Invasive mechanical ventilation is a remarkable advance, but the possibility of ventilator-induced lung injury exists, particularly if the ventilator settings are not optimized. The best methods to avoid lung injury during mechanical ventilation, either during ventilation of healthy lungs in the operating room or during ventilation as support during critical illness, are topics of debate. In this review, we summarize the current evidence and review a relatively new concept to prevent lung injury: targeting driving pressure defined by plateau pressure minus positive end-expiratory pressure (PEEP; see table 1) when setting and adjusting mechanical ventilation.
Table 1.
Terminology for Mechanical Ventilation
| Driving pressure (ΔP) | ΔP = Pplat - PEEP or ΔP = VT / CRS |
| Plateau pressure (Pplat) | Measured during an inspiratory pause on the ventilator, it reflects the mean pressure in the alveoli and small airways. |
| Respiratory system compliance (CRS) | Crs = VT / (Pplat - PEEP) |
| Ventilator-induced lung injury (VILI) | The term coined to emphasize that while the ventilator can be life saving it can also be injurious depending on the settings. VILI refers to worsening lung function caused by application of mechanical ventilation to predisposed lungs. |
| Elastance (E) | Elastance (stiffness): change in pressure for a given change in volume (E = ΔP / ΔV). Elastance is the inverse of Compliance (E = 1 / Compliance) |
| Transpulmonary pressure (PTP) | Airway pressure - pleural pressure or pressure difference inside versus outside the lung (PTP = Pairway - Ppleural). This is the pertinent distending pressure of the lung. |
| Pleural pressure (PPI) | Pressure within the pleural space surrounding the lung sometimes estimated using esophageal manometry. |
| Atelectrauma | Lung injury caused by high shear forces from cyclic opening and collapse of atelectatic but recruitable lung units. |
| Biotrauma | Extrapulmonary organ injury caused by proinflammatory and other circulating mediators systemically released during injurious ventilation. |
VT, tidal volume.
Lung injury
Lung injury results from excess transpulmonary pressure (stress on the lung), lung stretch, and atelectrauma (lung injury from repetitive alveolar collapse).1 Transpulmonary pressure is measured as the airway opening pressure (i.e., that measured on the ventilator) minus the pleural pressure (estimated with esophageal manometry).2 One cause of high transpulmonary pressures (inside pressure minus outside pressure) is the delivery of large tidal volumes (VT).3 This situation can lead to high lung stress, causing lung injury and inflammation, and is associated with poor outcomes, particularly in acute respiratory distress syndrome (ARDS). In addition to lung stress from tidal inflation, atelectrauma is the damage caused by repetitive collapse of the lung at end-exhalation, and low PEEP settings may contribute to this pathophysiology.4
The importance of protecting the lung is emphasized by the systemic consequences of lung injury: the term biotrauma has been coined to describe the concept that lung injury may contribute to remote organ injury and multisystem organ failure through the release of inflammatory factors and other mediators.5 Thus, optimization of mechanical ventilation settings can have benefits beyond lung protection and may be protective for systemic organ function.6,7
How to Protect the Lung?
