Gas6 promotes inflammation by enhancing interactions between endothelial cells, platelets, and leukocytes, Blood 111 (2008) 4096–4105. [DOI] [PubMed] [Google Scholar]
- [83].Ito S, Osaka M, Edamatsu T, Itoh Y, Yoshida M, Crucial role of the aryl hydrocarbon receptor (AhR) in indoxyl sulfate-induced vascular inflammation, J. Atheroscler. Thromb 23 (2016) 960–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cho YN, Kee SJ, Kim TJ, Jin HM, Kim MJ, Jung HJ, et al. , Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus, J. Immunol 193 (2014) 3891–3901. [DOI] [PubMed] [Google Scholar]
- [85].Yang X, Yang J, Chu Y, Xue Y, Xuan D, Zheng S, et al. , T follicular helper cells and regulatory B cells dynamics in systemic lupus erythematosus, PloS One 9 (2014) e88441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lei H, Xue Y, Yiyun Y, Weiguo W, Ling L, Zou H, Associations of circulating CXCR3(−)PD-1(+)CD4(+)T cells with disease activity of systemic lupus erythematosus, Mod. Rheumatol (2018) 1–20. [DOI] [PubMed] [Google Scholar]
- [87].Kristjansdottir H, Steinsson K, Gunnarsson I, Grondal G, Erlendsson K, Alarcon-Riquelme ME, Lower expression levels of the programmed death 1 receptor on CD4+CD25+ T cells and correlation with the PD-1.3A genotype in patients with systemic lupus erythematosus, Arthritis Rheum. 62 (2010) 1702–1711. [DOI] [PubMed] [Google Scholar]
- [88].McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG, T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection, Nature 523 (2015) 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Shi H, Ye J, Teng J, Yin Y, Hu Q, Wu X, et al. , Elevated serum autoantibodies against co-inhibitory PD-1 facilitate T cell proliferation and correlate with disease activity in new-onset systemic lupus erythematosus patients, Arthritis Res. Ther. 19 (2017) 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, et al. , IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE, Nat. Commun 9 (2018) 1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jenks S, Cashman K, Zumaquero E, Margorta U, Patel A, Wang X, et al. , Identification of a New Effector B Cell Pathway with Defective Negative Regulation of TLR7 Signaling in Human SLE Immunity, (2018). [Google Scholar]
- [92].Chen H, Peng Q, Yang H, Yin L, Shi J, Zhang Y, et al. , Increased levels of soluble programmed death ligand 1 associate with malignancy in patients with dermatomyositis, J. Rheumatol 45 (2018) 835–840. [DOI] [PubMed] [Google Scholar]
- [93].Prokunina L, Castillejo-Lopez C, Oberg F, Gunnarsson I, Berg L, Magnusson V, et al. , A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans, Nat. Genet 32 (2002) 666–669. [DOI] [PubMed] [Google Scholar]
- [94].Gao J, Gai N, Wang L, Liu K, Liu XH, Wei LT, et al. , Meta-analysis of programmed cell death 1 polymorphisms with systemic lupus erythematosus risk, Oncotarget 8 (22) (2017. May 30) 36885–36897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cho HY, Lee SW, Seo SK, Choi IW, Choi I, Lee SW, Interferon-sensitive response element (ISRE) is mainly responsible for IFN-alpha-induced upregulation of programmed death-1 (PD-1) in macrophages, Biochim. Biophys. Acta 1779 (2008) 811–819. [DOI] [PubMed] [Google Scholar]
- [96].Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, et al. , IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity, J. Immunol 186 (2011) 2772–2779. [DOI] [PubMed] [Google Scholar]
- [97].Thibult ML, Mamessier E, Gertner-Dardenne J, Pastor S, Just-Landi S, Xerri L, et al. , PD-1 is a novel regulator of human B-cell activation, Int. Immunol 25 (2013) 129–137. [DOI] [PubMed] [Google Scholar]
