Abstract
Background
Limited information is available about the proportion of patients with degenerative lumbar spinal disease (DLSD) who have gastrointestinal (GI) and cardiovascular (CV) risk factors. Many DLSD patients are prescribed nonsteroidal anti-inflammatory drugs (NSAIDs) that are known to carry risks to the GI and CV systems by increasing GI bleeding and thromboembolic events. This study aimed to measure the prevalence of GI and CV risk in patients with DLSD and to ascertain whether the prescription of NSAIDs is in line with current guidelines.
Methods
This study included 153 patients with symptomatic DLSD who were planning to undergo lumbar spinal surgery. The GI profile was checked using the GI Standardized Calculator of Risk for Event system and CV risk was evaluated using the presence of metabolic syndrome. The conformity of the prescription of NSAIDs was investigated according to the recommendations in current guidelines.
Results
More than half of the patients (59.5%) had high or very high GI risk, and 66% of the patients were diagnosed with metabolic syndrome, which corresponds with CV risk. The rate of simultaneous GI and CV risk was 40.5% (n = 62 / 153; gastrointestinal Standardized Calculator of Risk for Event, > high and metabolic syndrome, yes). The actual prescription of NSAIDs was not in accordance with current guidelines.
Conclusions
Two out of 3 patients had GI or CV risk factors, and approximately 40% of patients had both. Detailed assessment of GI and CV risk in patients with DLSD by using effective evaluation tools is mandatory for optimal medical treatment.
Keywords: Gastrointestinal disorder, Cardiovascular disease, Risk assessment, Spine disease, Non-steroidal anti-inflammatory agents
Lower back pain in degenerative spinal lumbar disease (DSLD) is strongly linked with the inflammatory reaction that is associated with intervertebral disc degeneration1) and back muscle degeneration.2) Nonsteroidal anti-inflammatory drugs (NSAIDs) are most frequently prescribed for pain management in DSLD.3,4) NSAIDs inhibit the activity of cyclo-oxygenase, thereby inhibiting the synthesis of prostaglandins and thromboxane. There are 2 categories: non-selective NSAIDs (ns-NSAIDs) and cyclooxygenase 2 (COX-2) selective inhibitors (coxibs). These drugs are frequently used for long periods of time by elderly patients.5) However, NSAIDs are not always appropriately prescribed, and some authors have called attention to the high prevalence of inappropriate prescription of these drugs, especially in elderly patients, because NSAIDs are known to carry risks to the gastrointestinal (GI) and cardiovascular (CV) systems by increasing GI bleeding and thromboembolic events.6) It is well established that ns-NSAIDs increase the risk of serious GI events. A person treated with NSAIDs has a 3–5 times higher risk of developing GI complications than a person not treated with these drugs.7) Furthermore, in severe cases, ns-NSAIDs can cause death due to GI bleeding.8,9) Mortality caused by GI complications is 16,500 deaths per year in the USA, which is comparable to the number of deaths from acquired immunodeficiency syndrome (AIDS).10) While coxibs have a better GI safety profile than ns-NSAIDs,11) long-term use and high dose of coxibs may increase CV risk.12,13,14) In addition, ns-NSAIDs may have a similar deleterious CV effect.15,16,17)
Recently, some meta-analyses have tried to identify specific NSAIDs that are associated with relatively lower CV risk.18,19) Appropriate selection of NSAIDs should be driven by the assessment of the GI and CV risks of individual patients.3,20) Many authors have recommended specific therapeutic guidelines using various tools to evaluate GI and CV risks.9,21) Despite the frequent use of NSAIDs for patients with degenerative lumbar spinal disease (DLSD) and the known risks on the GI and CV systems, the prevalence of such risks and current prescription patterns of NSAIDs have not been fully investigated.
Therefore, we sought to (1) identify the prevalence of GI and CV risks in patients with DLSD; (2) investigate the relationship between these risks and patient demographic variables such as age, sex, and body mass index (BMI); and (3) analyze the pattern of NSAID prescription in our cohort of subjects with these risks.