Various strategies guide optimal ventilator settings and may mitigate the deleterious effects of mechanical ventilation and high transpulmonary pressures.8
Low Tidal Volumes
Low VT mechanical ventilation is a well-established method that limits ventilator-induced lung injury and has been shown to improve mortality in clinical trials. An original study by the ARDS Network published in 2000 compared a low versus high VT strategy and demonstrated a clear mortality benefit with the low VT (6 ml/kg ideal body weight) approach compared to a higher delivered VT (12 ml/kg ideal body weight).9
Positive End-Expiratory Pressure
A promising single-center study looked at adjusting PEEP settings based on measuring transpulmonary pressures.2 The authors used an esophageal balloon manometer to estimate pleural pressures in patients with ARDS. Results suggested improved gas exchange and respiratory compliance using this strategy. Recently however, Beitler et al. published results of another study which used a similar strategy of esophageal pressure monitoring to estimate transpulmonary pressure and titrate to an optimal PEEP10 In this latter study, the control arm used a higher PEEP/Fio2 titration ladder compared to the former study. They enrolled 200 patients with moderate to severe ARDS (Pao2:Fio2 ≤ 200 mmHg). When compared with the control patients, there was no difference in the composite endpoint of death and days free from mechanical ventilation between groups. That is, titrating ventilator settings to an optimal transpulmonary pressure based on measuring esophageal pressure was not more effective than simple PEEP titrations based on Fio2. Interestingly, airway driving pressure was not different between the two groups at baseline or with treatment, suggesting that both strategies had similar mechanical effects. Although further research is required,11–13 the routine use of esophageal manometry in clinical practice is not generally needed.14,15
Driving Pressure
Recently, adjusting settings using a targeted driving pressure has been advanced as a method that may minimize subsequent lung injury and has many appealing features. Driving pressure is defined by plateau pressure (end-inflation hold pressure) minus PEEP and is thus a key determinant in the delivered VT (fig. 1). The distinction between driving pressure and transpulmonary pressure is an important one since the former reflects the pressure difference within a breath as opposed to the latter which reflects the pressure difference across the lung. Both values are purported to be implicated in lung stress, although transpulmonary pressure more directly conveys this concept based on Newtonian principles. Driving pressure, although directly measured from the ventilator, is a function of both VT and respiratory system compliance (driving pressure = VT / respiratory system compliance). Both increases in VT and decreases in respiratory system (lung, chest wall) compliance can increase driving pressure. Similarly, driving pressure increases with higher plateau pressure as well as with lower PEEP all other things being equal. Thus, practicing clinicians should be aware of the components of driving pressure and consider underlying mechanisms rather than treating a single variable in isolation.
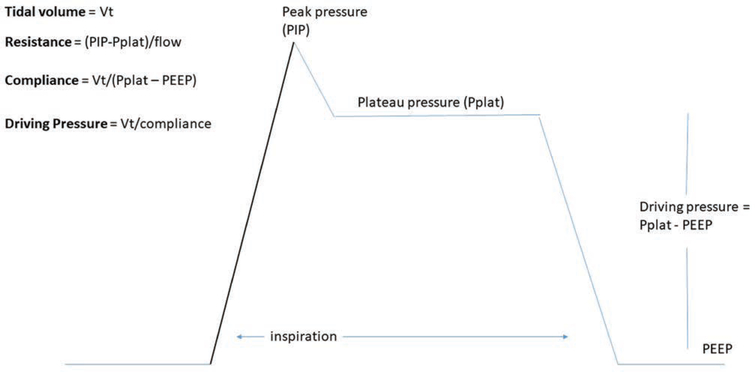
Fig. 1.
A schematic diagram of an inspiratory waveform delivered during typical volume cycled ventilation. Pplat is based on an end-inspiratory hold. The driving pressure can be seen as the difference between the Pplat and the PEEP, but can also be calculated as the ratio of the VT to the respiratory system compliance. PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure; Pplat, plateau pressure; VT, tidal volume.