- [98].Nishimura H, Nose M, Hiai H, Minato N, Honjo T, Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor, Immunity 11 (1999) 141–151. [DOI] [PubMed] [Google Scholar]
- [99].Zhen Y, Priest SO, Shao WH, Opposing roles of tyrosine kinase receptors mer and Axl determine clinical outcomes in experimental immune-mediated nephritis, J. Immunol 197 (2016) 2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zhen Y, Lee IJ, Finkelman FD, Shao WH, Targeted inhibition of Axl receptor tyrosine kinase ameliorates anti-GBM-induced lupus-like nephritis, J. Autoimmun 93 (2018) 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Guo Z, Li Y, Zhang D, Ma J, Axl inhibition induces the antitumor immune response which can be further potentiated by PD-1 blockade in the mouse cancer models, Oncotarget 8 (2017) 89761–89774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Skinner HD, Giri U, Yang LP, Kumar M, Liu Y, Story MD, et al. , Integrative analysis identifies a novel AXL-PI3 kinase-PD-L1 signaling Axis Associated with radiation resistance in head and neck cancer, Clin. Cancer Res. 23 (2017) 2713–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, et al. , Adverse renal effects of immune checkpoint inhibitors: a narrative review, Am. J. Nephrol 45 (2017) 160–169. [DOI] [PubMed] [Google Scholar]
- [104].Abelson AK, Johansson CM, Kozyrev SV, Kristjansdottir H, Gunnarsson I, Svenungsson E, et al. , No evidence of association between genetic variants of the PDCD1 ligands and SLE, Genes Immun. 8 (2007) 69–74. [DOI] [PubMed] [Google Scholar]
- [105].Clarke EV, Weist BM, Walsh CM, Tenner AJ, Complement protein C1q bound to apoptotic cells suppresses human macrophage and dendritic cell-mediated Th17 and Th1 T cell subset proliferation, J. Leukoc. Biol 97 (2015) 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Liao W, Zheng H, Wu S, Zhang Y, Wang W, Zhang Z, et al. , The systemic activation of programmed death 1-PD-L1 Axis protects systemic lupus erythematosus model from nephritis, Am. J. Nephrol 46 (2017) 371–379. [DOI] [PubMed] [Google Scholar]
- [107].Sung SJ, Ge Y, Dai C, Wang H, Fu SM, Sharma R, et al. , Dependence of glomerulonephritis induction on novel intraglomerular alternatively activated bone marrow-derived macrophages and mac-1 and PD-L1 in lupus-prone NZM2328 mice, J. Immunol 198 (2017) 2589–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Esser C, Rannug A, The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology, Pharmacol. Rev 67 (2015) 259–279. [DOI] [PubMed] [Google Scholar]
- [109].Kung T, Murphy KA, White LA, The aryl hydrocarbon receptor (AhR) pathway as a regulatory pathway for cell adhesion and matrix metabolism, Biochem. Pharmacol 77 (2009) 536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kado S, Chang WLW, Chi AN, Wolny M, Shepherd DM, Vogel CFA, Aryl hydrocarbon receptor signaling modifies Toll-like receptor-regulated responses in human dendritic cells, Arch. Toxicol 91 (2017) 2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Vogel CF, Khan EM, Leung PS, Gershwin ME, Chang WL, Wu D, et al. , Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-kappaB, J. Biol. Chem 289 (2014) 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Noakes R, The aryl hydrocarbon receptor: a review of its role in the physiology and pathology of the integument and its relationship to the tryptophan metabolism, Int. J. Tryptophan Res. 8 (2015) 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Seok SH, Ma ZX, Feltenberger JB, Chen H, Chen H, Scarlett C, et al. , Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (AHR), J. Biol. Chem 293 (2018) 1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Badawy AA, Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects, Int. J. Tryptophan Res. 10 (2017) 1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Diani-Moore S, Shoots J, Singh R, Zuk JB, Rifkind AB, NAD(+) loss, a new player in AhR biology: prevention of thymus atrophy and hepatosteatosis by NAD (+) repletion, Sci. Rep 7 (2017) 2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Liu Y, Liang X, Dong W, Fang Y, Lv J, Zhang T, et al. , Tumor-repopulating cells induce PD-1 expression in CD8(+) T cells by transferring kynurenine and AhR activation, Cancer Cell 33 (2018) 480–494 e7. [DOI] [PubMed] [Google Scholar]