METHODS
Patients
We conducted a retrospective cross-sectional observational study of patients with symptomatic DLSD who were scheduled for lumbar decompression surgery with or without posterolateral fusion. Data from patients were collected from June 2013 to July 2015. Patients were considered for inclusion in the study if they met the following criteria: (1) diagnosis of symptomatic DLSD; (2) age ≥ 50 years; (3) scheduled for decompression surgery with or without posterolateral fusion; and (4) verbal consent from the patients or their legal representatives. Exclusion criteria were (1) age < 50 years and (2) refusal to participate in the study. A total of 153 patients were enrolled in this study. This study was approved by the Institutional Review Board of Severance Hospital (IRB No. 4-2014-0571).
GI and CV Risk Evaluation
To assess the risk factors for GI complications, we used the GI Standardized Calculator of Risk for Event (SCORE) system for each patient (Appendix 1). Developed by the Division of Immunology and Rheumatology at Stanford University,22) the SCORE tool assigns points to different patient characteristics to stratify patients according to the risk of developing serious GI complications. A Northern California Health Maintenance Organization (HMO) developed a treatment guideline, indicating that different NSAIDs should be recommended for different patients, depending on the total number of points assigned by the SCORE tool.23) For the purpose of this study, 6 predictors of the SCORE system were investigated: age, diagnosis of rheumatoid arthritis, current health status, proportion of time taking steroids, history of previous GI side effects, and history of previous GI hospitalization.23) This GI SCORE was determined on each patient's admission day for surgery.
CV risk was also evaluated by checking the presence of metabolic syndrome. Metabolic syndrome comprises a number of conditions, including obesity, atherogenic dyslipidemia, impaired fasting glucose, and hypertension (HTN), the prevalence of which has rapidly increased.24) Among patients with metabolic syndrome, a significantly increased risk of CV disease25) and mortality26) are reported. Therefore, identifying metabolic syndrome is thought to be a simple method of CV risk evaluation. The presence of metabolic syndrome was defined using the National Cholesterol Education Program (NCEP ATPIII).27) The cutoffs established for Korean adults, as proposed by The Korean Society for the Study of Obesity, were adopted for the diagnosis of abdominal obesity.28) The criterion for diagnosis of high glucose was adopted from the guidelines established by the American Diabetes Association.29) Subjects with 3 or more of the following characteristics were considered to have metabolic syndrome: abdominal obesity (waist circumference [WC] ≥ 90 cm in men and ≥ 80 cm in women); hypertriglyceridemia ≥ 150 mg/dL; low high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL in men and < 50 mg/dL in women; high blood pressure ≥ 130/85 mmHg or use of antihypertensive medication; and high fasting glucose ≥ 100 mg/dL or under treatment for diabetes. The measurement of WC and a laboratory test were performed on the day of hospital admission. Based on these criteria, we assigned to each patient a metabolic syndrome score between 0 and 5 points (Appendix 2).
Evaluation of the Relationship between GI and CV Risks and Demographic Variables
Demographic data such as age, sex, and BMI were collected on the day of admission. The relationship between GI and CV risks and these variables was evaluated by statistical methods.
Evaluation of the Pattern of NSAID Prescription
Data on the types of NSAID and gastroprotective agents prescribed by physicians were obtained at the outpatient clinic office. To evaluate patterns of NSAID prescription, patients underwent assessment of GI and CV risks and were classified into 6 subgroups according to the guidelines for prevention of NSAID-related ulcer complications.21) Then, we investigated the conformity of the current prescription to the recommendations of the guidelines.
Statistical Analysis
The descriptive analysis of the patients included demographic variables, diagnosis, duration of symptoms, and duration of medication. All continuous variables were expressed as median with interquartile range (IQR) because data were tested for normality using the Shapiro-Wilk test and were found to be non-normally distributed. Prevalence of GI and CV risks was expressed as median with IQR for continuous variables and frequency and percentage for discrete data. Patient distribution of GI and metabolic syndrome scores was plotted using an x-y plot. In the analysis of the relationship between GI / CV risks and demographic variables, a Spearman correlation analysis and nonparametric regression analysis using locally estimated scatterplot smoothing curves with a span of 80% were conducted for continuous variables such as age and BMI. Differences in GI and metabolic syndrome scores between sexes were tested using the Mann-Whitney U-test. To identify prescription patterns of NSAIDs in DLSD patients, a 2-dimensional scatter plot with GI SCORE as the X-axis and metabolic syndrome score as the Y-axis was drawn. It was divided into 6 regions according to guidelines for the prevention of NSAID-related ulcer complications. Conformity of actual prescriptions with recommendations in the guidelines was calculated as percentage. MedCalc ver. 11.6 (MedCalc Software, Mariakerke, Belgium) and R ver. 3.1.0 (Comprehensive R Archive Network, GNU General Public License) were used for all statistical analyses.