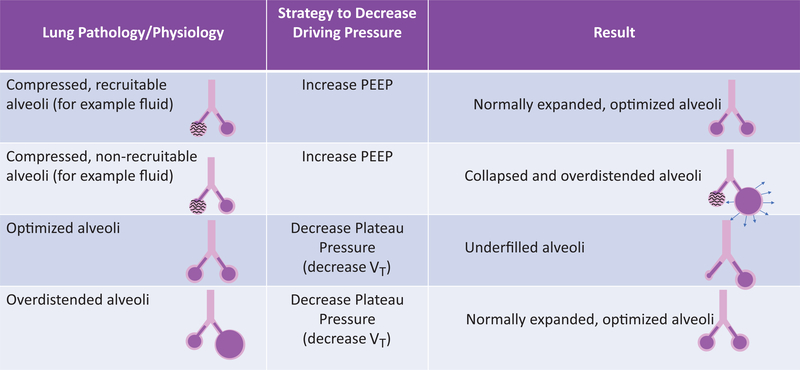
In 2015 Amato et al.16 demonstrated that driving pressure may be the most important factor in determining lung injury during mechanical ventilation: In an individual patient meta-analysis, a composite of 3,562 patients with ARDS who were previously enrolled in nine separate randomized trials was analyzed to determine predictors of survival. Among ventilator settings, survival was most strongly associated with driving pressure, while plateau pressure, PEEP, and VT did not independently predict survival when driving pressure was already a covariate. In their mediation analyses, higher driving pressure predicted lower survival while increases in PEEP or reductions in VT were only beneficial when they were associated with reductions in driving pressure. Even though such a mediation analysis is unable to establish causality, the authors conclude that, based on their study, driving pressure is the ventilatory variable that best predicts survival. Others have pointed toward important limitations of this study.17 First, driving pressure can be most accurately determined in passively ventilated patients, but is less clear during spontaneous breathing.18 Second, reductions in driving pressure can be generally achieved by either a decrease in VT or an increase in PEEP (or a combination thereof), resulting in potentially very different clinical scenarios (e.g., from the standpoint of hemodynamics and/or atelectrauma). Of note, driving pressure can be reduced by different strategies (fig. 2) and the mere reduction of driving pressure, depending on which strategy is used, may not adequately optimize lung mechanics. Another limitation of the Amato analyses is that the parent studies were generally designed with a relatively low and fixed VT within a narrow range, making its predictive value quite weak on a statistical basis. Thus, as stated, driving pressure is an important variable but potentially problematic to guide patient management when used in isolation.19
Fig. 2.
The impact of reduced driving pressure depends greatly on how it is achieved and on the underlying biology of the lung. The figure illustrates the varying results that could occur with reduced driving pressure, emphasizing the need to avoid assessing variables in isolation and to assess individual patients at the bedside after any mechanical ventilator changes. PEEP, positive end-expiratory pressure; VT, tidal volume.
Subsequent to the Amato et al. analyses, the Large Observational Cohort Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNG SAFE) study20 was a 4-week investigation (conducted in the winter of 2014) of 459 intensive care units (ICU) from 50 countries across five continents, and was designed to evaluate ICU incidence and outcome of ARDS. While the authors’ primary outcome was the incidence of ARDS in the studied ICUs, other outcomes, such as clinician recognition of ARDS, ventilatory management, the use of adjunctive therapies, and clinical outcomes, were also included. The results may be surprising to some: the authors found ARDS to be an underrecognized syndrome. The incidence was 10.4% of all admitted patients and 23.4% of (invasively or noninvasively) ventilated ICU admissions studied (29,144 admitted 13,566 ventilated and enrolled). Clinician recognition of ARDS at the time of fulfillment of ARDS criteria was only 34.0% and ARDS was eventually diagnosed in only 60.2% of patients who met Berlin definition criteria.21 In addition, the recognition of ARDS had a limited effect on ventilation strategies. While the recognition of ARDS did not significantly affect VT or plateau pressure, the ARDS diagnosis was associated with higher levels of PEEP and increased use of adjunctive therapies (e.g., prone positioning or neuromuscular blockade), as compared to no ARDS diagnosis. However, PEEP and adjunctive measure use were still lower than expected in this cohort.22 Because ARDS was associated with high mortality rates (unadjusted ICU mortality from ARDS was 35.5%; hospital mortality was 40.0%) and clinician recognition did no consistently resulta in changes in ventilator strategies, the authors point to the potential for major improvement in ARDS recognition and management. Given the variability in the recognition of ARDS, some have argued for the more routine use of lung protective strategies (such as low VT and/or low driving pressure), such that inadvertent lung injurious ventilation is avoided in patients who have deteriorating gas exchange.23 An additional analysis24 of the LUNG SAFE study focused on the potentially modifiable risk factors for mortality in ARDS. Data from 2,377 patients originally enrolled in the LUNG SAFE trial were analyzed. Patients were included if they met ARDS criteria on day 1 or 2 after the development of failure and initiation of mechanical ventilation. Multivariate analysis revealed that lower PEEP, higher peak inspiratory pressures, higher plateau pressures, and higher driving pressure, as well as increased respiratory rate, were associated with higher ARDS mortality. VT did not differ between patients who survived ARDS and those who did not. In patients with moderate and severe ARDS, lower driving pressures were associated with lower hospital mortality. In aggregate, these data—when coupled with the previous findings of Amato et al.—suggest that targeting a lower driving pressure in patients with ARDS may improve outcomes.16