- [117].Kleczynska W, Jakiela B, Plutecka H, Milewski M, Sanak M, Musial J, Imbalance between Th17 and regulatory T-cells in systemic lupus erythematosus, Folia Histochem. Cytobiol. 49 (2011) 646–653. [DOI] [PubMed] [Google Scholar]
- [118].Redd PS, Lu C, Klement JD, Ibrahim ML, Zhou G, Kumai T, et al. , H3K4me3 mediates the NF-kappaB p50 homodimer binding to the pdcd1 promoter to activate PD-1 transcription in T cells, Oncoimmunology 7 (2018) e1483302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ruan Q, Chen YH, Nuclear factor-kappaB in immunity and inflammation: the Treg and Th17 connection, Adv. Exp. Med. Biol 946 (2012) 207–221. [DOI] [PubMed] [Google Scholar]
- [120].Gutierrez-Vazquez C, Quintana FJ, Regulation of the immune response by the aryl hydrocarbon receptor, Immunity 48 (2018) 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T, Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 9721–9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ouaked N, Mantel PY, Bassin C, Burgler S, Siegmund K, Akdis CA, et al. , Regulation of the foxp3 gene by the Th1 cytokines: the role of IL-27-induced STAT1, J. Immunol 182 (2009) 1041–1049. [DOI] [PubMed] [Google Scholar]
- [123].Villarino AV, Gallo E, Abbas AK, STAT1-activating cytokines limit Th17 responses through both T-bet-dependent and -independent mechanisms, J. Immunol 185 (2010) 6461–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Khajehdehi P, Zanjaninejad B, Aflaki E, Nazarinia M, Azad F, Malekmakan L, et al. , Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study, J. Ren. Nutr 22 (2012) 50–57. [DOI] [PubMed] [Google Scholar]
- [125].Wang ZL, Luo XF, Li MT, Xu D, Zhou S, Chen HZ, et al. , Resveratrol possesses protective effects in a pristane-induced lupus mouse model, PloS One 9 (2014) e114792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Han SS, Chung ST, Robertson DA, Ranjan D, Bondada S, Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, cmyc, bcl-XL, NF-kappa B, and p53, Clin. Immunol 93 (1999) 152–161. [DOI] [PubMed] [Google Scholar]
- [127].Ma C, Wang Y, Dong L, Li M, Cai W, Anti-inflammatory effect of resveratrol through the suppression of NF-kappaB and JAK/STAT signaling pathways, Acta Biochim. Biophys. Sin. (Shanghai) 47 (2015) 207–213. [DOI] [PubMed] [Google Scholar]
- [128].Jeong YI, Kim SW, Jung ID, Lee JS, Chang JH, Lee CM, et al. , Curcumin suppresses the induction of indoleamine 2,3-dioxygenase by blocking the Janus-activated kinase-protein kinase Cdelta-STAT1 signaling pathway in interferon-gamma-stimulated murine dendritic cells, J. Biol. Chem 284 (2009) 3700–3708. [DOI] [PubMed] [Google Scholar]
- [129].Ma C, Wang Y, Shen A, Cai W, Resveratrol upregulates SOCS1 production by lipopolysaccharide-stimulated RAW264.7 macrophages by inhibiting miR-155, Int. J. Mol. Med 39 (2017) 231–237. [DOI] [PubMed] [Google Scholar]
- [130].Mohammadi-Bardbori A, Bengtsson J, Rannug U, Rannug A, Wincent E, Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AHR), Chem. Res. Toxicol 25 (2012) 1878–1884. [DOI] [PubMed] [Google Scholar]