RESULTS
Patient Characteristics
This study included 153 patients older than 50 years who were planning to have lumbar decompression surgery. The patient demographic and clinical characteristics are summarized in Table 1. The median age was 70 years, and most patients were women (male : female = 109 : 44). The median value of BMI was 24.4 kg/cm2. The diagnosis of DLSD was spinal stenosis in 75.1% (n = 115 / 153), herniated lumbar disc in 13.7% (n = 21 / 153), and spondylolisthesis in 13% (n = 13 / 153) of patients. The duration of symptoms before surgery was 3.2 years and the duration of medication was 0.7 years.
Table 1. Demographic Data (n = 153).
| Variable | Value |
|---|---|
| Age (yr) | 70.0 (64.0–74.0) |
| Sex (female : male) | 109 : 44 |
| Body mass index (kg/cm2) | 24.4 (22.7–26.7) |
| Diagnosis | |
| Spinal stenosis | 115 (75.1) |
| Herniated disc | 21 (13.7) |
| Spondylolisthesis | 13 (8.5) |
| Duration of symptom (yr) | 3.2 (2.9–3.6) |
| Duration of medication (yr) | 0.7 (0.5–0.9) |
Values are presented as median (interquartile range) or number (%).
Prevalence of GI and CV Risks
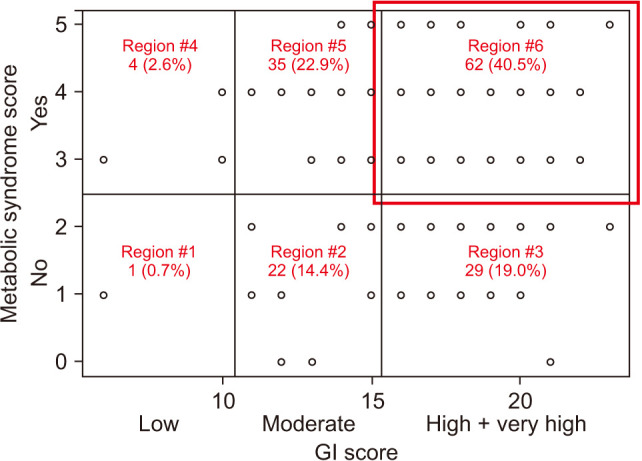
The median GI SCORE was 16.0. The GI SCORE system was used to classify subjects into 4 groups: low (< 10), moderate (11–15), high (16–20), or very high (> 20) GI risk groups. More than half of patients (59.5%; n = 92 / 153) had high or very high GI risk, whereas only 3.3% had low GI risk. The median metabolic syndrome score was 3.0 and 66% (n = 101 / 153) of patients had metabolic syndrome, which corresponded with CV risk (Table 2). The rate of simultaneous GI and CV risk was 40.5% (n = 62 / 153; GI SCORE, > high and metabolic syndrome, yes) (Fig. 1).
Table 2. Prevalence of GI and CV Risks.
| Variable | Value |
|---|---|
| GI score | 16 (15–18) |
| Grade of GI score | |
| Low risk (≤ 10) | 5 (3.3) |
| Moderate risk (11–15) | 57 (37.2) |
| High risk (16–20) | 80 (52.3) |
| Very high risk (> 20) | 11 (7.2) |
| Metabolic syndrome score | 3 (2–4) |
| Presence of metabolic syndrome | |
| No | 52 (34) |
| Yes | 101 (66) |
Values are presented as median (interquartile range) or number (%).
GI: gastrointestinal, CV: cardiovascular.
Fig. 1. The scatter plot according to gastrointestinal (GI) and cardiovascular risk. Each region is marked according to American College of Gastroenterology guidelines for prevention of nonsteroidal anti-inflammatory drug-related ulcer complications.19).