Blondonnet et al.25 recently published a prospective observational study of patients at risk for the development of ARDS by analyzing driving pressure at baseline and at 24 h. Interestingly, the entire cohort had a baseline Pao2:Fio2 ratio of 269 ± 88 mmHg, suggesting some already had ARDS based on current consensus definitions. Two hundred twenty-one patients were enrolled and 34 (15%) were diagnosed with ARDS by day 7. There was no statistical difference between baseline VT (7.7 ± 1.2 ml/kg [no ARDS] vs. 7.4 ± 1.4 ml/kg [ARDS]; P = 0.4) or in baseline plateau pressures (nonsignificantly higher in the ARDS vs. no ARDS group). Both baseline driving pressure and respiratory rate were statistically lower in the no ARDS group. Subsequent analyses suggested that a baseline driving pressure greater than16.5 cm H2O was predictive of the development of incident ARDS with a specificity of 90%. Similarly, a baseline driving pressure less than 7.5 cm H2O was highly sensitive in predicting those who would not develop ARDS. While ventilator settings were not titrated to a specific driving pressure, this study suggests that elevated driving pressure may be a marker for risk of subsequent lung injury. The predictive value of intermediate driving pressure values (7.5 to 16.5 cm H2O) is less clear, but in general, we target low driving pressures, recognizing that other clinical factors (including hemodynamics and gas exchange) may contribute to these decisions.
Although the concepts underlying the use of driving pressure were conceived for passively ventilated patients, some have argued the utility of driving pressure during spontaneous breathing. Driving pressure may be controlled by the human body during spontaneous breathing as a protective mechanism: one retrospective review examined whether patients may have innate control over driving pressure. Georgopoulos et al.26 found that when critically ill mechanically ventilated patients were switched from controlled mechanical ventilation (with a set VT) to proportional assist ventilation (a mode which adjusts pressure based on respiratory effort), patients controlled their driving pressure (with plateau pressure measured in a modified way to account for patients’ inspiratory effort as previously described27) within a narrow “safe” window (e.g., for those with driving pressure greater than or equal to 15 cm H2O on controlled mechanical ventilation with low VT; on switch to proportional assist ventilation, patients controlled driving pressure without constraining VT), suggesting neural control mechanisms limit lung stretch. Thus, in theory, a neuronal feedback loop may be present which helps to reduce respiratory effort by limiting a maximal transpulmonary pressure. The authors concluded that lung protective mechanical ventilation may be practiced without VT restriction. The findings give credibility to the use of driving pressure targets in patients who are spontaneously breathing. Moreover, the work of Bellani et al. has supported the efforts to interpret driving pressure and plateau airway pressure in the face of spontaneous breathing.18,28 In general, the plateau pressure can still be interpreted and measured during spontaneous breathing by analyzing the waveforms, as the impact of respiratory effort can be identified.18,29 Of note, regarding spontaneous breathing, recent studies have questioned the use of neuromuscular blockade in the management of patients with ARDS. Papazian et al. had previously shown benefits to 48 h of muscle relaxant therapy in patients with ARDS; however, a more recent study showed no benefit to a similar approach.30,31 As a result, the current trend in ARDS is to avoid heavy sedation (defined as nonresponsive patients, e.g., Richmond Agitation and Sedation Scale of −4 or −5) and neuromuscular blockade; the resulting increase in spontaneously breathing ARDS patients will potentially complicate the interpretation of driving pressure. Until further data are available, however, limiting the use of driving pressure to patients who are passively ventilated would seem reasonable.