- [131].Handono K, Pratama MZ, Endharti AT, Kalim H, Treatment of low doses curcumin could modulate Th17/Treg balance specifically on CD4+ T cell cultures of systemic lupus erythematosus patients, Cent. Eur. J. Immunol 40 (2015) 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Yao J, Wei C, Wang JY, Zhang R, Li YX, Wang LS, Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice, World J. Gastroenterol. 21 (2015) 6572–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing Y, et al. , FOXP3 and RORgammat: transcriptional regulation of Treg and Th17, Int. Immunopharmacol 11 (2011) 536–542. [DOI] [PubMed] [Google Scholar]
- [134].Chang MR, Dharmarajan V, Doebelin C, Garcia-Ordonez RD, Novick SJ, Kuruvilla DS, et al. , Synthetic RORgammat agonists enhance protective immunity, ACS Chem. Biol. 11 (2016) 1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Busbee PB, Rouse M, Nagarkatti M, Nagarkatti PS, Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders, Nutr. Rev 71 (2013) 353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ehrlich AK, Kerkvliet NI, Is chronic AhR activation by rapidly metabolized ligands safe for the treatment of immune-mediated diseases? Curr. Opin. Toxicol 2 (2017) 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Domeier PP, Chodisetti SB, Soni C, Schell SL, Elias MJ, Wong EB, et al. , IFN-gamma receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity, J. Exp. Med 213 (2016) 715–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Klein U, Heise N, Unexpected functions of nuclear factor-kappaB during germinal center B-cell development: implications for lymphomagenesis, Curr. Opin. Hematol 22 (2015) 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Vaidyanathan B, Chaudhry A, Yewdell WT, Angeletti D, Yen WF, Wheatley AK, et al. , The aryl hydrocarbon receptor controls cell-fate decisions in B cells, J. Exp. Med 214 (2017) 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA, An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells, J. Immunol 185 (2010) 3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Villa M, Gialitakis M, Tolaini M, Ahlfors H, Henderson CJ, Wolf CR, et al. , Aryl hydrocarbon receptor is required for optimal B-cell proliferation, EMBO J. 36 (2017) 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Nishimura H, Minato N, Nakano T, Honjo T, Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses, Int. Immunol 10 (1998) 1563–1572. [DOI] [PubMed] [Google Scholar]
- [143].Moisini I, Davidson A, BAFF: a local and systemic target in autoimmune diseases, Clin. Exp. Immunol 158 (2009) 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, et al. , Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB, Proc. Natl. Acad. Sci. U. S. A 106 (2009) 14978–14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Balfour HH Jr., Sifakis F, Sliman JA, Knight JA, Schmeling DO, Thomas W, Age-specific prevalence of Epstein-Barr virus infection among individuals aged 6–19 years in the United States and factors affecting its acquisition, J. Infect. Dis 208 (2013) 1286–1293. [DOI] [PubMed] [Google Scholar]
- [146].Larochelle B, Flamand L, Gourde P, Beauchamp D, Gosselin J, Epstein-Barr virus infects and induces apoptosis in human neutrophils, Blood 92 (1998) 291–299. [PubMed] [Google Scholar]
- [147].Coleman CB, Lang J, Sweet LA, Smith NA, Freed BM, Pan Z, et al. , Epstein-Barr virus type-2 infects T-cells and induces B-cell lymphomagenesis in humanized mice, J. Virol 92 (21) (2018. October 12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Tsao SW, Tsang CM, Pang PS, Zhang G, Chen H, Lo KW, The biology of EBV infection in human epithelial cells, Semin. Cancer Biol. 22 (2012) 137–143. [DOI] [PubMed] [Google Scholar]