Relationship between GI / CV Risk and Demographic Variables
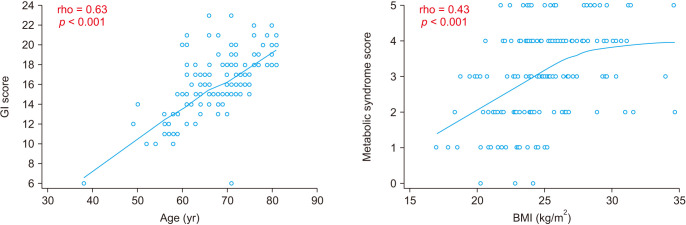
GI risk showed a positive relationship with age (rho = 0.63, p < 0.001) and CV risk showed a positive relationship with BMI (rho = 0.43, p < 0.001) (Fig. 2). Sex was not correlated with GI risk (p = 0.177; GI SCORE: median, 15.5 [IQR, 14.5–18.0] for male vs. median, 16.0 [IQR, 15.0–18.0] for female) or CV risk (p = 0.184; metabolic syndrome score: median, 3.0 [IQR, 2.0–4.0] for male vs. median, 3.0 [IQR, 2.0–4.0] for female) (Fig. 3). GI risk was more positively correlated with age in patients with metabolic syndrome (rho = 0.65, p < 0.001) than patients without metabolic syndrome (rho = 0.59, p < 0.001). However, there was no significant correlation between GI risk and BMI regardless of the presence of metabolic syndrome (p > 0.05) (Fig. 4).
Fig. 2. A scatter plot with regression curve. Gastrointestinal (GI) score and metabolic syndrome score are positively correlated with age and body mass index (BMI), respectively. Correlation analysis was performed using a Spearman method, which produces a correlation coefficient (rho) with a significance level (p). Nonparametric regression analysis was performed using locally estimated scatterplot smoothing curves with a span of 80%.
Fig. 3. Relationship between GI/CV risk and demographic variables (age, sex, and BMI). The arrows marked with O indicate a significant correlation, otherwise the correlation is nonsignificant. Spearman correlation analysis was performed for age and BMI. A Mann-Whitney U-test was performed for sex. GI: gastrointestinal, CV: cardiovascular, IQR: interquartile range, BMI: body mass index.
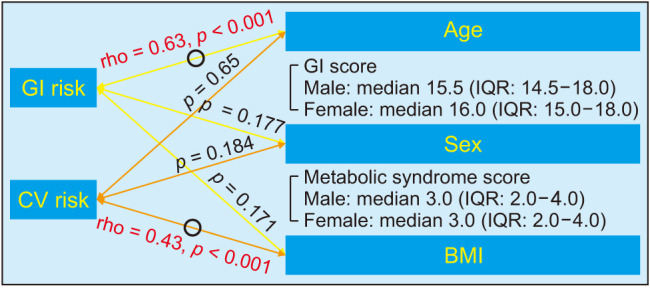
Fig. 4. A Co-plot according to the presence of metabolic syndrome. Gastrointestinal (GI) score correlates positively with age more strongly in patients with metabolic syndrome (rho = 0.65) than in patients without metabolic syndrome (rho = 0.59). There is no significant correlation between GI score and body mass index (BMI) regardless of the presence of metabolic syndrome.
Pattern of NSAID Prescription
The most commonly prescribed pattern of NSAIDs in DLSD patients was ns-NSAIDs alone (n = 71, 46.4%) followed by alternative therapy (n = 48, 31.4%) regardless of the presence of GI and CV risk factors. All alternative therapy comprised the prescription of opioids. The prescription of NSAIDs was not in accordance with current guidelines or recommendations (Table 3).
Table 3. Pattern of NSAID Prescription.
| Variable | Region #1 | Region #2 | Region #3 | Region #4 | Region #5 | Region #6 | Total (%) |
|---|---|---|---|---|---|---|---|
| Recommendation | NSAIDs alone | NSAIDs + PPI/misoprostol | COX-2 inhibitor or alternative therapy | Naproxen + PPI/misoprostol | Naproxen + PPI/misoprostol | Alternative therapy | |
| Number (%) | 1 (0.7) | 22 (14.4) | 29 (19.0) | 4 (2.6) | 35 (22.9) | 62 (40.5) | 153 (100) |
| Current prescription | |||||||
| NSAIDs alone | 0 | 6 | 13 | 4 | 20 | 28 | 71 (46.4) |
| NSAIDs + PPI/misoprostol | 0 | 1 | 2 | 0 | 3 | 7 | 13 (8.5) |
| COX-2 inhibitor or alternative therapy | 0 | 1 | 2 | 0 | 3 | 13 | 19 (12.4) |
| Naproxen + PPI/misoprostol | 0 | 1 | 0 | 0 | 0 | 1 | 2 (1.3) |
| Alternative therapy | 1 | 13 | 12 | 0 | 9 | 13 | 48 (31.4) |
| Conformity (%) | 0 | 5 | 7 | 0 | 0 | 21 |
NSAID: nonsteroidal anti-inflammatory drug, PPI: proton pump inhibitor, COX-2: cyclooxygenase 2.