Driving pressure has also been studied in the operative patient. Park et al32 randomized 291 patients scheduled for elective thoracic surgery requiring one-lung ventilation to two different ventilatory management strategies: one group was ventilated with a standard low VT (6 ml/kg ideal body weight), PEEP of 5 cm H2O and recruitment maneuvers as needed, while the other group used low VT, but titrated PEEP based on targeting driving pressure. Both postoperative pulmonary complications (12.2% in conventional vs. 5.5% in driving pressure group) and ARDS by postoperative day 3 (five patients in the conventional group; zero patients in the driving pressure group) were noted; however, there was no difference in ARDS by day 7 or ICU or hospital length of stay. Of note, the median difference in driving pressure between the two groups was only 1 cm H2O, and the median optimum PEEP in the driving pressure group was only 3 cm H2O (vs. the set PEEP in the lung protective strategy of 5 cm H2O). The authors argued that individualized ventilation, not driving pressure per se, was key for their findings. Although these data suggest possible benefit in the driving pressure targeted group, the minor difference in exposure and lack of significant difference in outcome make a major clinical impact unlikely and thus, have not changed clinical practice to date. As a result, driving pressure can be monitored in the operating room, but again, data do not support using it in isolation to guide mechanical ventilation.
Targeting driving pressure may also be helpful in patients on extracorporeal membrane oxygenation: although comprehensive human studies are sparse to date, studies utilizing pig models may point to the usefulness of limiting driving pressure in such setting. Araos et al.33 induced lung injury in a pig model, placing them on venovenous extracorporeal membrane oxygenation and randomizing them to one of three mechanical ventilation strategies—one group with a target driving pressure of 10 cm H2O and low RR and VT, and the other two with various PEEP levels, higher RRs and VTs, and no driving pressure target. Gas exchange was managed by the extracorporeal membrane oxygenation circuit, but driving pressure was significantly higher in the latter two groups (14 to 15 cm H2O in the 6 ml/kg group, and 21 to 24 cm H2O in the 10 ml/kg group). Histologic lung injury scores were lowest in the group ventilated with a low driving pressure strategy. Future studies in humans on extracorporeal membrane oxygenation using driving pressure compared with the current standard of low respiratory rate, minimal VT, and moderate PEEP are warranted. However, a low driving pressure target would seem reasonable for respiratory failure patients receiving extracorporeal membrane oxygenation until further data are available.
Future Directions
Although the concept of driving pressure has gained traction in recent years, questions remain about how best to implement strategies guided by this approach in the operating room and the ICU. A number of issues could be addressed in the future by clinicians and by researchers.
Although some data support the limitation of driving pressure in the case of patients at risk of ARDS, currently there are insufficient data to support this strategy in patients in the operating room. Thus, one future direction could be to study driving pressure–based approaches in the operating room (for various kinds of surgery) to test whether this approach helps to improve the risk of postoperative respiratory failure, duration of mechanical ventilation, or incidence of ARDS.
Could a mechanical ventilation strategy using a driving pressure based approach provide better outcomes than a traditional approach as recommended by the ARDS Network in patients with or at risk of developing ARDS? Isolating a particular variable such as driving pressure will be difficult to achieve independent ofVT and Paco2, but experimental pathways could be developed depending on which independent variable is to be prioritized. In the research context, studies during extracorporeal support could be used to isolate a variable during mechanical ventilation since gas exchange could be independently controlled.
From the standpoint of implementation, is a driving pressure-based strategy superior to a traditional approach that might require manipulation of PEEP, plateau pressure, and VT34? Manipulating a mechanical ventilator to target a low driving pressure may be easier to automate and to teach. Adherence with a particular protocol could be an outcome of interest since a driving pressure based approach may be easier to implement at the bedside.