- [149].Thorley-Lawson DA, Epstein-Barr virus: exploiting the immune system, Nat. Rev. Immunol 1 (2001) 75–82. [DOI] [PubMed] [Google Scholar]
- [150].Xia LP, Li BF, Shen H, Lu J, Interleukin-27 and interleukin-23 in patients with systemic lupus erythematosus: possible role in lupus nephritis, Scand. J. Rheumatol 44 (2015) 200–205. [DOI] [PubMed] [Google Scholar]
- [151].He B, Raab-Traub N, Casali P, Cerutti A, EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching, J. Immunol 171 (2003) 5215–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Najjar I, Baran-Marszak F, Le Clorennec C, Laguillier C, Schischmanoff O, Youlyouz-Marfak I, et al. , Latent membrane protein 1 regulates STAT1 through NF-kappaB-dependent interferon secretion in Epstein-Barr virus-immortalized B cells, J. Virol 79 (2005) 4936–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Bi XW, Wang H, Zhang WW, Wang JH, Liu WJ, Xia ZJ, et al. , PD-L1 is upregulated by EBV-driven LMP1 through NF-kappaB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma, J. Hematol. Oncol 9 (2016) 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Li Y, Mahajan NP, Webster-Cyriaque J, Bhende P, Hong GK, Earp HS, et al. , The C-mer gene is induced by Epstein-Barr virus immediate-early protein BRLF1, J. Virol 78 (2004) 11778–11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Adamson AL, Darr D, Holley-Guthrie E, Johnson RA, Mauser A, Swenson J, et al. , Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases, J. Virol 74 (2000) 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Li Y, Long X, Huang L, Yang M, Yuan Y, Wang Y, et al. , Epstein-barr virus BZLF1-mediated downregulation of proinflammatory factors is essential for optimal lytic viral replication, J. Virol 90 (2015) 887–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. , The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27, Nat. Immunol 11 (2010) 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, et al. , Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1, Immunity 36 (2012) 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chien CH, Yu HC, Chen SY, Chiang BL, Characterization of c-maf(+)foxp3(−) regulatory T cells induced by repeated stimulation of antigen-presenting B cells, Sci. Rep 7 (2017) 46348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Nemerow GR, Mullen JJ 3rd, Dickson PW, Cooper NR, Soluble recombinant CR2 (CD21) inhibits Epstein-Barr virus infection, J. Virol 64 (1990) 1348–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Martin DR, Marlowe RL, Ahearn JM, Determination of the role for CD21 during Epstein-Barr virus infection of B-lymphoblastoid cells, J. Virol 68 (1994) 4716–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Masilamani M, Kassahn D, Mikkat S, Glocker MO, Illges H, B cell activation leads to shedding of complement receptor type II (CR2/CD21), Eur. J. Immunol 33 (2003) 2391–2397. [DOI] [PubMed] [Google Scholar]
- [163].Vereshchagina LA, Tolnay M, Tsokos GC, Multiple transcription factors regulate the inducible expression of the human complement receptor 2 promoter, J. Immunol 166 (2001) 6156–6163. [DOI] [PubMed] [Google Scholar]
- [164].Larcher C, Kempkes B, Kremmer E, Prodinger WM, Pawlita M, Bornkamm GW, et al. , Expression of Epstein-Barr virus nuclear antigen-2 (EBNA2) induces CD21/CR2 on B and T cell lines and shedding of soluble CD21, Eur. J. Immunol 25 (1995) 1713–1719. [DOI] [PubMed] [Google Scholar]
- [165].Rickert RC, Regulation of B lymphocyte activation by complement C3 and the B cell coreceptor complex, Curr. Opin. Immunol 17 (2005) 237–243. [DOI] [PubMed] [Google Scholar]