DISCUSSION
Our results show that the prevalence of GI and CV risks or both in patients with DLSD was relatively high compared to that in the general population. The GI risk in DLSD patients was about 60% in this study, which was higher than the average prevalence of high GI risk (45%) among the general population of orthopedic patients visiting outpatient clinics in Korea.30) The CV risk in this study was 66% and this was higher than that for patients with knee osteoarthritis in Korea (53%).31) Our study is the first to describe the prevalence of DLSD patients with both GI and CV risks, and the rate was about 40%.
These figures underscore the need for more careful assessment of GI and CV risks in DLSD patients since they are relatively older and are often chronic users of NSAIDs. Lanas et al.20) reported that among patients with osteoarthritis who need treatment with NSAIDs, 22.3% had a high GI risk, 44.2% had a high CV risk, and 15.5% had a high combined risk. Compared to that study, the results of the current study show a much higher GI and CV risk prevalence among patients with DLSD, although different tools were used to evaluate GI and CV risks in the 2 studies. According to the National Health Insurance Database of Korea, among 11,000 patients who underwent spine surgery, about 42% of patients had GI comorbidities, such as peptic ulcer disease32) and 37% of patients had CV comorbidity, such as myocardial infarction or congestive heart failure.31) Although these figures are lower than the prevalence of the risks revealed in this study, this still represents a relatively high rate of GI and CV comorbidities after spinal surgery. These results suggest that GI and CV risks should be recognized and evaluated as comorbid factors of spinal surgery in patients with symptomatic DLSD. More attention should be paid to selecting the proper NSAIDs for postoperative pain or persisting symptoms after surgery.
We also found that the GI risk has a positive relationship with age in DLSD patients. It is well known that old age and chronic use of NSAIDs are the most common risk factors for the development of serious GI adverse reactions.3) A previous study found that GI risk in patients over 75 years is approximately twice that of patients over 60 years.10) The current study showed that most patients had taken NSAIDs for quite a long period (mean, 7 months) before spinal surgery and found that GI SCORE correlated positively and strongly with age. These results suggest the need for mandatory GI screening, especially for older patients. On the other hand, CV risk had a positive relationship with BMI in DLSD patients. Considering that obesity is a well-established CV risk factor, this result was predictable, but it implies that decreased physical activity in DLSD patients can have a negative influence on a patient's BMI and we should pay more attention to CV risk in DLSD patients with higher BMI.
Finally, prescription patterns of NSAIDs in clinical practice showed relatively low conformity to recommendations. Current guidelines recommend the use of selective COX-2 inhibitors or conventional NSAIDs with proton pump inhibitors in patients with high GI risk.21) However, this study showed that NSAIDs or gastroprotectives were often prescribed according to the physician's habits and preferences rather than according to the guidelines. Moreover, according to the current guidelines, patients with combined GI and CV risk should not take NSAIDs or coxibs unless absolutely necessary.21) Nevertheless, this study revealed that most patients with DLSD were prescribed NSAIDs regardless of their GI or CV risk factors. These results suggest that more attention should be paid to the guidelines to reduce these risks.
The limitations of the current study were the same as those of any cross-sectional observational study. The number of enrolled patients was small because we investigated only those subjects planning spinal surgery in a single spine center. Furthermore, the prevalence might differ depending on which screening tool is used to assess GI and CV risks. Despite these limitations, the high prevalence of GI and CV risks in patients with surgically indicated symptomatic DLSD underscores the need for spine surgeons to execute routine GI and CV screening before medical treatment, especially when prescribing NSAIDs.
In conclusion, this study documented the prevalence and baseline profile of GI and CV risks in patients with symptomatic DLSD. The prevalence of GI and CV risks or both in patients with DLSD was relatively high compared to that in the general population. Two out of 3 patients had a high GI or CV risk, and approximately 40% of patients had both. In DLSD patients, GI risk showed a positive relationship with age, whereas CV risk showed a positive relationship with BMI. These results highlight the need for individual assessment of GI and CV risks and selection or limitation of NSAIDs under suggested guidelines. However, current prescription patterns of NSAIDs showed relatively low conformity to the recommendations. Detailed recognition of GI and CV risks in patients with symptomatic DLSD by using effective evaluation tools is mandatory for optimal medical treatment.