Physiological research using imaging modalities such as electrical impedance tomography could be used to assess the impact of various manipulations in driving pressure.35 Because increases in PEEP or a reduction in plateau pressure impacts driving pressure, differences in lung injury/atelectasis distribution as a result may be important to evaluate,36 but may also differ depending on the amount of lung available for gas exchange based on imaging of the recruitable lung volume.37
Basic studies regarding cell stretch and deformation of the lung are ongoing but could be used to identify which forces may have the greatest impact on injury and repair. Further insights regarding cellular stress and strain could be used to guide strategies to optimize lung protection.38,39
Summary
Despite persistent questions, the existing data provide some reassurance that driving pressure is a useful addition to our armamentarium (table 2), but should not be used in isolation due to the multitude of factors that influence its value, interpretation, and changes through time. In general, mechanical ventilator settings should be individualized based on the nature of the lung disease, the patient’s hemodynamics, gas exchange, body habitus, and other factors. Until more definitive data are available, the targeting of driving pressure in ARDS should be considered in the context of other mechanical ventilation variables and clinical factors, including hemodynamics, as well as gas exchange.
Table 2.
Clinical Recommendations Regarding Driving Pressure and ARDS
| Driving pressure should not be used in isolation but should be interpreted in the context of PEEP, VT, and lung mechanics. |
| Elevated driving pressure should prompt a bedside assessment regarding how best to change settings and lower the driving pressure, e.g., reducing VT, diuresis, or sedation. |
| To the extent possible, driving pressure should be reduced through time, recognizing that hypercapnia, dyspnea, and patient/ventilator dyssynchrony may occur with low-minute ventilation. |
| In patients at risk of ARDS, minimizing driving pressure may be associated with reduced risk of incident ARDS. Data regarding clinical benefits are less compelling than established ARDS. |
ARDS, acute respiratory distress syndrome; PEEP, positive end-expiratory pressure; VT, tidal volume.
Acknowledgments
Research Support
ResMed (San Diego, California) provided a philanthropic donation to University of California San Diego in support of a sleep center. Dr. Malhotra is funded by the National Heart, Lung, and Blood Institute (Bethesda, Maryland) and received income from Merck (Kenilworth, New Jersey) related to medical education for drug discovery. Dr. Meier is funded by the National Institutes of Health (Bethesda, Maryland; 1KL2TR001444).
Footnotes
Competing Interests
Dr. Meier reports receiving an International Anesthesia Research Society (San Francisco, California) Mentored Training Grant and the Altman Clinical and Translational Research Institute (La Jolla, California) Pilot Grant outside of the submitted work, as well as a speaker fee from the California Society of Anesthesiologists (Sacramento, California), and reviews grants for the International Anesthesia Research Society. The other authors declare no competing interests.
References
- 1.Brower RG, Hubmayr RD, Slutsky AS: Lung stress and strain in acute respiratory distress syndrome: Good ideas for clinical management? Am J Respir Crit Care Med 2008; 178:323–4 [DOI] [PubMed] [Google Scholar]
- 2.Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH: Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008; 359:2095–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malhotra A: Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med 2007; 357:1113–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kneyber MC, Zhang H, Slutsky AS: Ventilator-induced lung injury. Similarity and differences between children and adults. Am J Respir Crit Care Med 2014; 190:258–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hepokoski M, Englert JA, Baron RM, Crotty- Alexander LE, Fuster MM, Beitler JR, Malhotra A, Singh P: Ventilator-induced lung injury increases expression of endothelial inflammatory mediators in the kidney. Am J Physiol Renal Physiol 2017; 312:F654–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall JC, Ranieri VM, Slutsky AS: Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 2003; 289:2104–12 [DOI] [PubMed] [Google Scholar]