- [166].Thorarinsdottir K, Camponeschi A, Gjertsson I, Martensson IL, CD21 -/low B cells: a snapshot of a unique B cell subset in Health and disease, Scand. J. Immunol 82 (2015) 254–261. [DOI] [PubMed] [Google Scholar]
- [167].Masilamani M, Nowack R, Witte T, Schlesier M, Warnatz K, Glocker MO, et al. , Reduction of soluble complement receptor 2/CD21 in systemic lupus erythomatosus and Sjogren’s syndrome but not juvenile arthritis, Scand. J. Immunol 60 (2004) 625–630. [DOI] [PubMed] [Google Scholar]
- [168].Haas KM, Poe JC, Tedder TF, CD21/35 promotes protective immunity to Streptococcus pneumoniae through a complement-independent but CD19-dependent pathway that regulates PD-1 expression, J. Immunol 183 (2009) 3661–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Das R, Bar N, Ferreira M, Newman AM, Zhang L, Bailur JK, et al. , Early B cell changes predict autoimmunity following combination immune checkpoint blockade, J. Clin. Investig 128 (2018) 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Rossille D, Azzaoui I, Feldman AL, Maurer MJ, Laboure G, Parrens M, et al. , Soluble programmed death-ligand 1 as a prognostic biomarker for overall survival in patients with diffuse large B-cell lymphoma: a replication study and combined analysis of 508 patients, Leukemia 31 (2017) 988–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Azrielant S, Tiosano S, Watad A, Mahroum N, Whitby A, Comaneshter D, et al. , Correlation between systemic lupus erythematosus and malignancies: a cross-sectional population-based study, Immunol. Res 65 (2017) 464–469. [DOI] [PubMed] [Google Scholar]
- [172].Chihara D, Nastoupil LJ, Williams JN, Lee P, Koff JL, Flowers CR, New insights into the epidemiology of non-Hodgkin lymphoma and implications for therapy, Expert Rev. Anticancer Ther. 15 (2015) 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Knight JS, Blayney DW, Somers EC, Patients with systemic lupus erythematosus and haematological malignancy at a tertiary care centre: timing, histopathology and therapy, Lupus Sci. Med. 1 (2014) e000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Lugassy G, Lishner M, Polliack A, Systemic lupus erythematosus and chronic lymphocytic leukemia: rare coexistence in three patients, with comments on pathogenesis, Leuk. Lymphoma 8 (1992) 243–245. [DOI] [PubMed] [Google Scholar]
- [175].Sinha S, Boysen J, Nelson M, Secreto C, Warner SL, Bearss DJ, et al. , Targeted Axl inhibition primes chronic lymphocytic leukemia B cells to apoptosis and shows synergistic/additive effects in combination with BTK inhibitors, Clin. Cancer Res. 21 (2015) 2115–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Niemann CU, Wiestner A, B-cell receptor signaling as a driver of lymphoma development and evolution, Semin. Cancer Biol. 23 (2013) 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Teras LR, Rollison DE, Pawlita M, Michel A, Brozy J, de Sanjose S, et al. , Epstein-Barr virus and risk of non-Hodgkin lymphoma in the cancer prevention study-II and a meta-analysis of serologic studies, Int. J. Cancer 136 (2015) 108–116. [DOI] [PubMed] [Google Scholar]
- [178].Bassig BA, Zheng T, Zhang Y, Berndt SI, Holford TR, Hosgood HD 3rdet al., Polymorphisms in complement system genes and risk of non-Hodgkin lymphoma, Environ. Mol. Mutagen 53 (2012) 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Rahman HA, Khorshied MM, Khorshid OM, Mahgoub SM, Toll-like receptor 2 and 9 genetic polymorphisms and the susceptibility to B cell Non-Hodgkin Lymphoma in Egypt, Ann. Hematol 93 (2014) 1859–1865. [DOI] [PubMed] [Google Scholar]
- [180].Puente XS, Pinyol M, Quesada V, Conde L, Ordonez GR, Villamor N, et al. , Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia, Nature 475 (2011) 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]