Appendix 1
NSAIDs GI Risk: Standardized Calculator of Risk for Event (SCORE) Card
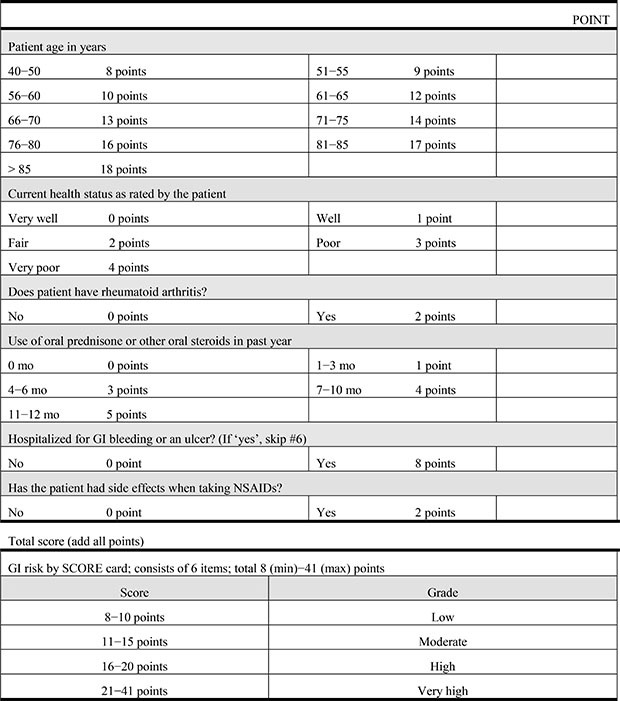
NSAID: nonsteroidal anti-inflammatory drug, GI: gastrointestinal.
Appendix 2
NSAIDs CV Risk: Definition of Metabolic Syndrome
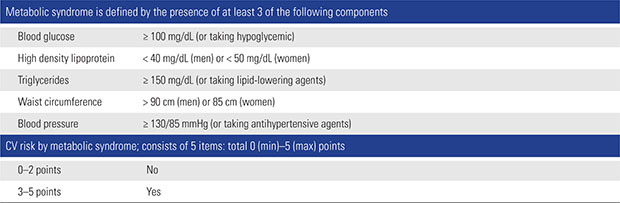
NSAID: nonsteroidal anti-inflammatory drug, CV: cardiovascular.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Rahyussalim AJ, Zufar ML, Kurniawati T. Significance of the association between disc degeneration changes on imaging and low back pain: a review article. Asian Spine J. 2020;14(2):245–257. doi: 10.31616/asj.2019.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim WJ, Kim KJ, Song DG, et al. Sarcopenia and back muscle degeneration as Risk factors for back pain: a comparative study. Asian Spine J. 2020;14(3):364–372. doi: 10.31616/asj.2019.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patricio JP, Barbosa JP, Ramos RM, Antunes NF, de Melo PC. Relative cardiovascular and gastrointestinal safety of non-selective non-steroidal anti-inflammatory drugs versus cyclo-oxygenase-2 inhibitors: implications for clinical practice. Clin Drug Investig. 2013;33(3):167–183. doi: 10.1007/s40261-013-0052-6. [DOI] [PubMed] [Google Scholar]
- 4.van Tulder MW, Scholten RJ, Koes BW, Deyo RA. Nonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine (Phila Pa 1976) 2000;25(19):2501–2513. doi: 10.1097/00007632-200010010-00013. [DOI] [PubMed] [Google Scholar]
- 5.Scheiman JM, Fendrick AM. Practical approaches to minimizing gastrointestinal and cardiovascular safety concerns with COX-2 inhibitors and NSAIDs. Arthritis Res Ther. 2005;7 Suppl 4(Suppl 4):S23–S29. doi: 10.1186/ar1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juby AG, Davis P. Utility of published guidelines on the use of nonsteroidal anti-inflammatory drugs in the elderly. Clin Rheumatol. 2008;27(9):1191–1194. doi: 10.1007/s10067-008-0952-7. [DOI] [PubMed] [Google Scholar]
- 7.Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24(2):121–132. doi: 10.1016/j.bpg.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Lanas A. Prevention and treatment of NSAID-induced gastroduodenal injury. Curr Treat Options Gastroenterol. 2006;9(2):147–156. doi: 10.1007/s11938-006-0033-4. [DOI] [PubMed] [Google Scholar]
- 9.Schlansky B, Hwang JH. Prevention of nonsteroidal anti-inflammatory drug-induced gastropathy. J Gastroenterol. 2009;44 Suppl 19:44–52. doi: 10.1007/s00535-008-2275-5. [DOI] [PubMed] [Google Scholar]
- 10.Gene E, Calvet X, Moron A, Iglesias ML. Recommendations for the use of anti-inflammatory drugs and indications for gastrointestinal protection in emergency departments. Emergencias. 2009;21(4):295–300. [Google Scholar]
- 11.Rostom A, Muir K, Dube C, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: a Cochrane Collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5(7):818–828. doi: 10.1016/j.cgh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, Wittes J, Finn PV, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117(16):2104–2113. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia Rodriguez LA, Gonzalez-Perez A. Long-term use of non-steroidal anti-inflammatory drugs and the risk of myocardial infarction in the general population. BMC Med. 2005;3:17. doi: 10.1186/1741-7015-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antman EM, DeMets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation. 2005;112(5):759–770. doi: 10.1161/CIRCULATIONAHA.105.568451. [DOI] [PubMed] [Google Scholar]