- 7.Ranieri VM, Giunta F, Suter PM, Slutsky AS: Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA 2000; 284:43–4 [DOI] [PubMed] [Google Scholar]
- 8.Talmor D, Sarge T, O’Donnell CR, Ritz R, Malhotra A, Lisbon A, Loring SH: Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med 2006; 34:1389–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–8 [DOI] [PubMed] [Google Scholar]
- 10.Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, Loring SH, Talmor D, Group EP-S : Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baedorf Kassis E, Loring SH, Talmor D: Should we titrate peep based on end-expiratory transpulmonary pressure?-yes. Ann Transl Med 2018; 6:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baedorf Kassis E, Loring SH, Talmor D: Lung volumes and transpulmonary pressure are decreased with expiratory effort and restored with passive breathing in ARDS: A reapplication of the traditional Campbell diagram. Intensive Care Med 2018; 44:534–6 [DOI] [PubMed] [Google Scholar]
- 13.Baedorf Kassis E, Loring SH, Talmor D: Esophageal pressure:research or clinical tool? Med Klin Intensivmed Notfmed 2018; 113(Suppl 1):13–20 [DOI] [PubMed] [Google Scholar]
- 14.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, Pelosi P, Talmor D, Grasso S, Chiumello D, Guérin C, Patroniti N, Ranieri VM, Gattinoni L, Nava S, Terragni PP, Pesenti A, Tobin M, Mancebo J, Brochard L; PLUG Working Group (Acute Respiratory Failure Section of the European Society of Intensive Care Medicine): The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014; 189:520–31 [DOI] [PubMed] [Google Scholar]
- 15.Chiumello D, Carlesso E, Brioni M, Cressoni M: Airway driving pressure and lung stress in ARDS patients. Crit Care 2016; 20:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG:Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015; 372:747–55 [DOI] [PubMed] [Google Scholar]
- 17.Loring SH, Malhotra A: Driving pressure and respiratory mechanics in ARDS. N Engl J Med 2015; 372:776–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellani G, Grassi A, Sosio S, Foti G: Plateau and driving pressure in the presence of spontaneous breathing. Intensive Care Med 2019; 45:97–8 [DOI] [PubMed] [Google Scholar]
- 19.Lanspa MJ, Peltan ID, Jacobs JR, Sorensen JS, Carpenter L, Ferraro JP, Brown SM, Berry JG, Srivastava R, Grissom CK: Driving pressure is not associated with mortality in mechanically ventilated patients without ARDS. Crit Care 2019; 23:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A; LUNG SAFE Investigators; ESICM Trials Group: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 21.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS; ARDS Definition Task Force: Acute respiratory distress syndrome: The Berlin definition. JAMA 2012; 307:2526–33 [DOI] [PubMed] [Google Scholar]
- 22.Cornejo RA, Diaz JC, Tobar EA, Bruhn AR, Ramos CA, Gonzalez RA, Repetto CA, Romero CM, Galvez LR, Llanos O, Arellano DH, Neira WR, Diaz GA, Zamorano AJ, Pereira GL: Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2013; 188:440–8 [DOI] [PubMed] [Google Scholar]
- 23.Rubenfeld GD, Shankar-Hari M: Lessons from ARDS for non-ARDS research: Remembrance of trials past. JAMA 2018; 320:1863–5 [DOI] [PubMed] [Google Scholar]
- 24.Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, Brochard L, Clarkson K, Esteban A, Gattinoni L, van Haren F, Heunks LM, Kurahashi K, Laake JH, Larsson A, McAuley DF, McNamee L, Nin N, Qiu H, Ranieri M, Rubenfeld GD, Thompson BT, Wrigge H, Slutsky AS, Pesenti A; LUNG SAFE Investigators and the ESICM Trials Group: Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: The LUNG SAFE study. Intensive Care Med 2016; 42:1865–76 [DOI] [PubMed] [Google Scholar]