- 15.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332(7553):1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirayama A, Tanahashi N, Daida H, et al. Assessing the cardiovascular risk between celecoxib and nonselective nonsteroidal antiinflammatory drugs in patients with rheumatoid arthritis and osteoarthritis. Circ J. 2014;78(1):194–205. doi: 10.1253/circj.cj-12-1573. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Diaz S, Varas-Lorenzo C, Garcia Rodriguez LA. Non-steroidal antiinflammatory drugs and the risk of acute myocardial infarction. Basic Clin Pharmacol Toxicol. 2006;98(3):266–274. doi: 10.1111/j.1742-7843.2006.pto_302.x. [DOI] [PubMed] [Google Scholar]
- 18.McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med. 2011;8(9):e1001098. doi: 10.1371/journal.pmed.1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanas A, Tornero J, Zamorano JL. Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Ann Rheum Dis. 2010;69(8):1453–1458. doi: 10.1136/ard.2009.123166. [DOI] [PubMed] [Google Scholar]
- 21.Lanza FL, Chan FK, Quigley EM; Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728–738. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 22.Fries JF, Williams CA, Bloch DA, Michel BA. Nonsteroidal anti-inflammatory drug-associated gastropathy: incidence and risk factor models. Am J Med. 1991;91(3):213–222. doi: 10.1016/0002-9343(91)90118-h. [DOI] [PubMed] [Google Scholar]
- 23.Bull SA, Conell C, Campen DH. Relationship of clinical factors to the use of Cox-2 selective NSAIDs within an arthritis population in a large HMO. J Manag Care Pharm. 2002;8(4):252–258. doi: 10.18553/jmcp.2002.8.4.252. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 25.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 26.Trevisan M, Liu J, Bahsas FB, Menotti A. Syndrome X and mortality: a population-based study. Risk Factor and Life Expectancy Research Group. Am J Epidemiol. 1998;148(10):958–966. doi: 10.1093/oxfordjournals.aje.a009572. [DOI] [PubMed] [Google Scholar]
- 27.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 28.Lee SY, Park HS, Kim DJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75(1):72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Han CD, Yang IH, Ha CW. Prescription pattern of NSAIDs and the prevalence of NSAID-induced gastrointestinal risk factors of orthopaedic patients in clinical practice in Korea. J Korean Med Sci. 2011;26(4):561–567. doi: 10.3346/jkms.2011.26.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han CD, Yang IH, Lee WS, Park YJ, Park KK. Correlation between metabolic syndrome and knee osteoarthritis: data from the Korean National Health and Nutrition Examination Survey (KNHANES) BMC Public Health. 2013;13:603. doi: 10.1186/1471-2458-13-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim CH, Chung CK, Park CS, et al. Reoperation rate after surgery for lumbar spinal stenosis without spondylolisthesis: a nationwide cohort study. Spine J. 2013;13(10):1230–1237. doi: 10.1016/j.spinee.2013.06.069. [DOI] [PubMed] [Google Scholar]