- 25.Blondonnet R, Joubert E, Godet T, Berthelin P, Pranal T, Roszyk L, Chabanne R, Eisenmann N, Lautrette A, Belville C, Cayot S, Gillart T, Souweine B, Bouvier D, Blanchon L, Sapin V, Pereira B, Constantin JM, Jabaudon M: Driving pressure and acute respiratory distress syndrome in critically ill patients. Respirology 2019; 24:137–45 [DOI] [PubMed] [Google Scholar]
- 26.Georgopoulos D, Xirouchaki N, Tzanakis N, Younes M: Driving pressure during assisted mechanical ventilation: Is it controlled by patient brain? Respir Physiol Neurobiol 2016; 228:69–75 [DOI] [PubMed] [Google Scholar]
- 27.Younes M, Webster K, Kun J, Roberts D, Masiowski B: A method for measuring passive elastance during proportional assist ventilation. Am J Respir Crit Care Med 2001; 164:50–60 [DOI] [PubMed] [Google Scholar]
- 28.Bellani G, Grasselli G, Teggia-Droghi M, Mauri T, Coppadoro A, Brochard L, Pesenti A: Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care 2016; 20:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellani G, Grassi A, Sosio S, Gatti S, Kavanagh BP, Pesenti A, Foti G: Driving pressure is associated with outcome during assisted ventilation in acute respiratory distress syndrome. Anesthesiology 2019; 131:594–604 [DOI] [PubMed] [Google Scholar]
- 30.National Heart L, Blood Institute PCTN, Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, Grissom CK, Gundel S, Hayden D, Hite RD, Hou PC, Hough CL, Iwashyna TJ, Khan A, Liu KD, Talmor D, Thompson BT, Ulysse CA, Yealy DM, Angus DC: Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med 2019; 380: 1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guérin C, Prat G, Morange S, Roch A; ACURASYS Study Investigators: Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–16 [DOI] [PubMed] [Google Scholar]
- 32.Park M, Ahn HJ, Kim JA, Yang M, Heo BY, Choi JW, Kim YR, Lee SH, Jeong H, Choi SJ, Song IS: Driving pressure during thoracic surgery: A randomized clinical trial. Anesthesiology 2019; 130:385–93 [DOI] [PubMed] [Google Scholar]
- 33.Araos J, Alegria L, Garcia P, Cruces P, Soto D, Erranz B, Amthauer M, Salomon T, Medina T, Rodriguez F, Ayala P, Borzone GR, Meneses M, Damiani F, Retamal J, Cornejo R, Bugedo G, Bruhn A: Near-apneic ventilation decreases lung injury and fibroproliferation in an acute respiratory distress syndrome model with extracorporeal membrane oxygenation. Am J Respir Crit Care Med 2019; 199:603–12 [DOI] [PubMed] [Google Scholar]
- 34.Carson SS, Goss CH, Patel SR, Anzueto A, Au DH, Elborn S, Gerald JK, Gerald LB, Kahn JM, Malhotra A, Mularski RA, Riekert KA, Rubenfeld GD, Weaver TE, Krishnan JA; American Thoracic Society Comparative Effectiveness Research Working Group: An official American Thoracic Society research statement: Comparative effectiveness research in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med 2013; 188:1253–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida T, Piraino T, Lima CAS, Kavanagh BP, Amato MBP, Brochard L: Regional ventilation displayed by electrical impedance tomography as an incentive to decrease positive end-expiratory pressure. Am J Respir Crit Care Med 2019; 200:933–7 [DOI] [PubMed] [Google Scholar]
- 36.Malhotra A, Drazen JM: High-frequency oscillatory ventilation on shaky ground. N Engl J Med 2013; 368:863–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beitler JR, Majumdar R, Hubmayr RD, Malhotra A, Thompson BT, Owens RL, Loring SH, Talmor D: Volume delivered during recruitment maneuver predicts lung stress in acute respiratory distress syndrome. Crit Care Med 2016; 44:91–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hubmayr RD, Malhotra A: Still looking for best PEEP. Anesthesiology 2014; 121:445–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hubmayr RD, Shore SA, Fredberg JJ, Planus E, Panettieri RA Jr, Moller W, Hey der J, Wang N: Pharmacological activation changes stiffness of cultured human airway smooth muscle cells. Am J Physiol 1996; 271(5 Pt 1):C1660–8 [DOI] [PubMed] [Google Scholar]